Key Points
The V600E kinase-activating mutation of BRAF profoundly shapes the distinct identity of HCL among B-cell neoplasms.
Clinically available BRAF and MEK inhibitors exert potent antileukemic activity in patients’ HCL cells in vitro and in vivo.
Abstract
Hairy cell leukemia (HCL) shows unique clinicopathological and biological features. HCL responds well to purine analogs but relapses are frequent and novel therapies are required. BRAF-V600E is the key driver mutation in HCL and distinguishes it from other B-cell lymphomas, including HCL-like leukemias/lymphomas (HCL-variant and splenic marginal zone lymphoma). The kinase-activating BRAF-V600E mutation also represents an ideal therapeutic target in HCL. Here, we investigated the biological and therapeutic importance of the activated BRAF–mitogen-activated protein kinase kinase (MEK)–extracellular signal-regulated kinase (ERK) pathway in HCL by exposing in vitro primary leukemic cells purified from 26 patients to clinically available BRAF (vemurafenib; dabrafenib) or MEK (trametinib) inhibitors. Results were validated in vivo in samples from vemurafenib-treated HCL patients within a phase 2 clinical trial. BRAF and MEK inhibitors caused, specifically in HCL (but not HCL-like) cells, marked MEK/ERK dephosphorylation, silencing of the BRAF-MEK-ERK pathway transcriptional output, loss of the HCL-specific gene expression signature, downregulation of the HCL markers CD25, tartrate-resistant acid phosphatase, and cyclin D1, smoothening of leukemic cells’ hairy surface, and, eventually, apoptosis. Apoptosis was partially blunted by coculture with bone marrow stromal cells antagonizing MEK-ERK dephosphorylation. This protective effect could be counteracted by combined BRAF and MEK inhibition. Our results strongly support and inform the clinical use of BRAF and MEK inhibitors in HCL.
Introduction
Hairy cell leukemia (HCL) is a mature B-cell malignancy with unique clinicopathological, immunophenotypic, and gene expression features among other B-cell leukemias/lymphomas.1-5 Patients with HCL typically present with pancytopenia, splenomegaly in the absence of significant lymphadenopathy, and infiltration of the bone marrow, spleen, and liver by leukemic cells with peculiar hairy projections emanating from their cell membrane. These leukemic hairy cells circulate usually in low numbers in the peripheral blood and are difficult to aspirate from the bone marrow due to HCL-induced marrow fibrosis.1,4 HCL responds well to chemotherapy with the purine analogs cladribine and pentostatin, but ∼40% of patients relapse and become progressively less responsive to these myelotoxic and immune-suppressive drugs.6,7 Thus, new therapeutic approaches are needed. Recently, by whole-exome sequencing, we discovered the genetic lesion underlying HCL, that is, the V600E phosphomimetic substitution in the activation segment of the BRAF kinase domain.8 The BRAF-V600E mutation defines HCL among B-cell leukemias and lymphomas, as it is clonally present in almost 100% of HCL patients and in almost no patients with other B-cell malignancies.8-10 The latter include HCL-like neoplasms, such as HCL-variant and splenic marginal zone lymphoma with villous lymphocytes, that have clinicopathological features similar to HCL but do not respond well to purine analogs and require a different therapeutic strategy.8-10
The BRAF-V600E mutation is known to be an oncogenic driver in cutaneous melanoma and other solid tumors through constitutive phosphorylation of its downstream kinase targets mitogen-activated protein kinase kinases (MEKs) MEK1 and MEK2, which in turns phosphorylate the extracellular signal-regulated kinases (ERKs) ERK1 and ERK2, leading to cell transformation, proliferation, and inhibition of apoptosis.11,12 Thus, the BRAF-MEK-ERK pathway appears an ideal candidate to illuminate the peculiar biology of HCL and an ideal therapeutic target in HCL13 to be attacked by small-molecule BRAF inhibitors or MEK inhibitors, which have already proven effective in clinical trials of BRAF-V600E+ melanoma patients.14-16 However, comprehensive dissection of the biochemical, molecular, phenotypic, and cellular effects of the BRAF-MEK-ERK pathway in a hematologic malignancy such as HCL is thus far lacking, as are mechanistic studies on the effects of clinically available BRAF and MEK inhibitors in a large number of HCL patients.
Putative “HCL” cell lines lack BRAF-V600E (questioning their true HCL origin) and HCL animal models are missing.17,18 Therefore, to comprehensively explore the biological and therapeutic relevance of the BRAF-MEK-ERK pathway in HCL, we used a variety of assays to study leukemic cells purified from a total of 26 HCL patients. We unraveled features of this pathway that are specific of HCL (ie, regulation of the hairy morphology and expression of the molecular markers of the disease), beyond what might have been predicted from previous work on BRAF-mutated solid tumors.
Materials and methods
Overall study design
Primary leukemic cells, purified (≥85%) from 26 HCL patients and 10 HCL-like patients (4 HCL-variant, 2 splenic marginal zone lymphomas, 4 unclassifiable splenic lymphoma/leukemias), were exposed in vitro to active BRAF inhibitors (vemurafenib or dabrafenib) or the MEK inhibitor trametinib for 30 minutes to 96 hours at various concentrations (up to 1 µM), and were then monitored for: (1) the activation status of MEK and ERK by western blotting (in 25 HCL and 10 HCL-like patients); (2) downstream transcriptional changes by genome-wide expression profiling (in 6 HCL patients); (3) surface morphology changes by confocal microscopy after phalloidin/annexin V staining to highlight the F-actin–rich hairy projections in still living cells (in 9 HCL and 4 HCL-like patients); (4) viability (by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide] or WST [4-(3-[4-iodophenyl]-2-[4-nitrophenyl]-2H-5-tetrazolio)-1,3-benzene disulfonate] metabolic assays) and apoptosis (by annexin V/propidium iodide staining) in 14 HCL and 4 HCL-like patients (analyzed in technical triplicates, except in the few instances noted in the figure legends). Patient samples were obtained from HCL and HCL-like cases seen at our institution or sent from other hospitals. Of the 26 HCL patients analyzed, 14 and 10 were sampled before and after treatment with purine analogs, respectively (for the remaining 2 patients this information was not available), and we did not observe any major differences in the respective response to BRAF inhibition (not shown).
The in vitro results were then validated in vivo in HCL patients refractory to or relapsed after standard chemotherapy with purine analogs and treated with oral Vemurafenib 960 mg twice daily within an ongoing phase 2 clinical trial (Eudract no. 2011-005487-13; A Phase II, Multi-Center, Open Label Study of the Clinical Activity and Safety of the BRAF-V600 Inhibitor Vemurafenib in Previously Treated Patients With Hairy Cell Leukemia Carrying the BRAF-V600E Mutation).
Purification of primary leukemic cells from HCL and HCL-like patients
Peripheral blood leukemic cells were purified from 26 HCL patients and 10 HCL-like patients using Ficoll-Hypaque (GE Healthcare) density gradient centrifugation, followed by magnetic-activated cell sorting to select CD19+ cells (CD19 Microbeads; Miltenyi Biotec). HCL cell purity, as analyzed by flow cytometry for κ/λ light chain restriction or coexpression of CD20+ CD11c, CD103, and/or CD25, was always ≥85%. The study was approved by the Perugia University ethics committee (Prot. no. 2013-039R).
Flow cytometry and immunostichemistry
Full details of immunophenotyping characterization of HCL and HCL-like cells are given in the supplemental Methods (see supplemental Data available on the Blood Web site).
Western blot analysis
The phosphorylation status of MEK and ERK was investigated in HCL patients treated with vemurafenib (n = 23), dabrafenib (n = 12), and trametinib (n = 12), respectively, and in HCL-like patients treated with vemurafenib (n = 10) and dabrafenib (n = 3), respectively (supplemental Table 1). Details are specified in the supplemental Methods.
Genome-wide expression profiling
Primary leukemic cells (7.5 × 105) purified from 6 HCL patients (denoted with the letters A-F in Figure 2C) were seeded in flat-bottom 24-well plates and treated with vemurafenib 1000 nM, or vehicle (dimethylsulfoxide [DMSO]) as control, for 48 hours (6 patients: A-F) or 72 hours (5 patients: A, C, D, E, and F). HCL cells from patient A were treated with vemurafenib in duplicate for 48 and 72 hours. Full details of the genome-wide expression profiling study and bioinformatic analyses are described in the supplemental Methods.
Confocal immunofluorescence microscopy
The morphology of leukemic cells was analyzed in HCL patients treated with vemurafenib (n = 9), dabrafenib (n = 1), PLX4720 (n = 1), or trametinib (n = 1) and in HCL-like patients treated with vemurafenib (n = 4) or PLX4720 (n = 2) (supplemental Table 3, supplemental Methods). Full details are described in the supplemental Methods.
Cytotoxicity studies
The metabolic viability of leukemic cells was analyzed in HCL patients treated with vemurafenib (n = 11), dabrafenib (n = 2), or trametinib (n = 2) and in HCL-like patients treated with vemurafenib (n = 4), using the MTT or WST assays (supplemental Table 5, supplemental Methods).
Flow cytometry with annexin V/propidium iodide was used to evaluate apoptosis in HCL patients treated with vemurafenib (n = 12), dabrafenib (n = 7), or trametinib (n = 6), respectively, and in HCL-like patients treated with vemurafenib (n = 4) (supplemental Table 5, supplemental Methods).
We note the technical challenge posed by working with primary nonproliferating HCL cells, which are not adapted to the growth in vitro and were often collected from patient samples traveling overnight from other hospitals, resulting in spontaneous apoptosis in vehicle-treated cells (35.2%-86.0% cells at 48 hours, 43.1%-84.3% at 72 hours, 41.9%-72.4% at 96 hours). Thus, we cannot exclude that the challenges in cellular procurement and viability may have introduced some unpredictable effects. However, no major differences between samples traveling (n = 12) vs not traveling overnight (n = 14) were observed in terms of basal ERK phosphorylation levels or viability (as evaluated in 12 and 13 samples, respectively, for both assays), nor in terms of drug-modulated ERK phosphorylation (as evaluated in 12 and 13 samples, respectively) or drug-modulated viability (as evaluated in 4 and 10 samples, respectively) (not shown).
Statistical analyses
Details are given in the supplemental Methods.
Results
Vemurafenib, dabrafenib, and trametinib cause strong MEK/ERK dephosphorylation and silence the transcriptional output of the BRAF-MEK-ERK pathway in HCL
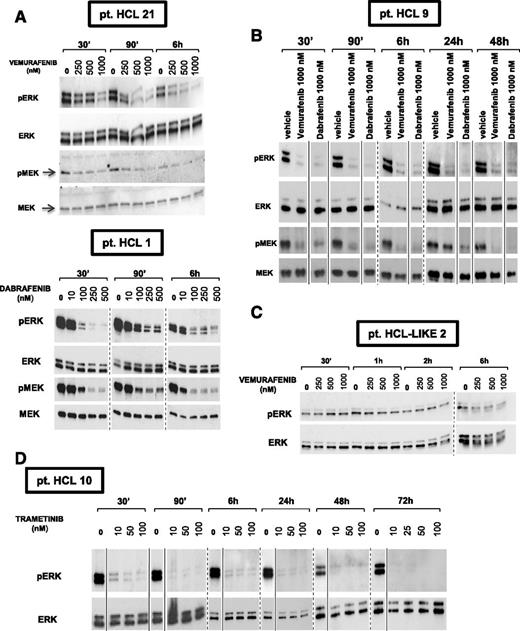
We exposed in vitro primary leukemic cells purified from 25 HCL patients to the BRAF inhibitors vemurafenib and dabrafenib or the MEK inhibitor trametinib14-16 at various concentrations (up to 1 µM) for 30 minutes to 72 hours. A consistent, dose-dependent and sustained MEK/ERK dephosphorylation was observed in all HCL cases, without major differences among the 3 inhibitors (Figure 1A-B,D, supplemental Table 1). Conversely, these effects were not detected in any of 10 HCL-like patients (Figure 1C, supplemental Table 1), which had much lower basal levels of phospho-MEK/phospho-ERK. This demonstrates the specificity of BRAF inhibitors for mutated BRAF.
Vemurafenib, dabrafenib, and trametinib cause sustained, dose-dependent MEK and ERK dephosphorylation in primary HCL but not HCL-like cells. (A-B, D) Western blot analysis of purified HCL cells from 4 representative patients shows strong phosphorylation of both MEK and ERK under basal conditions (0 nM drug, ie, DMSO vehicle only) and their dose-dependent dephosphorylation after 30 minutes to 72 hours of incubation with the specific active BRAF inhibitors vemurafenib and dabrafenib (A-B) at concentrations ranging from 100 nM to 1000 nM, and with the MEK inhibitor trametinib (D) at concentrations ranging from 10 nM to 100 nM. (C) Conversely, primary leukemic cells from a representative HCL-like patient express a relatively low basal level of phospho-ERK which is not influenced by treatment with up to 1000 nM vemurafenib for 30 minutes to 6 hours. Membranes were probed with antibodies against phospho-ERK1/2 (pERK), phospho-MEK1/2 (pMEK), total ERK1/2, and total MEK1/2 as indicated on the left of each panel. Please note that to obtain, from the HCL-like cell lysates, the pERK bands representatively shown in panel C, the exposure of the pERK blot had to be prolonged (tens of minutes) as compared with HCL cell lysates (tens of seconds in A-B). Solid and dashed lines separate lanes repositioned from the same gel and, respectively, lanes taken from different gels.
Vemurafenib, dabrafenib, and trametinib cause sustained, dose-dependent MEK and ERK dephosphorylation in primary HCL but not HCL-like cells. (A-B, D) Western blot analysis of purified HCL cells from 4 representative patients shows strong phosphorylation of both MEK and ERK under basal conditions (0 nM drug, ie, DMSO vehicle only) and their dose-dependent dephosphorylation after 30 minutes to 72 hours of incubation with the specific active BRAF inhibitors vemurafenib and dabrafenib (A-B) at concentrations ranging from 100 nM to 1000 nM, and with the MEK inhibitor trametinib (D) at concentrations ranging from 10 nM to 100 nM. (C) Conversely, primary leukemic cells from a representative HCL-like patient express a relatively low basal level of phospho-ERK which is not influenced by treatment with up to 1000 nM vemurafenib for 30 minutes to 6 hours. Membranes were probed with antibodies against phospho-ERK1/2 (pERK), phospho-MEK1/2 (pMEK), total ERK1/2, and total MEK1/2 as indicated on the left of each panel. Please note that to obtain, from the HCL-like cell lysates, the pERK bands representatively shown in panel C, the exposure of the pERK blot had to be prolonged (tens of minutes) as compared with HCL cell lysates (tens of seconds in A-B). Solid and dashed lines separate lanes repositioned from the same gel and, respectively, lanes taken from different gels.
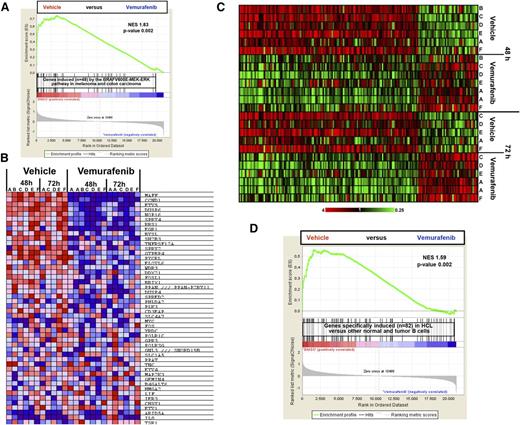
We then investigated the molecular consequences of BRAF inhibition through genome-wide expression profiling (GEP) of leukemic cells purified from 6 HCL cases and exposed in vitro to vemurafenib for 48 to 72 hours. Gene set enrichment analysis19 showed a strong vemurafenib-induced silencing of the BRAF-MEK-ERK pathway transcriptional output as defined in BRAF-mutated melanoma and colorectal carcinoma20 (48 genes, Figure 2A-B). This suggests that a core signature of this pathway exists which is largely shared across highly different tumors.
Vemurafenib silences the transcriptional output of the BRAF-MEK-ERK pathway in HCL and the whole expression signature distinguishing HCL from normal B cells and other B-cell neoplasms. (A) The overall expression of the 48 genes induced by the BRAF-MEK-ERK pathway in melanoma and colorectal carcinoma20 is considerably depleted in the profiles of HCL cells treated for 48 and/or 72 hours with vemurafenib (13 samples from 6 patients) vs drug vehicle (11 samples from the same 6 patients), according to GSEA. (B) Color-coded heat map showing, in the individual HCL samples, the expression (red = high; blue = low) of these 48 genes, ranked by their signal-to-noise ratio (the default GSEA metric) in vemurafenib-treated vs vehicle-treated samples. (C) Color-coded expression heat map of genes significantly modulated (twofold change, corrected P < .05) in HCL cells from 6 patients (A-F) treated with vemurafenib 1 µM vs DMSO for 48 and/or 72 hours. (D) The overall expression of the HCL-specific signature (distinguishing HCL from normal B cells and other B-cell neoplasms3 ) is considerably depleted in the profiles of HCL cells (from 6 patients) treated with vemurafenib vs drug vehicle (DMSO) for 48 and/or 72 hours, according to GSEA.19 NES, normalized enrichment score.
Vemurafenib silences the transcriptional output of the BRAF-MEK-ERK pathway in HCL and the whole expression signature distinguishing HCL from normal B cells and other B-cell neoplasms. (A) The overall expression of the 48 genes induced by the BRAF-MEK-ERK pathway in melanoma and colorectal carcinoma20 is considerably depleted in the profiles of HCL cells treated for 48 and/or 72 hours with vemurafenib (13 samples from 6 patients) vs drug vehicle (11 samples from the same 6 patients), according to GSEA. (B) Color-coded heat map showing, in the individual HCL samples, the expression (red = high; blue = low) of these 48 genes, ranked by their signal-to-noise ratio (the default GSEA metric) in vemurafenib-treated vs vehicle-treated samples. (C) Color-coded expression heat map of genes significantly modulated (twofold change, corrected P < .05) in HCL cells from 6 patients (A-F) treated with vemurafenib 1 µM vs DMSO for 48 and/or 72 hours. (D) The overall expression of the HCL-specific signature (distinguishing HCL from normal B cells and other B-cell neoplasms3 ) is considerably depleted in the profiles of HCL cells (from 6 patients) treated with vemurafenib vs drug vehicle (DMSO) for 48 and/or 72 hours, according to GSEA.19 NES, normalized enrichment score.
BRAF inhibition impacts on the genome-wide expression profile of HCL by downregulating its specific expression signature and markers
A broader supervised analysis of the GEP data identified as many as 30 and 105 genes robustly upregulated and downregulated by vemurafenib in HCL, respectively (Figure 2C, supplemental Table 2), indicating a pronounced influence of BRAF-V600E in driving the GEP of HCL. Notably, besides known transcriptional targets of the BRAF-MEK-ERK pathway (eg, MAFF, CCND1, ETV5, DUSP6, EGR1, SPRY2), several other interesting genes were downregulated by vemurafenib in HCL (supplemental Table 2) and most of them did not emerge as being transcriptionally modulated by BRAF-V600E in solid tumors.20 They included genes encoding for proteins that are HCL diagnostic markers, for example, CD25 (IL2RA) and tartrate-resistant acid phosphatase (TRAP; ACP5),1,21 or being upregulated in HCL in a relatively specific manner across mature B-cell neoplasms, for example, CCND1 (cyclin D1), ACTB (β-actin), THBS1 (thrombospondin-1), AIF1 (allograft inflammatory factor-1), and SPRY2.3,22 We previously showed that HCL is characterized by a unique transcriptional signature, which distinguishes HCL from normal mature B cells and other mature B-cell leukemias/lymphomas, and which resembles that of memory B cells with altered expression of chemokine and adhesion receptors.3 Thus, we asked how this HCL-specific expression signature is modulated as a whole by BRAF inhibition. Strikingly, such a signature was strongly silenced in vemurafenib-treated HCL cells (Figure 2D), supporting a major role of BRAF-V600E in shaping the specific GEP of HCL among normal B cells and other B-cell neoplasms.
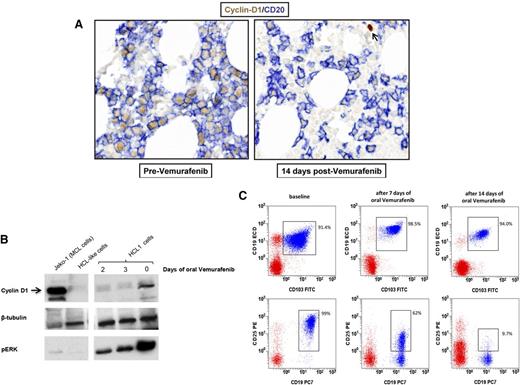
To externally validate at the protein level and in vivo the messenger RNA GEP results obtained in vitro, we monitored cyclin D1 and CD25 protein expression in 3 HCL patients (not analyzed by GEP) who were plurirelapsed after purine analogs and were treated with oral vemurafenib (960 mg twice daily) in a phase 2 clinical trial (EudraCT 2011-005487-13). In one patient, cyclin D1 expression in HCL cells by immunohistochemistry shifted from strong to weak in bone marrow biopsies taken at baseline and after 14 days of vemurafenib, respectively (Figure 3A). In a second case, basal cyclin D1 expression considerably decreased on western blotting after 2 and 3 days of vemurafenib (Figure 3B). In the third patient, CD25 expression by flow cytometry switched from 99% of leukemic cells at baseline to 62% and 9.7% after 7 and 14 days of vemurafenib, respectively (Figure 3C). These phenomena were specific to cyclin D1 and CD25 (both downregulated in vitro by vemurafenib; supplemental Table 2) indeed, leukemic cells maintained the protein expression of genes (CD20/MS4A1, TUBB/tubulin β, CD19, and CD103/ITGAE; Figure 3) not modulated by vemurafenib in vitro (supplemental Table 2).
BRAF inhibition downregulates the expression of HCL-specific markers in vivo. (A) Immunohistochemistry shows strong cyclin D1 downregulation (brown nuclear staining) by HCL cells (defined by the blue membrane staining for CD20) in bone marrow biopsies of a trial HCL patient taken before and after 2 weeks of oral treatment with vemurafenib 960 mg twice daily. The arrow in the right panel indicates a cyclin D1+ non-B cell as internal control. (B) Western blotting for cyclin D1, phospho-ERK, and (as loading control) tubulin β in primary leukemic cells purified from the blood of a HCL patient (HCL 1) before and after 2 and 3 days of oral vemurafenib. A mantle cell lymphoma cell line (Jeko-1) and primary purified leukemic cells from a HCL-like patient were used as positive and negative control for cyclin D1 expression, respectively. (C) Flow cytometric expression of surface CD25 in blood leukemic cells (coexpressing CD19 and CD103; blue events in the CD45+ gate) of HCL patient 4 before and after treatment with oral vemurafenib for 7 and 14 days. Red events represent the rest of CD45+ blood cells.
BRAF inhibition downregulates the expression of HCL-specific markers in vivo. (A) Immunohistochemistry shows strong cyclin D1 downregulation (brown nuclear staining) by HCL cells (defined by the blue membrane staining for CD20) in bone marrow biopsies of a trial HCL patient taken before and after 2 weeks of oral treatment with vemurafenib 960 mg twice daily. The arrow in the right panel indicates a cyclin D1+ non-B cell as internal control. (B) Western blotting for cyclin D1, phospho-ERK, and (as loading control) tubulin β in primary leukemic cells purified from the blood of a HCL patient (HCL 1) before and after 2 and 3 days of oral vemurafenib. A mantle cell lymphoma cell line (Jeko-1) and primary purified leukemic cells from a HCL-like patient were used as positive and negative control for cyclin D1 expression, respectively. (C) Flow cytometric expression of surface CD25 in blood leukemic cells (coexpressing CD19 and CD103; blue events in the CD45+ gate) of HCL patient 4 before and after treatment with oral vemurafenib for 7 and 14 days. Red events represent the rest of CD45+ blood cells.
BRAF or MEK inhibition causes loss of the hairy morphology in living HCL but not HCL-like cells
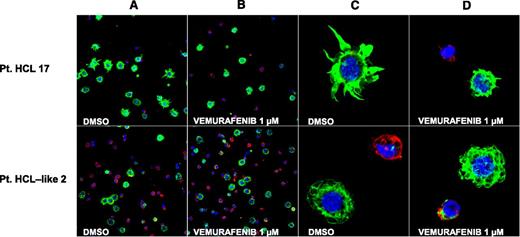
Interestingly, vemurafenib-induced transcriptional changes (supplemental Table 2) included the downregulation of ACTB and LST1. ACTB is enriched in the cytoskeleton of hairy projections4 and LST1 is important in forming actin-containing long-membrane protrusions.23,24 To address whether BRAF-V600E influences the morphology of HCL cells in vitro, we cultured them with vemurafenib, dabrafenib, PLX4720 (a nonclinical BRAF inhibitor),25 or trametinib, and then monitored them for 24 to 72 hours for potential morphologic changes. In particular, leukemic cells purified from 9 HCL and 4 HCL-like patients were analyzed by confocal microscopy following immunostaining with phalloidin (marking the F-actin–rich hairy projections) and ANXA5 (to exclude apoptotic cells that may loose any preexisting morphologic peculiarity). On visual microscopic examination, BRAF inhibitor-treated HCL (but not HCL-like) cells showed a considerable loss of their hairy projections and became smaller and smoother while still alive (Figure 4). Furthermore, a quantitative analysis of various cell shape-related parameters (area, perimeter, and circularity) confirmed a significant smoothening and shrinking of HCL (n = 9) but not HCL-like cells (n = 4) (supplemental Table 3). Importantly, loss of hairy projections in living leukemic cells (before the appearance of any membrane positivity for the apoptotic marker ANXA5) was observed only after exposure to vemurafenib, and not in vehicle-treated HCL cells undergoing spontaneous apoptosis over time. In other words, the latter cells switched from their living hairy phenotype directly to the apoptotic one without passing through the intermediate, vemurafenib-specific, phenotype of living smooth cells. Indeed, in all 6 HCL-evaluable cases, we observed that, although the proportion of apoptotic HCL cells increased over the course of vehicle treatment, the proportion of leukemic cells with the living smooth phenotype was overall low and did not increase with time (supplemental Table 4). This finding excludes that vemurafenib simply accelerates in HCL cells an apoptotic program intrinsically characterized by the intermediate step of “hair loss.”
BRAF inhibition trims the hairy projections of HCL but not HCL-like cells. Confocal immunofluorescence staining for phalloidin (green), annexin V (red) and Draq5 (blue) in primary leukemic cells purified from the blood of a representative HCL patient (no. 17, top panels) and a representative HCL-like patient (no. 2, bottom panels) and treated in vitro with vehicle (DMSO) or vemurafenib 1 µM (for 48 and 72 hours in the HCL and HCL-like patient, respectively). (A-B) Two-dimensional (2D) images of a representative field (×63 optical magnification with oil immersion). (C-D) Electronically magnified 2D-image of a representative cell for each condition.
BRAF inhibition trims the hairy projections of HCL but not HCL-like cells. Confocal immunofluorescence staining for phalloidin (green), annexin V (red) and Draq5 (blue) in primary leukemic cells purified from the blood of a representative HCL patient (no. 17, top panels) and a representative HCL-like patient (no. 2, bottom panels) and treated in vitro with vehicle (DMSO) or vemurafenib 1 µM (for 48 and 72 hours in the HCL and HCL-like patient, respectively). (A-B) Two-dimensional (2D) images of a representative field (×63 optical magnification with oil immersion). (C-D) Electronically magnified 2D-image of a representative cell for each condition.
Overall, these data strongly implicate the deregulated BRAF activity in shaping the hairy morphology selectively in HCL cells.
BRAF or MEK inhibition induces loss of viability due to apoptosis in primary HCL but not HCL-like cells
Then, to evaluate the antileukemic effect of BRAF-MEK-ERK pathway inhibition, we assessed the viability of leukemic cells from 12 HCL patients exposed in vitro to BRAF or MEK inhibitors, using MTT/WST metabolic assays. A consistent loss of viability (ranging from 11.5% to 54% reduction of viability relative to vehicle-treated cells) was observed at all time points (48-96 hours) and drug concentrations (100-1000 nM) in all cases (supplemental Table 5, supplemental Figure 1). In a trial patient unusually featuring a leukemic lymphocytosis (35 190 white blood cells [WBCs]/mm3, 83% leukemic cells), the latter drastically diminished after a week of vemurafenib (6200 WBC/mm3, 38% leukemic cells), confirming ongoing loss of viability in vivo during BRAF inhibition. Because the HCL proliferation rate is very low (<1%)4,26 and vemurafenib-induced transcriptional changes in vitro (supplemental Table 2) included upregulation of the apoptotic activators BCL2L11/BIM and CDKN1C/p57-KIP2,27 we asked whether viability loss was due to apoptosis. Indeed, BRAF or MEK inhibition significantly reduced living ANXA5-negative cells (12.4%-84.4% reduction, relative to vehicle) in all 13 HCL patients studied (Figure 5A, supplemental Table 5). To account for possible decreases of drug stability and/or availability in vitro over time, in 2 HCL cases we refreshed daily the drug-supplemented medium, keeping fixed the drug concentration to simulate the steady state in daily dosed patients. In both instances, this caused an even greater reduction of viability (up to twofold) and of ANXA5-negative cells (up to 1.35-fold) than observed without drug renewal (supplemental Figure 2).
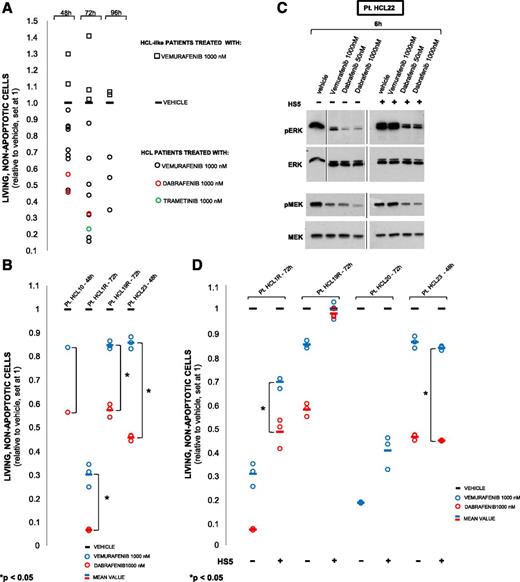
BRAF inhibition induces apoptosis in primary HCL (but not HCL-like cells), which is partially rescued by coculture with bone marrow stromal cells blunting MEK-ERK dephosphorylation. (A) Quantification of apoptosis (by flow cytometry for ANXA5) in leukemic cells from 12 HCL (circles) and 4 HCL-like (squares) patients (supplemental Table 5), treated in vitro for 48 to 96 hours with the indicated drugs in triplicate (average shown), except 2 HCL cases run in duplicate or in single. All HCL cases showed drug-induced reduction of living cells from 1 (vehicle; horizontal bar) down to a maximum of 0.156 (ie, 84.4% relative decrease; P < .05 in all patients analyzed in replicate; supplemental Table 5). Conversely, no HCL-like cases featured such a reduction (and rather displayed a paradoxical increase in some instances). (B) Apoptosis by vemurafenib vs dabrafenib in 4 HCL patients (including 2 relapsed after vemurafenib; denoted with R and not included in panel A), performed in triplicate (circles; horizontal line: average) except in patient HCL 10 (single replicate). In all cases, dabrafenib reduced living cells more (1.3- to 3.8-fold) than vemurafenib in a statistically significant manner (P < .05) for all 3 cases analyzed in triplicate. (C) Western blotting for phospho-ERK1/2 and phospho-MEK1/2 of hairy cell protein lysates from a representative HCL patient (HCL 22; supplemental Table 1) exposed in vitro to the indicated drugs for 6 hours, in the presence or absence of HS5 bone marrow stromal cells. (D) Drug-induced apoptosis in the absence of HS5 cells was reduced (1.2- to 2.3-fold for vemurafenib, 1.7- to 7.3-fold for dabrafenib; P < .05, not shown) upon coculture with HS5 cells in all 4 cases tested except HCL 23. However, in the presence of HS5 cells dabrafenib produced more apoptosis than vemurafenib in 2 of 3 cases (HCL 1R, 1.7-fold; HCL 23, 3.4-fold).
BRAF inhibition induces apoptosis in primary HCL (but not HCL-like cells), which is partially rescued by coculture with bone marrow stromal cells blunting MEK-ERK dephosphorylation. (A) Quantification of apoptosis (by flow cytometry for ANXA5) in leukemic cells from 12 HCL (circles) and 4 HCL-like (squares) patients (supplemental Table 5), treated in vitro for 48 to 96 hours with the indicated drugs in triplicate (average shown), except 2 HCL cases run in duplicate or in single. All HCL cases showed drug-induced reduction of living cells from 1 (vehicle; horizontal bar) down to a maximum of 0.156 (ie, 84.4% relative decrease; P < .05 in all patients analyzed in replicate; supplemental Table 5). Conversely, no HCL-like cases featured such a reduction (and rather displayed a paradoxical increase in some instances). (B) Apoptosis by vemurafenib vs dabrafenib in 4 HCL patients (including 2 relapsed after vemurafenib; denoted with R and not included in panel A), performed in triplicate (circles; horizontal line: average) except in patient HCL 10 (single replicate). In all cases, dabrafenib reduced living cells more (1.3- to 3.8-fold) than vemurafenib in a statistically significant manner (P < .05) for all 3 cases analyzed in triplicate. (C) Western blotting for phospho-ERK1/2 and phospho-MEK1/2 of hairy cell protein lysates from a representative HCL patient (HCL 22; supplemental Table 1) exposed in vitro to the indicated drugs for 6 hours, in the presence or absence of HS5 bone marrow stromal cells. (D) Drug-induced apoptosis in the absence of HS5 cells was reduced (1.2- to 2.3-fold for vemurafenib, 1.7- to 7.3-fold for dabrafenib; P < .05, not shown) upon coculture with HS5 cells in all 4 cases tested except HCL 23. However, in the presence of HS5 cells dabrafenib produced more apoptosis than vemurafenib in 2 of 3 cases (HCL 1R, 1.7-fold; HCL 23, 3.4-fold).
The in vitro proapoptotic activity of vemurafenib and dabrafenib could be directly compared in leukemic cells from 4 patients. Dabrafenib reduced ANXA5-negative cells 1.4- to 3.8-fold more than vemurafenib in all cases, including 2 patients relapsed after vemurafenib (Figure 5B). These findings demonstrate the key role of BRAF-V600E in sustaining HCL growth by apoptosis inhibition, and qualify BRAF and MEK inhibitors as potent anti-HCL drugs (with dabrafenib potentially more effective than vemurafenib). This antileukemic activity appears HCL-specific because it was observed in none of the 4 HCL-like cases tested (Figure 5A; supplemental Figure 1, supplemental Table 5).
Apoptosis by BRAF inhibitors is partially rescued by coculture with bone marrow stromal cells blunting MEK-ERK dephosphorylation
To recapitulate in vitro the physiologic microenvironment of HCL cells in vivo, and to investigate its potential modulating effect on BRAF inhibitors’ activity, we cultured HCL cells with or without the bone marrow stromal cell line HS5 and exposed them to vemurafenib or dabrafenib. Interestingly, in all 3 cases tested, HS5 cells dampened ERK and MEK dephosphorylation, but to a lesser extent in dabrafenib-treated compared with vemurafenib-treated cells (Figure 5C). Also, apoptosis was blunted by HS5 cells in 3 of 4 patients tested (Figure 5D). However, dabrafenib appeared again less affected than vemurafenib by this protective stromal effect in 2 of 3 tested cases, including one trial patient relapsed after vemurafenib (in the other, neither BRAF inhibitor significantly induced apoptosis of HCL cells cocultured with stromal cells) (Figure 5D). Finally, in the presence of HS5 cells, combined BRAF and MEK blockade with dabrafenib and trametinib elicited stronger ERK dephosphorylation and more apoptosis than either agent alone (Figure 6). This suggests that the marrow microenvironment can partially rescue HCL cells from apoptosis induced by BRAF inhibitors, likely through antagonizing MEK and ERK dephosphorylation. Indeed, in 3 trial patients presenting with leukocytosis (>10 000 WBC/mm3) due to high numbers of circulating leukemic cells (59.8%-83% of all nucleated blood cells), we observed a rapid clearance (>90% reduction relative to baseline) of the leukemic burden in the blood within 14 days of vemurafenib treatment in all 3 cases. However, a significant leukemic infiltration persisted in the bone marrow biopsy after 8 weeks of vemurafenib in 2 of 3 cases (showing only 17% and 56% reduction relative to baseline), whereas in the third patient the relative reduction was >90%.
Combined BRAF and MEK inhibition counteracts the protective effect of bone marrow stromal cells against ERK dephosphorylation and HCL cell apoptosis induced by single BRAF or MEK inhibition. (A) Western blot analysis of phospho-ERK1/2 levels in hairy cells purified from patient HCL 24 (supplemental Table 1) and exposed in vitro to the indicated BRAF and MEK inhibitors alone or in combination, for 6 hours, in the presence of HS5 bone marrow stromal cells. After cotreatment with dabrafenib 50 nM and trametinib 1 nM, the phospho-ERK bands, relative to the total ERK bands as loading control, are weaker than after either drug alone (twofold and fivefold weaker, respectively, as quantified in the phospho-ERK/ERK ratio indicated below each lane and obtained by densitometry using the ImageJ software). A similar result was obtained in another HCL patient (no. 26, supplemental Table 1) (B) Flow cytometric quantification of living (ANXA5-negative) cells in primary blood leukemic cells purified from a HCL patient (HCL 25) and in vitro exposed in triplicate for 48 hours to the indicated drugs, in the presence or absence of HS5 stromal cells. In the absence of HS5 cells, trametinib 1 nM and dabrafenib 50 nM were able to induce significant (P < .05, supplemental Table 5) HCL cell apoptosis and their combination was not more effective than dabrafenib alone. Conversely, in the presence of HS5 cells drug-induced apoptosis was dampened but combined BRAF and MEK inhibition resulted in more apoptosis than BRAF or MEK inhibition alone, as quantified in the histograms of panel C (mean ± standard deviation [SD], relative to trametinib 1 nM). A similar result was obtained in another HCL patient (no. 26, supplemental Table 5).
Combined BRAF and MEK inhibition counteracts the protective effect of bone marrow stromal cells against ERK dephosphorylation and HCL cell apoptosis induced by single BRAF or MEK inhibition. (A) Western blot analysis of phospho-ERK1/2 levels in hairy cells purified from patient HCL 24 (supplemental Table 1) and exposed in vitro to the indicated BRAF and MEK inhibitors alone or in combination, for 6 hours, in the presence of HS5 bone marrow stromal cells. After cotreatment with dabrafenib 50 nM and trametinib 1 nM, the phospho-ERK bands, relative to the total ERK bands as loading control, are weaker than after either drug alone (twofold and fivefold weaker, respectively, as quantified in the phospho-ERK/ERK ratio indicated below each lane and obtained by densitometry using the ImageJ software). A similar result was obtained in another HCL patient (no. 26, supplemental Table 1) (B) Flow cytometric quantification of living (ANXA5-negative) cells in primary blood leukemic cells purified from a HCL patient (HCL 25) and in vitro exposed in triplicate for 48 hours to the indicated drugs, in the presence or absence of HS5 stromal cells. In the absence of HS5 cells, trametinib 1 nM and dabrafenib 50 nM were able to induce significant (P < .05, supplemental Table 5) HCL cell apoptosis and their combination was not more effective than dabrafenib alone. Conversely, in the presence of HS5 cells drug-induced apoptosis was dampened but combined BRAF and MEK inhibition resulted in more apoptosis than BRAF or MEK inhibition alone, as quantified in the histograms of panel C (mean ± standard deviation [SD], relative to trametinib 1 nM). A similar result was obtained in another HCL patient (no. 26, supplemental Table 5).
Discussion
HCL is unique among all hematologic malignancies due to a number of peculiar morphologic, immunophenotypic, molecular, and clinical features.1-5 The latter include not only exquisite initial sensitivity to myelotoxic chemotherapy with purine analogs, but also frequent relapses accompanied by a progressive loss of response to these drugs with time,6,7 calling for new therapeutic strategies. We recently discovered that HCL is a unique hematologic neoplasm also genetically, in that the BRAF-V600E kinase-activating mutation is present in virtually all HCL patients whereas it is exceptionally rare in all other blood tumors,28 including HCL mimics such as splenic marginal zone lymphoma with villous lymphocytes and HCL variant.8-10 Although the BRAF-MEK-ERK pathway has been already extensively characterized in solid tumors harboring the BRAF-V600E mutation,11,29 it is currently unknown whether its deregulation in a hematologic malignancy such as HCL has specific consequences of biological and therapeutic relevance. In this work, we comprehensively dissected in HCL the biology of the BRAF-MEK-ERK pathway and the molecular and cellular mechanisms of clinically available BRAF and MEK inhibitors, and uncovered several aspects that could not be anticipated from previous studies on BRAF-mutated solid tumors.
Notably, our work is based on the in vitro study of primary leukemic cells purified from a large number (n = 26) of HCL patients, followed by extensive in vivo validation in HCL patients refractory to or relapsed after standard chemotherapy with purine analogs and treated with oral vemurafenib within the first clinical trial of a BRAF inhibitor in HCL. Obtaining purified leukemic cells from patients is a technically challenging task in HCL, where usually (and unlike all other leukemias) few tumor cells circulate in the blood and are aspirable from the fibrotic marrow.1,4 However, the above experimental and validation strategy based on primary tumor cells representative of a sizable number of patients provides a more reliable view of tumor biology than that only derived from a few model cell lines (as has been often done in BRAF-mutated solid tumors), and is therefore likely to more meaningfully impact on and inform patient care.
Strikingly, we found that HCL relies heavily, and much more than BRAF-mutated solid tumors, on the BRAF-V600E mutation for most of its unique features. Indeed, BRAF and MEK inhibitors caused a marked and sustained MEK/ERK dephosphorylation followed, not only by the silencing of the BRAF-MEK-ERK pathway transcriptional output previously described in BRAF-mutated melanoma and colorectal carcinoma,20 but also, more importantly, by a broader reshaping of the HCL global gene expression profile affecting various peculiar aspects of this disease. In particular, BRAF inhibition caused a loss of the genome-wide expression signature discriminating HCL from the main normal peripheral B-cell subsets and from other mature B-cell malignancies,3 as well as the downregulation of established HCL diagnostic markers such as CD25/ILR2, TRAP/ACP5, and CCND1.1,21,22 This latter observation may also bear clinical consequences, in that these markers (and CD25 in particular) may not be suitable for monitoring residual disease during treatment with BRAF and/or MEK inhibitors. Further interesting transcripts modulated by vemurafenib in HCL included the downregulation of LST1 (leukocyte transcript 1), a gene involved in the formation of actin-containing long-membrane protrusions,23,24 as well as the downregulation of actin β itself (ACTB), which is enriched in the cytoskeleton of the hairy projections and is part of the HCL-specific expression signature.3,4 Prompted by this finding, we explored the influence of the BRAF-V600E mutation in determining the peculiar morphology of HCL, and we indeed observed a striking trimming of the hairy surface in living leukemic cells upon BRAF or MEK inhibition. Finally, loss of the hairy morphology was followed by marked apoptosis induction, again consistent with the upregulation by vemurafenib of some proapoptotic genes (ie, BIM/BCL2L11 and CDKN1C/p57-KIP2) and with the concept that the growth of the leukemic hairy clone is mainly sustained by apoptosis inhibition (being the HCL proliferation index, <1%, one of the lowest among hematologic malignancies4,26 ). In other words, the BRAF-V600E mutation plays a dominant role in shaping the distinct biochemical, molecular, immunophenotypic, morphologic, and antiapoptotic “identity” of HCL, in keeping with the observation that no other recurrent genetic lesions have been observed in this malignancy,4 as opposed to BRAF-mutated solid tumors.30 Notably, BRAF and MEK inhibitors did not elicit any of the above-described biological effects in leukemic cells purified from patients with HCL-like neoplasms (BRAF-unmutated), further confirming the specificity of these drug effects for HCL (BRAF-mutated).
Our study also provides a methodological framework for studying the pharmacodynamics of BRAF and MEK inhibitors in HCL patients. For example, given that these inhibitors are not devoid of side effects14-16 and that lower doses of vemurafenib have shown clinical activity in anecdotal HCL patients (reviewed in Sári et al31 ), alternative vemurafenib doses and/or schedules (as compared with the standard ones used in our and other clinical trials,14-16 960 mg twice daily on a continuous basis) might be explored to define the minimal exposure times and concentrations required to elicit the desired biochemical and/or cellular pharmacodynamic effect.
Importantly, from the standpoint of clinical translation, we observed that: (1) clinically available inhibitors of BRAF (vemurafenib and dabrafenib) or MEK (trametinib) exert potent antileukemic activity in patients’ HCL cells in vitro; (2) dabrafenib seems more effective at inducing apoptosis than vemurafenib even in HCL cells from patients relapsing after vemurafenib; and (3) the bone marrow microenvironment antagonizes both MEK-ERK dephosphorylation and apoptosis induced by BRAF inhibitors. Finally, by showing that dabrafenib is less affected than vemurafenib by such a protective stromal effect and that the latter is further reduced by combined BRAF and MEK inhibition (dabrafenib + trametinib), we offer viable strategies to inform the clinical use of BRAF and MEK inhibitors in HCL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Alessandro Brozzi (Department of Medicine, University of Perugia) for help with statistics, and to Roberta Pacini and Alessia Tabarrini (Institute of Hematology Perugia) for help with immunohistochemistry.
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC IG-14447 [E.T.]; AIRC MCO-10007 [B.F.]), European Research Council (ERC-FP7/2007-2013 CoG-617471 [E.T.]), Hairy Cell Leukemia Foundation (B.F. and E.T.), Ministero dell’Istruzione, Università e Ricerca (MIUR; PRIN 20104HBZ8E [B.F.]; Futuro in Ricerca 2010-RBFR10WT2K [E.T.]), Ministero della Salute (Giovani Ricercatori GR-2010-2303444 [E.T.]), and Associazione Umbra contro le Leucemie e i Linfomi (AULL [B.F.]).
E.T. is a scholar in Clinical Research of the Leukemia & Lymphoma Society (contract no. 2030-14).
Authorship
Contribution: B.F. and E.T. conceived the study, designed, directed, and interpreted experiments, and wrote the paper; V.P. supervised and partly performed wet-laboratory experiments, collected data, interpreted experiments, and contributed to the writing of the paper; A.S. performed and interpreted most wet-laboratory experiments and collected data; E.I. and R.M. performed and interpreted the confocal microscopy analyses; G.R., A.P., B.B., G.S., E.F., A.S.-R., P.S., and M.P.M. helped in performing wet-laboratory experiments and collecting data; and L.K.-H. performed gene expression profiling.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brunangelo Falini, Institute of Hematology, University of Perugia, Perugia, Italy; e-mail: brunangelo.falini@unipg.it; and Enrico Tiacci, Institute of Hematology, University of Perugia, Perugia, Italy; e-mail: enrico.tiacci@unipg.it.
References
Author notes
V.P. and A.S. share first authorship.
B.F. and E.T. share last authorship.

![Figure 6. Combined BRAF and MEK inhibition counteracts the protective effect of bone marrow stromal cells against ERK dephosphorylation and HCL cell apoptosis induced by single BRAF or MEK inhibition. (A) Western blot analysis of phospho-ERK1/2 levels in hairy cells purified from patient HCL 24 (supplemental Table 1) and exposed in vitro to the indicated BRAF and MEK inhibitors alone or in combination, for 6 hours, in the presence of HS5 bone marrow stromal cells. After cotreatment with dabrafenib 50 nM and trametinib 1 nM, the phospho-ERK bands, relative to the total ERK bands as loading control, are weaker than after either drug alone (twofold and fivefold weaker, respectively, as quantified in the phospho-ERK/ERK ratio indicated below each lane and obtained by densitometry using the ImageJ software). A similar result was obtained in another HCL patient (no. 26, supplemental Table 1) (B) Flow cytometric quantification of living (ANXA5-negative) cells in primary blood leukemic cells purified from a HCL patient (HCL 25) and in vitro exposed in triplicate for 48 hours to the indicated drugs, in the presence or absence of HS5 stromal cells. In the absence of HS5 cells, trametinib 1 nM and dabrafenib 50 nM were able to induce significant (P < .05, supplemental Table 5) HCL cell apoptosis and their combination was not more effective than dabrafenib alone. Conversely, in the presence of HS5 cells drug-induced apoptosis was dampened but combined BRAF and MEK inhibition resulted in more apoptosis than BRAF or MEK inhibition alone, as quantified in the histograms of panel C (mean ± standard deviation [SD], relative to trametinib 1 nM). A similar result was obtained in another HCL patient (no. 26, supplemental Table 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/8/10.1182_blood-2014-10-603100/5/m_1207f6.jpeg?Expires=1770498437&Signature=rbJAr89M0ZVQEpR2NshEKfLUteQjzd9uEtwChVsdQchy7nGno3G2zTW2VG5Btxc8PN1Vq2mOaC45cCtNEIWrkWnFtMdPUz86jdCnl3dY~GXsdJ35Les9locVFxMPNSoIcKob7kxk4D3nIK52ykGKyRveHnmMJjAULpkAlYGz-WHTzfo-vv~l9AW2wKXwJ4qyGmSQ8e0OYoKRjd8oZOgx3-gjTps7IecXSvkyQg~ZtFNCKGwTc0tUcU2SMpf9Cx30vzjhzXjALWtaAdSKKn8hgSh3kvei1vbd7kmw6y28p9wBrZ8y6hBCSF20WI60onNLHjwFXySIiu1qW3XNZ5XD9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)