In this issue of Blood, Lafouresse et al visualized the in vivo behavior of human chronic lymphocytic leukemia (CLL) cells within the mouse lymph node (LN) microcirculation and discovered that CLL cells bind to LN high endothelial venules (HEVs) via an L-selectin (CD62L)-dependent adhesion process. They also found that CLL cells from patients treated with the B-cell receptor (BCR) inhibitor idelalisib decrease their levels of L-selectin and reduce binding to LN HEVs.1
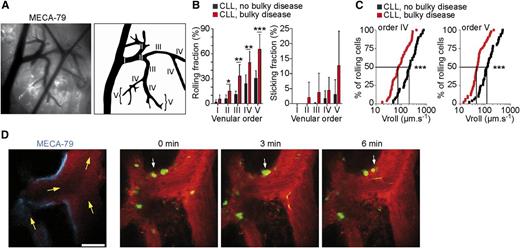
Human CLL cells roll, stick, and crawl within mouse LN HEVs in vivo. (A) Visualization of the mouse inguinal LN microcirculation by intravital microscopy. (B) Rolling and sticking of human CLL cells within mouse LN HEVs. (C) CLL cells from patients with bulky disease roll slower within LN HEVs. (D) Two-photon microscopy time-lapse images showing CLL cells from patients with bulky disease (arrowheads) crawling on HEV endothelium. See the complete Figure 3 in the article by Lafouresse et al that begins on page 1336.
Human CLL cells roll, stick, and crawl within mouse LN HEVs in vivo. (A) Visualization of the mouse inguinal LN microcirculation by intravital microscopy. (B) Rolling and sticking of human CLL cells within mouse LN HEVs. (C) CLL cells from patients with bulky disease roll slower within LN HEVs. (D) Two-photon microscopy time-lapse images showing CLL cells from patients with bulky disease (arrowheads) crawling on HEV endothelium. See the complete Figure 3 in the article by Lafouresse et al that begins on page 1336.
HEVs are specialized blood vessels that allow the entry of normal B lymphocytes into LN-specific microenvironments. Normal B cells bind to HEVs through a multistep adhesion cascade process that entails rolling, sticking, and crawling.2 The mechanisms that regulate CLL-cell migration in vivo remain incompletely understood even though it is known that chemokines and chemokine receptors play a central role in malignant B-cell traffic.3 As the microenvironment exerts a critical role in CLL development and progression,3 it is important to determine which elements direct CLL-cell trafficking and to identify which molecules control the initial capture of CLL cells by HEV walls, allowing their entry and re-entry in LNs. Understanding this mechanism would have 2 important implications. First, it might help in defining the molecular features that account for the clinical presentation of small lymphocytic lymphoma (SLL), which differs from CLL because of the presence of bulky LNs with only minimal-to-modest peripheral blood lymphocytosis. Second, it may shed some light onto the mechanisms that are at the basis of the profound CLL-cell redistribution observed in patients treated with BCR inhibitors such as the phosphoinositide 3-kinase δ inhibitor idelalisib.
By intravital microscopy and multiphoton in vivo imaging (see figure), the authors1 investigated the mechanisms that allow CLL-cell entry and/or re-entry in LNs. They observed that CLL cells from patients with bulky disease and elevated numbers of circulating lymphocytes endowed with high levels of L-selectin have a significantly increased rolling fraction and decreased rolling velocity. Conceivably, the decreased rolling velocity facilitates a chemokine-induced integrin-mediated arrest of CLL cells. Along this line an increased rolling velocity was evident in cells expressing lower levels of L-selectin obtained from CLL patients without bulky disease and a reduced rolling was observed in cells from patients with bulky disease but a low number of circulating lymphocytes expressing low levels of L-selectin. These data suggest that a defect in L-selectin–mediated cell entry in LNs through HEVs may explain the clinical features of these CLL/SLL patients. Furthermore, L-selectin was found to be downregulated in vivo on CLL cells from patients treated with idelalisib and this reduction was associated with increased rolling velocity and reduced sticking of CLL cells to HEV walls. The implication is that treatment with idelalisib delivers an in vivo inhibitory effect on the L-selectin–mediated CLL-cell entry into LNs.
The other side of the coin of B-cell trafficking is the LN egress, a process that in normal B cells depends on the sphingosine-1 phosphate receptor S1PR1.4,5 The expression of S1PR1 on CLL cells was found to be reduced in patients with unfavorable prognosis,4 suggesting that impaired S1PR1 expression might cause defective LN egress and a prolonged residency in the LN-conducive habitat. Egress occurs only minimally in SLL, and differential expression of S1PR1 may explain some of the differences between CLL and SLL. Of interest, idelalisib has been shown to increase the expression of S1PR1 on CLL cells.6 This implies that the malignant B-cell egress from LNs is favored by idelalisib treatment: it is plausible to envisage that this mechanism contributes to the observed rapid LN shrinkage and output of CLL cells into the blood.7
Idelalisib has been shown to inhibit chemotaxis of CLL cells toward lymphoid chemokines8 and to interfere with integrin-mediated adhesion of CLL cells to endothelial cells in vitro.9 The observation that idelalisib treatment decreases the levels of L-selectin on CLL cells in vivo and causes a reduced binding of CLL cells to HEV walls in LNs1 adds a further explanation for the profound idelalisib-induced CLL-cell redistribution.7 The drug could not only induce exit of CLL cells from LNs by increasing S1PR1 expression, but also inhibit their re-entry through HEVs by downregulating L-selectin and thus altering integrin-mediated adhesion and chemotaxis.
The finding that L-selectin is the key factor controlling CLL binding to HEVs1 suggests that interfering with L-selectin–mediated CLL-cell trafficking might represent a novel strategy to block dissemination of CLL cells to LNs. This strategy deserves investigation, especially in patients with advanced disease, bulky lymphadenopathy, and high levels of L-selectin on leukemic cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal