In this issue of Blood, Wu et al demonstrate that a cluster of microRNAs (miRs) previously shown to be important in malignant disease and B-cell development plays a critical role in the proinflammatory function of donor T cells that mediate acute graft-versus-host disease (GVHD).1
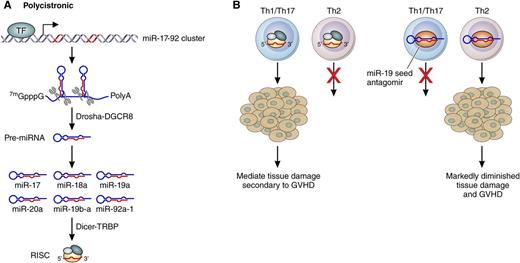
Mechanism of action of miR-17-92 cluster in acute GVHD. (A) Generation of the miR-17-92 pre-miR (denoted by 7mGppG polyadenylation tail species) by the enzymes Drosha-DGCR8 and the incorporation of the mature miR into the RNA-induced silencing complex (RISC) mediated by Dicer-TRBP. (B) The ability of the miR-17-92 complex to enhance the generation of Th1/Th17 cells and their ability to mediate tissue damage is shown. The miR-19 seed antagomir prevents GVHD by blocking the generation of Th1/Th17 cells and enhancing the expansion of Th2 cells. TF, transcription factor. Professional illustration by Patrick Lane, ScEYEnce Studios.
Mechanism of action of miR-17-92 cluster in acute GVHD. (A) Generation of the miR-17-92 pre-miR (denoted by 7mGppG polyadenylation tail species) by the enzymes Drosha-DGCR8 and the incorporation of the mature miR into the RNA-induced silencing complex (RISC) mediated by Dicer-TRBP. (B) The ability of the miR-17-92 complex to enhance the generation of Th1/Th17 cells and their ability to mediate tissue damage is shown. The miR-19 seed antagomir prevents GVHD by blocking the generation of Th1/Th17 cells and enhancing the expansion of Th2 cells. TF, transcription factor. Professional illustration by Patrick Lane, ScEYEnce Studios.
There are increasing levels of intricacy in the fundamental processes of messenger RNA (mRNA) transcription and protein translation. One such intricacy involves the ability of double-stranded RNA to inhibit the generation of or mediate the degradation of mRNA.2 These noncoding RNAs are 18 to 24 nucleotides and form a double-stranded hairpin generated by the RNase III Dicer enzyme3 (see figure). miRs regulate protein synthesis by binding to the 3′ untranslated region to target specific mRNAs. This inhibition is mediated by recruitment of the RNA-induced silencing complex that mediates repression of protein translation and/or deadenylation and subsequent degradation of mRNA targets. miRs have an effect on more than 60% of all protein coding genes.
There are possibly more than 1000 different human miRs. One important miR is a polycistronic region found in humans on chromosome 13 that encodes 6 different miRs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92-1. This miR cluster is conserved among all vertebrates.4 Previous investigators indicated a role for miR-17-92 in the development of several malignant diseases. It is tightly linked to the function of E2F proteins, and the activity of the proapoptotic protein Bim. This cluster of miRs is critical to B-cell development. Several different functions have been found for this miR cluster in T cells, including regulation of effector and memory CD8+ T cells and Th1 T cells, and inhibition of regulatory T-cell responses. However, a role for the miR-17-92 cluster in the biology of GVHD has not been shown previously.
Wu et al1 used mice lacking the expression of the miR-17-92 cluster only in T cells to investigate the function of this miR cluster in the biology of acute GVHD. The authors observed that miR-17-92 was important for the proliferation, survival, and effector function of T cells. Donor T cells that lacked miR-17-92 mediated substantially diminished acute GVHD in 2 separate models: a complete mismatch model and a minor major histocompatibility complex mismatch model. Long-term donor T-cell reconstitution using knockout T cells came from wild-type bone marrow cells, which indicates the profound effect of loss of this miR cluster on donor T-cell function. Interestingly, these effects were associated with impaired accumulation of donor CD4+ T cells in the spleen and liver but there was no impact on donor CD8+ T cells at these sites. This was not expected because previous work suggested a clear function for miR-17-92 in the cytolytic and proliferative activity of CD8+ T cells.
Acute GVHD is mediated predominantly by Th1 and Th17 cells. Wu et al found that the generation of donor Th1 and Th17 cells was markedly decreased in the gastrointestinal tract and mesenteric lymph nodes after the transplantation of T cells that were unable to generate miR-17-92. However, there was an increase in donor cells in the spleen that generated interleukin-4 (IL-4) and IL-5 consistent with a Th2 phenotype. Thus, one important function of the miR-17-92 cluster is polarizing T cells toward a proinflammatory Th1/Th17 profile and away from the generation of Th2 cells after allogeneic transplant.
To be clinically relevant, approaches that diminish GVHD cannot have a large impact on the graft-versus-tumor effect. Wu et al1 used 2 different tumor models to demonstrate that T cells lacking miR-17-92 were still functional in eliminating tumor cells. This may have been the result of the relative preservation of the cytolytic function of donor CD8+ T cells in the absence of miR-17-92. One criticism of genetic approaches in preclinical models is the ability to target a specific pathway only in donor T cells, which would not be possible using clinical approaches. To circumvent this concern, Wu et al made use of locked nucleic acid (LNA) antagomirs targeting miR-17-92. An LNA is an RNA molecule in which an additional methylene bridge fixes the conformation of the ribose moiety. Antagomirs are inhibitors of miRs that are altered to improve their resistance to degradation. Wu et al used 2 different antagomirs that targeted either the miR-17 or miR-19 seed family. The miR-19 antagomir was more effective than the miR-17 in decreasing interferon γ production by CD4+ and CD8+ T cells and improving survival (see figure). Thus, the use of the miR-19 antagomir represents a novel approach to preventing acute GVHD.
One question not addressed by the Wu et al study is the mechanism of action of miR-17-92 in the biology of acute GVHD. There are more than 5000 mRNA targets for miR-19a alone, and previous studies indicate that this miR cluster targets PTEN, TGFBRII, SMAD2, and SMAD4 as well as BCL2L11 (Bim), E2F2, and E2F3. If miR-17-92 is critical to diminishing the function of the transforming growth factor β (TGF-β) pathway, blocking its function could conceivably diminish GVHD via the generation of tolerogenic antigen-presenting cells, via the effects of TGF-β on the migration of immune cells, or perhaps through the expansion of inducible regulatory T cells. This will need to be investigated in future studies.
In terms of future approaches in transplantation biology, this cluster is critical for the generation of pre-B cells. Thus, it is important to determine whether blocking the function of miR-17-92 would have an impact on B-cell homeostasis and/or chronic GVHD.5 Are the antagomirs of miR-19 able to treat acute and/or chronic GVHD? Is this activity altered in the presence of calcineurin inhibitors and/or steroids? These and other questions will be important as these approaches move closer to use in the clinic.
The Wu et al1 study adds to a small but growing list of publications implicating miRs in the biology of acute GVHD.6,7 A previous publication demonstrated an increase in the plasma for specific miRs in patients with acute GVHD that included miR-423, miR-199a-3p, miR-93*, miR-377, miR-155, and miR-30a but, interestingly, not this miR cluster.8 It is anticipated that long noncoding RNA species will be shown to have an impact on the biology of acute GVHD in the not too distant future. These new revelations show that a complex disease is becoming more complex, but this complexity may offer clues to future therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal