In this issue of Blood, Jacque et al demonstrate that acute myeloid leukemia (AML) cells are selectively susceptible to disruption of glutamine metabolism through pharmacologic or genetic inhibition of the enzyme glutaminase C (GAC).1
Glutaminolysis and the consequences of its inhibition in AML. Professional illustration by Patrick Lane, ScEYEnce Studios.
Glutaminolysis and the consequences of its inhibition in AML. Professional illustration by Patrick Lane, ScEYEnce Studios.
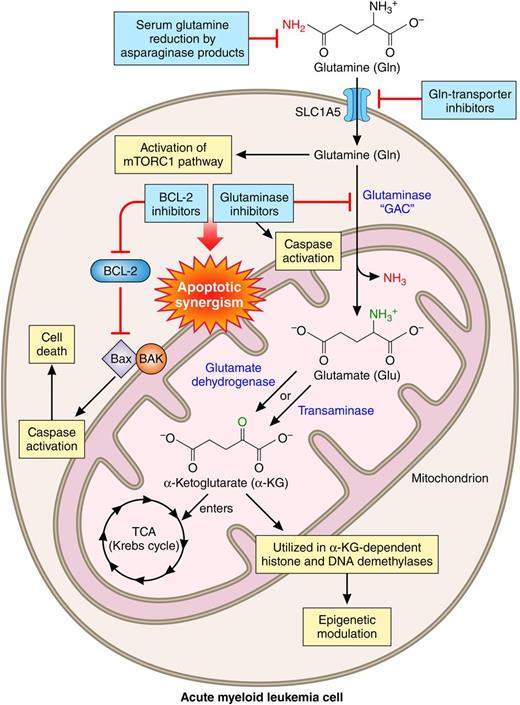
The second law of thermodynamics states that the entropy, or simply chaos, of any system in the universe will always increase over time. Then, how are cancer cells, as new creatures, able to decrease entropy when synthesizing complex and “ordered” biochemical molecules? The answer is one word and is similar to the way that life started on this planet: metabolism. Rapidly dividing cancer cells, including AML cells, genetically reprogram their nutritional requirements to match an increased metabolic demand. The 2 main nutrient sources for growth and survival of cancer cells are glucose, a sugar, and glutamine, an amino acid.2 In humans, glutamine is the most abundant amino acid inside cells, as well as in plasma, with concentrations of ∼20 and 0.6 to 0.9 mM, respectively.3 Glutamine supplies nitrogen for purine and pyrimidine synthesis, as well as for generation of nonessential amino acids for protein synthesis.4 Glutamine also stimulates the mammalian target of rapamycin complex 1 (mTORC1), and its deprivation inhibits mTORC1 and causes apoptosis of AML cells.5
Through a series of enzymatic reactions called glutaminolysis, glutamine, particularly in neoplastic cells, can feed mitochondria and contribute to the entire cancer cell metabolic machinery.6 After entering the cell through a special transporter, glutamine can be converted to glutamic acid by removal of its amide group. This important reaction is catalyzed by a family of enzymes called glutaminases. Subsequently, glutamic acid can be converted to α-ketoglutarate (αKG), either by transamination or by oxidation processes. In an elegant study, Jacque et al investigated the effect of perturbation of glutamine metabolism on mitochondrial function and its detrimental effect on AML cell survival. Glutamine depletion resulted in reduction of intracellular ATP and oxygen consumption rate and, eventually, induction of apoptosis in AML cells. A particular isoform of glutaminase, GAC, was shown to be overexpressed in primary AML cells isolated from patients, AML cell lines, and normal CD34+ hematopoietic cells. Genetic knockdown or pharmacologic inhibition of GAC negatively influenced mitochondrial respiration, cellular proliferation, and survival of AML cells but not normal CD34+ hematopoietic cells. AML cells undergoing these harmful events could be partially or completely rescued by αKG, confirming the mechanism of cellular injury by glutaminase blockade. The investigators also demonstrated the antileukemic effect of glutaminase inhibition in an in vivo mouse model. Finally, by taking advantage of the fact that cytotoxicity of glutaminolysis inhibition is contingent upon mitochondrial depolarization and caspase-dependent apoptosis, the investigators showed a synergistic antileukemic activity between a glutaminase inhibitor and a B-cell lymphoma 2 (BCL-2) inhibitor.
What is the clinical importance of these findings for patients with AML and how soon can they be translated into a therapeutic regimen? First, for patients with AML who are reading the original article or this commentary, it is important to know that these findings absolutely do not suggest that they should avoid a protein- and glutamine-containing diet.7 Second, based on research findings on glutamine addiction of AML cells, there are ongoing clinical trials exploring the therapeutic potential of interruption of glutamine metabolism in AML (#NCT01810705, #NCT02071927, and #NCT02283190). Third, the last 5 decades of hard work and trial after trial have sadly proved to clinicians, scientists, and patients that targeting a single aspect of the cellular apparatus in AML may not provide a significant clinical benefit. It stands to reason that one successful strategy for AML treatment might be to combine US Food and Drug Administration–approved and experimental agents that target specific aspects of glutamine metabolism and together may kill AML cells selectively and efficaciously. Such chemotherapeutic agents may include available inhibitors of glutaminase,1,8 mTOR,9 autophagy,10 and BCL-2,1,9 as well as asparaginase products.3 Let us not forget that further mechanistic studies such as the one reported by Jacque et al are necessary to enhance the diversity of drug candidates that can be logically added to the current list.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal