Key Points
Pharmacologic activation of executioner procaspases by B-PAC-1 in CLL bypasses antiapoptotic mechanisms and induces apoptosis.
B-PAC-1 activates apoptosis by abrogating the zinc ion-dependent inhibition of executioner procaspase activation.
Abstract
Intrinsic and extrinsic apoptotic pathways converge to activate common downstream executioner caspases (caspase-3, -6, and -7), resulting in cell death. In chronic lymphocytic leukemia (CLL), neoplastic B cells evade apoptosis owing to the overexpression of survival proteins. We hypothesized that direct activation of procaspases could bypass the apoptosis resistance induced by the upstream prosurvival proteins. The procaspase-activating compounds (PAC-1), including B-PAC-1 (L14R8), convert inactive executioner procaspases to their active cleaved forms by chelation of labile zinc ions. Both at transcript and protein levels, primary CLL cells express high levels of latent procaspases (3, -7, and -9). B-PAC-1 treatment induced CLL lymphocyte death which was higher than that in normal peripheral blood mononuclear cells or B cells, and was independent of prognostic markers and microenvironmental factors. Mechanistically, B-PAC-1 treatment activated executioner procaspases and not other Zn-dependent enzymes. Exogenous zinc completely, and pancaspase inhibitors partially, reversed B-PAC-1–induced apoptosis, elucidating the zinc-mediated mechanism of action. The cell demise relied on the presence of caspase-3/7 but not caspase-8 or Bax/Bak proteins. B-PAC-1 in combination with an inhibitor of apoptosis protein antagonist (Smac066) synergistically induced apoptosis in CLL samples. Our investigations demonstrated that direct activation of executioner procaspases via B-PAC-1 treatment bypasses apoptosis resistance and is a novel approach for CLL therapeutics.
Introduction
Chronic lymphocytic leukemia (CLL) is a prototype disease in which neoplastic B cells evade apoptosis owing to overexpression of Bcl-21 and inhibitor of apoptosis protein (IAP)2 family proteins. This evasion allows resistance to intrinsic or extrinsic programmed cell death (PCD). The intrinsic (or mitochondrial) pathway induces changes in the mitochondrial membrane resulting in the loss of transmembrane potential, causing the release of apoptosis-inducing factors into the cytosol. The released proapoptotic proteins in turn form apoptosome and activate the cascade-constituting initiator (caspase-9) and executioner caspases (caspase-3, -6, and -7) that transmit signals for cell demise. The regulation of apoptotic events in the mitochondria depends on the stoichiometry between proapoptotic and antiapoptotic signals of the Bcl-2 family proteins. In addition, release of second mitochondria-derived activator of caspase (smac; also known as DIABLO) and OMI (also known as HTRA2) from mitochondria neutralizes the caspase inhibitory function of IAP proteins. In the extrinsic apoptotic pathway, death receptors on the cell membrane are activated by their cognate ligands, leading to the recruitment of adaptor molecules such as first apoptosis signal (FAS)-associated death domain protein and initiator caspase-8. This results in the dimerization and activation of caspases-8, which can then directly cleave and activate executioner caspases, triggering apoptosis, or can cleave BH3 interacting domain death agonist (BID) to truncated BID (tBID) leading to a cross-talk with the intrinsic pathway.
Caspases are a family of cysteine-dependent aspartate-directed proteases that are key mediators of apoptosis. Of the 11 caspases that have been identified in humans to date, 7 are known to be involved in the apoptosis pathway. Among the 7, 4 are initiator caspases (caspase-2, -8, -9, and -10) and 3 are executioner caspases (caspase-3, -6, and -7). The caspase-9–mediated intrinsic apoptosis pathway (which heavily involves the mitochondria) and the caspase-8–dependent extrinsic apoptosis pathway (which originates from the death receptor axis) are the 2 major routes that execute PCD, by ultimately triggering the downstream executioner caspases.3 Importantly, the upstream Bcl-2 and IAP family proteins manipulate the activation of caspases, and have been implicated with significant oncogenic potential for their regulatory role on caspases. Collectively, the high expression of antiapoptotic proteins in CLL cells compels the need to develop alternative approaches for the terminal execution of apoptosis.
Executioner caspases are present in cells as inactive dimers or zymogen procaspases. Triggering of procaspases is a prerequisite to initiate PCD3 in which activated proteases cleave cellular substrates through recognition of a 4-aa substrate with a C-terminal aspartate residue. One key physiological regulator that maintains the executioner caspase in an inactive procaspase configuration is its inhibition by labile intracellular zinc.4 After the first demonstration that addition of zinc ion specifically inhibited caspase-3 cleavage activity and caspase-3–mediated apoptosis,5 a series of reports showed that addition of zinc increased cytoprotection6,7 and deprivation of zinc ion induced apoptosis.8-10 These findings provided an impetus to create small molecules to chelate the intracellular zinc to activate caspases.11 Procaspase-activating compounds of the PAC-1 class convert inactive dimers of executioner procaspases to their active cleaved forms by relieving zinc-mediated inhibition.12 These compounds bypass upstream survival factors11 and directly activate executioner caspases. PAC-1 (chemically, ortho-hydroxy N-acyl hydrazone) is the founding member of this class of compounds, and has shown promise in vitro,11,13,14 in vivo,11,15 and as a tool for studying procaspase-3 activation in various systems.16,17 PAC-1 inhibited growth in primary colon tumors and mouse xenograft models,11 through zinc ion chelation of executioner procaspase-3 and -7.12
Recently, a next-generation PAC-1 combinatorial library of 837 compounds was designed and synthesized to identify more potent and cancer cell–selective apoptosis inducers.13 B-PAC-1 (coded as 3{18,7} in Hsu et al13 ) was one of the most potent compounds with an 50% inhibitory concentration (IC50) of 1.4 µM in U937 lymphoma cells.13
We hypothesized that B-PAC-1 could directly activate executioner caspases and trigger apoptosis in CLL lymphocytes by overcoming the upstream checkpoints of PCD. We investigated the effects of B-PAC-1 in primary lymphocytes obtained from patients with CLL, and identified its mechanism of action, determined therapeutic index, and tested combination approaches.
Patients and methods
Drugs, ligands, and reagents
Healthy donor and CLL patient peripheral blood samples
Freshly isolated cells from peripheral blood samples obtained from CLL patients (n = 78; supplemental Table 2) or from healthy donors (n = 7) were used. All participants signed written informed consent forms in accordance with the Declaration of Helsinki, and the protocols were approved by the institutional review board at The University of Texas MD Anderson Cancer Center.
Isolation of lymphocytes and preparation of CLL cell and bone marrow stromal cell cocultures
The isolation of lymphocytes and their culture in suspension or coculture on NKTert human bone marrow stromal cells were previously described.19 CLL cell purity was above 90%. Normal B lymphocytes (freshly isolated negatively selected CD19+ B cells) were purchased from AllCells, and were kept in RPMI-1640 medium with 10% fetal bovine serum (FBS).
Cell lines
Isogenic cell lines are described in supplemental Table 3. The NKTert cell line (RIKEN Cell Bank) was authenticated by short tandem repeat DNA fingerprinting using the AmpFlSTR Identifiler kit (Applied Biosystems).
Treatment of caspase-deficient cell lines
Wild-type (WT) and caspase-3/7 (+/−) and (−/−) mouse embryonic fibroblasts (MEFs) and WT and caspase-8 (−/−) Jurkat cells were seeded for 16 hours, then incubated with B-PAC-1 for 24 hours, and apoptosis was tested.
Cell apoptosis and MOMP
Annexin V/propidium iodide (PI) binding assay19 and tetramethylrhodamine ethyl ester (TMRE) staining were used to measure apoptosis and mitochondrial outer membrane permeabilization (MOMP), respectively. Histograms of TMRE stains were determined using FACSCalibur (Becton Dickinson) and were quantified as geographical means using FlowJo software (TreeStar Inc).
Microarray and immunoblot analysis
To determine transcript expressions of caspase and IAPs, a TaqMan Human Apoptosis Array (microfluidic card; Applied Biosystems) was used.19 This array card contained 93 messenger RNA (mRNA) targets, including 11 in the caspase and 8 in the IAP family. The immunoblot analysis was performed as described.19 The primary antibodies are listed in supplemental Table 1. Protein band quantification was done using a LI-COR Odyssey CLx Infrared Imaging System.
Statistical analysis
Linear correlations were derived using GraphPad Prism 5 software (GraphPad Software, Inc). Different statistical tests were used and are listed in the figure legends. P values were determined using the following statistical methods: 1-sample t test for comparison with a hypothetical value (IC50); unpaired or paired Student t test for 2 groups; 1-way analyses of variance for >2 groups; and 2-way analysis of variance for >2 groups and >1 variable. The Tukey multiple comparisons test was applied to measure adjusted P value for 1-way and 2-way analyses of variance. For correlation studies, P values were determined using 2-tailed Pearson correlation.
Results
Expression of executioner caspases in CLL lymphocytes
Intracellular expression of executioner procaspases is the primary requisite for B-PAC-1 action. Our data showed mRNA expression of all the initiator (procaspase-2, -8, -9, and -10) and executioner (procaspase-3, -6, and -7) procaspases in CLL lymphocytes (Table 1). Among caspases involved in inflammation, procaspase-1 and -4 were detected in all samples, whereas procaspase-5 was detected in 4 of the 12 samples (Table 1).
Expression of procaspase mRNAs in CLL lymphocytes
| Function3 . | Gene name . | Protein name . | Positive samples/ Total samples . |
|---|---|---|---|
| Apoptosis initiator | CASP2 | Caspase-2 | 12/12 |
| Apoptosis initiator | CASP8 | Caspase-8 | 12/12 |
| Apoptosis initiator | CASP9 | Caspase-9 | 12/12 |
| Apoptosis initiator | CASP10 | Caspase-10 | 12/12 |
| Apoptosis effector | CASP3 | Caspase-3 | 12/12 |
| Apoptosis effector | CASP7 | Caspase-7 | 12/12 |
| Apoptosis effector | CASP6 | Caspase-6 | 12/12 |
| Proinflammatory and pyroptotic | CASP1 | Caspase-1 | 12/12 |
| Proinflammatory and pyroptotic | CASP4 | Caspase-4 | 12/12 |
| Proinflammatory and pyroptotic | CASP5 | Caspase-5 | 4/12 |
| Keratinocyte differentiation | CASP14 | Caspase-14 | 0/12 |
| Function3 . | Gene name . | Protein name . | Positive samples/ Total samples . |
|---|---|---|---|
| Apoptosis initiator | CASP2 | Caspase-2 | 12/12 |
| Apoptosis initiator | CASP8 | Caspase-8 | 12/12 |
| Apoptosis initiator | CASP9 | Caspase-9 | 12/12 |
| Apoptosis initiator | CASP10 | Caspase-10 | 12/12 |
| Apoptosis effector | CASP3 | Caspase-3 | 12/12 |
| Apoptosis effector | CASP7 | Caspase-7 | 12/12 |
| Apoptosis effector | CASP6 | Caspase-6 | 12/12 |
| Proinflammatory and pyroptotic | CASP1 | Caspase-1 | 12/12 |
| Proinflammatory and pyroptotic | CASP4 | Caspase-4 | 12/12 |
| Proinflammatory and pyroptotic | CASP5 | Caspase-5 | 4/12 |
| Keratinocyte differentiation | CASP14 | Caspase-14 | 0/12 |
Comprehensive analyses of procaspase mRNAs using a real time reverse transcription–polymerase chain reaction (RT-PCR) array (microfluidic card; Applied Biosystems) were carried out in leukemic lymphocytes from 12 CLL patients.
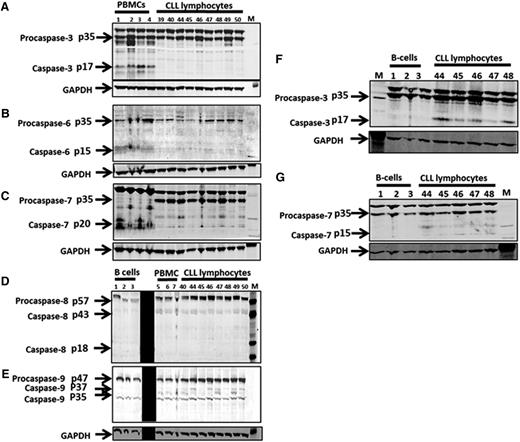
At the protein level, expressions of executioner procaspases in malignant CLL lymphocytes were compared with healthy peripheral blood mononuclear cells (PBMCs) or normal B cells (Figure 1). Procaspase-3 and -7 bands were confirmed using protein extracts of staurosporine-treated Jurkat cells (supplemental Figure 1A-B). Compared with levels in PBMCs (n = 4), CLL lymphocytes (n = 9) had: higher expression of procaspase-3 (median fold change = 1.5, P = .11), procaspase-7 (1.55, P = .0042), and procaspase-9 (1.57, P = .04); low expression of procaspases-6 (0.34, P = .006); and unchanged expression of procaspases-8 (1.14, P = .89). However, these procaspases were not significantly different compared with normal B lymphocytes (n = 3) (Figure 1; quantitation data not shown). The endogenous expression of active caspase-3 was detected in PBMCs but not in normal B cells (Figure 1).
Protein expression of executioner and effector procaspases in PBMCs, normal B lymphocytes, and CLL lymphocytes. Fresh samples of normal PBMCs from peripheral blood samples obtained from healthy donors (n = 4), leukemic lymphocytes from peripheral blood samples obtained from patients with CLL (n = 9), and commercially available pure B lymphocytes (n = 3) were collected and processed for immunoblot analysis. Executioner caspases, pro- and caspase-3 protein expression (A), pro- and caspase-6 protein expression (B), pro- and caspase-7 protein expression (C) in PBMCs and CLL lymphocytes are presented. Effector caspases, pro- and caspase-8 (D) and pro- and caspase-9 (E) are shown from B cells, PBMCs, and CLL cells. Executioner caspases, pro- and caspase-3 (F), and pro- and caspase-7 (G) from normal B and CLL lymphocytes are presented. GAPDH is used as loading control for all. M denotes protein molecular weight marker. The black strip in panels D and E was used to block technically weak lanes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Protein expression of executioner and effector procaspases in PBMCs, normal B lymphocytes, and CLL lymphocytes. Fresh samples of normal PBMCs from peripheral blood samples obtained from healthy donors (n = 4), leukemic lymphocytes from peripheral blood samples obtained from patients with CLL (n = 9), and commercially available pure B lymphocytes (n = 3) were collected and processed for immunoblot analysis. Executioner caspases, pro- and caspase-3 protein expression (A), pro- and caspase-6 protein expression (B), pro- and caspase-7 protein expression (C) in PBMCs and CLL lymphocytes are presented. Effector caspases, pro- and caspase-8 (D) and pro- and caspase-9 (E) are shown from B cells, PBMCs, and CLL cells. Executioner caspases, pro- and caspase-3 (F), and pro- and caspase-7 (G) from normal B and CLL lymphocytes are presented. GAPDH is used as loading control for all. M denotes protein molecular weight marker. The black strip in panels D and E was used to block technically weak lanes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
B-PAC-1 effect on Zn-dependent enzymes
B-PAC-1 is a PAC-1 derivative containing benzyloxy and di-t-butyl functionality (supplemental Figure 2A). Because B-PAC-1 chelates zinc ion on procaspases, we tested the effect of B-PAC-1 on other zinc-dependent enzymes such as carboxypeptidase A and histone deacetylase (HDAC) using biochemical assays (supplemental Figure 2B-C). In both cases, B-PAC-1 did not affect activity of these enzymes indicating selectivity of B-PAC-1 on procaspases.
Induction of cell death in CLL and normal lymphocytes by B-PAC-1
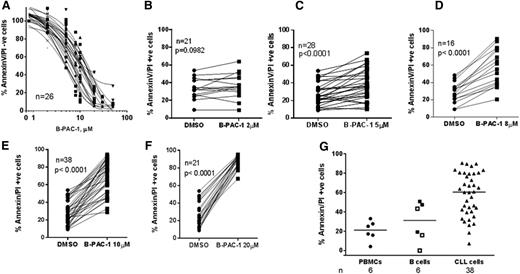
B-PAC-1 induced dose-dependent cell death in the CLL lymphocytes (IC50 = ∼10 µM, n = 26; Figure 2A); data with individual concentration are shown in Figure 2B-F. Dimethylsulfoxide (DMSO)-treated samples with <40% viability were not included (n = 2). When CLL cells were incubated with 10 µM B-PAC-1, cell demise was detected as early as 6 hours and was significant at 10 hours (n = 5; P < .05; data not shown).
Effect of B-PAC-1 treatment on apoptosis in CLL lymphocytes and normal PBMCs and B cells. (A-F) Freshly isolated CLL lymphocytes were treated with the indicated concentrations of B-PAC-1 for 24 hours (n = 26; A); vehicle alone or B-PAC-1 at 2 µM (n = 21; B); 5 µM (n = 28; C); 8 µM (n = 16; D); 10 µM (n = 39; E); or 20 µM (n = 21; F). (G) Comparison of B-PAC-1–induced apoptosis in normal vs malignant cells. Freshly isolated CLL cells and healthy donor PBMCs and B cells (CD19+ negatively selected) were treated with the 10 µM B-PAC-1 for 24 hours. Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells. □, B cell stained with CD19+ using flow cytometry; ▪, isolated CD19+ (negatively selected) B cells. For panel A, Annexin V/PI− cells in DMSO control were set as 100% and appropriately cell viability was calculated for B-PAC-1–treated cells. For panels B-F, raw cell death data for DMSO or B-PAC-1–treated cells are presented. For panel G, DMSO cell death was subtracted from the drug-treated value and plotted.
Effect of B-PAC-1 treatment on apoptosis in CLL lymphocytes and normal PBMCs and B cells. (A-F) Freshly isolated CLL lymphocytes were treated with the indicated concentrations of B-PAC-1 for 24 hours (n = 26; A); vehicle alone or B-PAC-1 at 2 µM (n = 21; B); 5 µM (n = 28; C); 8 µM (n = 16; D); 10 µM (n = 39; E); or 20 µM (n = 21; F). (G) Comparison of B-PAC-1–induced apoptosis in normal vs malignant cells. Freshly isolated CLL cells and healthy donor PBMCs and B cells (CD19+ negatively selected) were treated with the 10 µM B-PAC-1 for 24 hours. Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells. □, B cell stained with CD19+ using flow cytometry; ▪, isolated CD19+ (negatively selected) B cells. For panel A, Annexin V/PI− cells in DMSO control were set as 100% and appropriately cell viability was calculated for B-PAC-1–treated cells. For panels B-F, raw cell death data for DMSO or B-PAC-1–treated cells are presented. For panel G, DMSO cell death was subtracted from the drug-treated value and plotted.
To evaluate the therapeutic index of B-PAC-1, freshly isolated CD19+ (negatively selected) B cells (and PBMCs from healthy donors) and CLL patient lymphocytes were incubated with 10 µM B-PAC-1 for 24 hours (Figure 2G). At this concentration, CLL lymphocytes had 60% median death which was significantly higher than PBMCs (21%, P = .0007) and normal B lymphocytes (31%, P = .0114), indicating some selectivity of B-PAC-1 toward malignant lymphocytes.
B-PAC-1 induced apoptosis in CLL lymphocytes regardless of microenvironmental factors
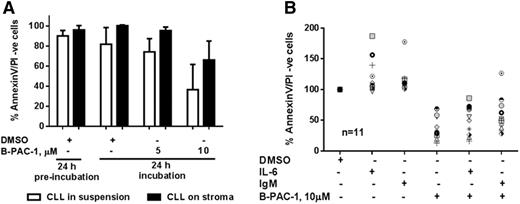
The survival and chemoresistance of CLL lymphocytes have been previously demonstrated to be associated with microenvironmental factors.19-21 To evaluate the impact of these factors, first, CLL lymphocytes were cocultured with NKTert cells for 24 hours (to acclimatize CLL cells in this new environment before adding B-PAC-1), followed by B-PAC-1 incubation for additional 24 hours. B-PAC-1 (10 µM) treatment induced apoptosis in CLL lymphocytes in both conditions: the absence (P = .0237; n = 4) and the presence (P = .0111; n = 4) of stromal cells, albeit with a lower apoptosis rate in the presence of stromal cells (Figure 3A). B-PAC-1–induced toxicity on stromal cells was <25% (median, 22.4%; range, 16%-33%; n = 4; data not shown), which might have underestimated the stroma-mediated protection during B-PAC-1–induced CLL cell death in cocultures. mRNA analysis of 93 apoptotic pathway genes in CLL lymphocytes cocultured for 72 hours with stromal cells indicated that expression of procaspase-3, -6, -7, -8, and -9 was only marginally changed (<25%).22 Second, evaluation on the effects of other factors such as interleukin-6 (IL-6; cytokine) and anti-immunoglobulin M (IgM; B-cell receptor [BCR] activation) demonstrated that the percentage of viable CLL cells was significantly lower after B-PAC-1 treatment in the presence of each of these factors (P < .0001 for both the factors; n = 11) and did not differ significantly compared with untreated CLL cells (P = .66, 0.25 for IL-6 and IgM, respectively), indicating that B-PAC-1 could overcome the apoptosis resistance induced by these factors (Figure 3B).
Effect of microenvironmental factors on B-PAC-1–induced apoptosis in CLL lymphocytes. Freshly isolated primary CLL cells were incubated in suspension or on NKTert bone marrow stromal cells for 24 hours (n = 4; A, termed preincubation) or with IL-6 or IgM (n = 11; B) for 48 hours. Cells were then incubated with DMSO or with 5 or 10 µM B-PAC-1 for an additional 24 hours. Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control.
Effect of microenvironmental factors on B-PAC-1–induced apoptosis in CLL lymphocytes. Freshly isolated primary CLL cells were incubated in suspension or on NKTert bone marrow stromal cells for 24 hours (n = 4; A, termed preincubation) or with IL-6 or IgM (n = 11; B) for 48 hours. Cells were then incubated with DMSO or with 5 or 10 µM B-PAC-1 for an additional 24 hours. Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control.
Role of cytogenetic and prognostic factors in B-PAC-1–induced apoptosis
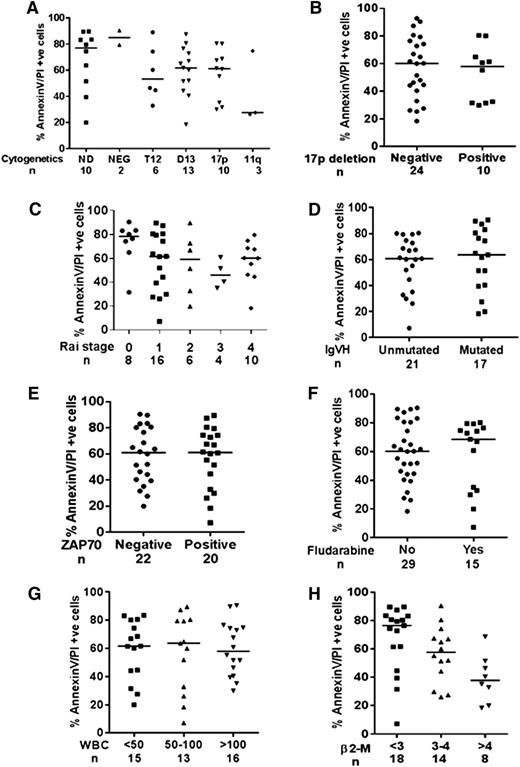
Samples with trisomy-12 mutations exhibited a trend of being more resistant (median, 53% cell death; n = 6) to B-PAC-1 treatment than samples with deletions in chromosome 13q14 (62% cell death; n = 13) and deletions in chromosome 17p (62% cell death; n = 10). The number of samples analyzed for deletions in chromosome 11q22/q23 was not sufficient to draw conclusions (n = 3; P = .69; Figure 4A). Samples with (n = 10) and without (n = 24) 17p deletion had similar responses to B-PAC-1, suggesting that the mechanism of B-PAC-1–induced response in CLL lymphocytes is p53 independent (Figure 4B; P = .59). Early Rai stage samples (0, n = 8) were more sensitive to B-PAC-1 (∼80% apoptosis) than those at other Rai stages (1, 62%; 2, 59%; 3, 46%; 4, 60% apoptosis; P = .0094, Figure 4C). In addition, CLL lymphocytes with mutated (64% apoptosis; n = 17) and unmutated (61% apoptosis; n = 21) immunoglobulin heavy chain variable gene status responded similarly to B-PAC-1 treatment (P = .68; Figure 4D). Similarly, ζ-chain–associated protein kinase 70 (ZAP70) status (P = .81; Figure 4E), prior fludarabine treatment (P = .744; Figure 4F), and white blood cell count in peripheral blood (P = .9; Figure 4G) did not affect B-PAC-1–mediated apoptosis. However, samples with high levels of β2-microglobulin (unit ≥4, 38% apoptosis, n = 8) were more resistant to B-PAC-1 treatment than those with low levels of β2-microglobulin (unit ≤3, 76% apoptosis, n = 18; P = .0094; Figure 4H).
Effect of prognostic markers on B-PAC-1–induced apoptosis in CLL lymphocytes. B-PAC-1–induced apoptosis was compared in CLL samples obtained from patients with different cytogenetics (A), with 17p chromosome deletion (B), different Rai stages (C), with or without IgVH mutation (D), ZAP70 status (E), with or without prior fludarabine exposure (F), peripheral white blood cell count (G), and level of β2-microglobulin (H). Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control. 11q, chromosome 11q deletion; 17p, chromosome 17p deletion; β2-M, β2-microglobulin; D13, chromosome 13q deletion; ND, not determined; NEG, negative for any cytogenetic factor; T12, trisomy 12; WBC, white blood cell.
Effect of prognostic markers on B-PAC-1–induced apoptosis in CLL lymphocytes. B-PAC-1–induced apoptosis was compared in CLL samples obtained from patients with different cytogenetics (A), with 17p chromosome deletion (B), different Rai stages (C), with or without IgVH mutation (D), ZAP70 status (E), with or without prior fludarabine exposure (F), peripheral white blood cell count (G), and level of β2-microglobulin (H). Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control. 11q, chromosome 11q deletion; 17p, chromosome 17p deletion; β2-M, β2-microglobulin; D13, chromosome 13q deletion; ND, not determined; NEG, negative for any cytogenetic factor; T12, trisomy 12; WBC, white blood cell.
Activation of procaspases in CLL lymphocytes after B-PAC-1 treatment
Among the executioner procaspases, B-PAC-1 treatment induced significant cleavage of both procaspase-3 and -7 but not procaspase-6 (Figure 5A, supplemental Figure 3). Among initiator procaspases, both procaspase-8 and -9 were cleaved (Figure 5A, supplemental Figure 4) in CLL cells, indicating activation of both intrinsic and extrinsic pathways with B-PAC-1 treatment. In addition, poly (ADP-ribose) polymerase (PARP), which is a known substrate of caspase-3, showed significantly more cleavage in B-PAC-1–treated cells in comparison with untreated cells (Figure 5A, supplemental Figure 5). In contrast to apoptotic procaspases, inflammatory procaspase-1 (α isoform) was not impacted by B-PAC-1 treatment (supplemental Figure 6).
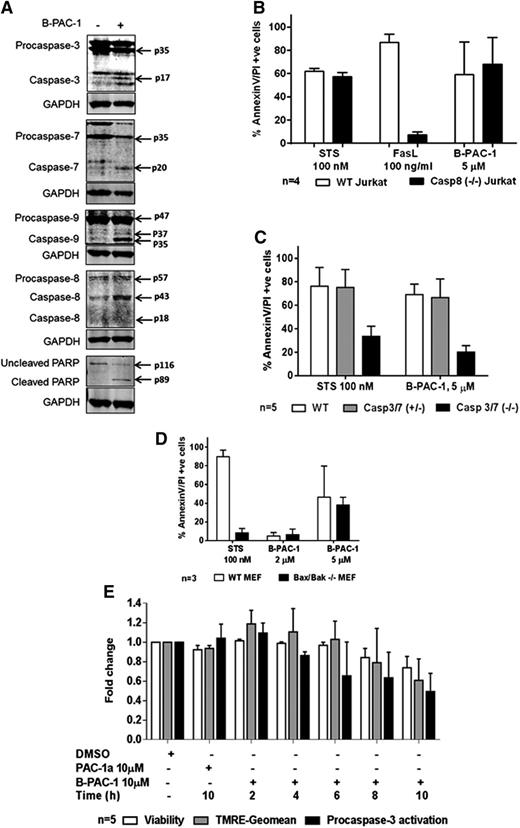
Effect of B-PAC-1 on procaspase activation and role of caspases in B-APC-1–mediated apoptosis in CLL lymphocytes. Representative immunoblots of executioner procaspase-3 and -7, initiator procaspase-8 and -9, and downstream target PARP protein before and 24 hours after B-PAC-1 treatment (A); quantitation of several immunoblots for these caspases are included as supplemental Figures 2-7. Effect of 24-hour treatment of B-PAC-1 on apoptosis in WT or caspase-8–deficient Jurkat cells (B), in WT, single allele caspase-3 and -7, or caspase-3 and -7 double knockout MEFs (C), and in WT or Bax and Bak double knockout MEF (D). Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control. Temporal relationship of B-PAC-1–induced reduction in viability (measured by AnnexinV/PI− cells), reduction in mitochondrial outer membrane staining (measured by TMRE staining), and reduction in procaspase-3 protein expression (cleavage in procaspase-3 protein expression measured by immunoblots), after 2 hours to 10 hours of incubation with 10 µM B-PAC-1 (E). Staurosporine, FasL, and PAC-1a were used as positive or negative controls. FasL, Fas ligand; Geomean, geometric mean; STS, Staurosporine.
Effect of B-PAC-1 on procaspase activation and role of caspases in B-APC-1–mediated apoptosis in CLL lymphocytes. Representative immunoblots of executioner procaspase-3 and -7, initiator procaspase-8 and -9, and downstream target PARP protein before and 24 hours after B-PAC-1 treatment (A); quantitation of several immunoblots for these caspases are included as supplemental Figures 2-7. Effect of 24-hour treatment of B-PAC-1 on apoptosis in WT or caspase-8–deficient Jurkat cells (B), in WT, single allele caspase-3 and -7, or caspase-3 and -7 double knockout MEFs (C), and in WT or Bax and Bak double knockout MEF (D). Apoptosis was measured by Annexin V/PI staining assay and unstained cells were considered viable cells; time-matched DMSO-treated cells served as control. Temporal relationship of B-PAC-1–induced reduction in viability (measured by AnnexinV/PI− cells), reduction in mitochondrial outer membrane staining (measured by TMRE staining), and reduction in procaspase-3 protein expression (cleavage in procaspase-3 protein expression measured by immunoblots), after 2 hours to 10 hours of incubation with 10 µM B-PAC-1 (E). Staurosporine, FasL, and PAC-1a were used as positive or negative controls. FasL, Fas ligand; Geomean, geometric mean; STS, Staurosporine.
Our studies indicated that B-PAC-1 activates both initiator and executioner caspases in the implementation of apoptosis. To test whether B-PAC-1 induced apoptosis through cascade of initiator caspases or through direct activation of executioner caspases, caspase-8 (−/−) Jurkat cells were treated with B-PAC-1. B-PAC-1 treatment induced a significant and similar extent of apoptosis in both caspase-8 WT and null cells, albeit at lower levels in the caspase-8 (−/−) cells (P = .95, Figure 5B). Staurosporine also induced a similar percentage of apoptosis in both cell lines (P = .99). In contrast, FasL-induced cell death was significantly higher in WT cells than that in caspase-8 null cells (P < .0001), consistent with the involvement of an extrinsic apoptotic pathway. We also checked expression of tBid to find involvement of caspase-8–mediated Bid cleavage. Expression of Bid protein was unchanged and tBid was below the level of detection under B-PAC-1 treatment (n = 6; data not shown).
Similar experiments were carried out to test the role of executioner caspases-3 and -7 in B-PAC-1–induced apoptosis. MEFs WT for caspase-3 and caspase-7, single knockout (caspase-3 [+/−] caspase-7 [+/−], referred as caspase-3/7 [+/−]; supplemental Table 3), and double knockout (caspase-3/7 [−/−]) were treated with 5 µM B-PAC-1. B-PAC-1 significantly promoted cell death in WT (P < .0001) and caspase-3/7 (+/−) (P < .0001) MEFs compared with caspase-3/7 (−/−) MEFs (Figure 5C). These results indicated that although initiator caspase-8 was not necessary, executioner caspases-3 and -7 were required for B-PAC-1–induced cell demise.
To rule out the possibility that Bcl-2 family proapoptotic factors that are upstream of caspases were involved in B-PAC-1–induced apoptosis, WT and Bax/Bak (−/−) MEFs were treated with B-PAC-1 for 24 hours. B-PAC-1 (5 µM) induced similar (∼40%) apoptosis in both WT and Bax/Bak (−/−) MEFs (P = .97, Figure 5D). In contrast, staurosporine-induced cell death required Bax/Bak in these MEFs (P = .0002).
To determine the sequence of events, that is, B-PAC-1 activates caspase-3 and -7 first, then mitochondrial permeabilization, and then caspase-8 and -9 activation, we quantitated caspase-3/7, caspase-8, and caspase-9 in a time- and dose-dependent manner (supplemental Figures 7-8). We found no difference among quantitated caspase-3/7, -8, and -9 stained cells. The results suggested that these events were fast and beyond our experimental detection limits to identify ordering of caspase activation. Along the same line, it will be beyond our experimental detection limit to differentiate ordering of caspase-3 activation and mitochondrial permeabilization. Hence, we hypothesized that B-PAC-1–induced caspase-3/7 activation shall strongly correlate with mitochondrial permeabilization. We evaluated B-PAC-1–induced changes in MOMP (which was measured by the geographic means of TMRE stains), apoptosis rate (decrease in viability; AnnexinV/PI− cells), and procaspase-3 over time within samples (Figures 5E, supplemental Figure 9). PAC-1a, a structurally similar but inactive version of PAC-1,12 was used as a negative control. The decreases in MOMP over time correlated strongly and significantly with procaspase-3 activation (r = 0.78; P = .0466; data not shown). Similarly, the decrease in MOMP and decrease in cell survival over time correlated strongly and significantly (r = 0.67; P < .0001; data not shown).
Reversibility of B-PAC-1–induced apoptosis and the role of zinc chelation
The proposed mechanism of action of B-PAC-1 is to relieve the zinc ion-mediated inhibition of procaspase-3 and -7.13 Adding exogenous zinc completely abrogated B-PAC-1–induced cell death (P = .0063) but did not affect ABT-199– or staurosporine-induced apoptosis (Figure 6A).
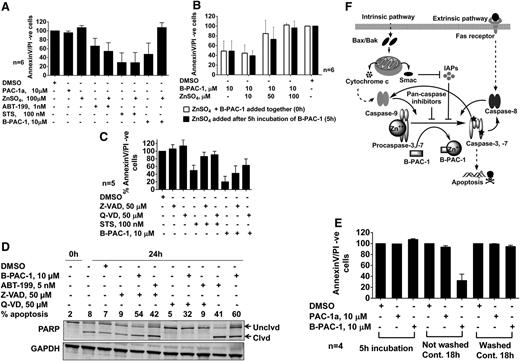
Mechanism of B-PAC-1–mediated apoptosis. Role of exogenous zinc ions in B-PAC-1–mediated apoptosis in CLL lymphocytes (A). Cells were treated with 100 µM exogenous zinc ions in the presence or absence of PAC-1a, ABT199, STS, or B-PAC-1 at indicated concentrations for 24 hours. Titration of zinc concentrations (B). ZnSO4 at 10 µM, 50 µM, and 100 µM was added to CLL lymphocytes in the presence of B-PAC-1 at 0 hours (□) or after 5 hours of B-PAC-1 incubation (▪). Role of caspase inhibitors in B-PAC-1–induced apoptosis (C-D). Cells were treated with 50 µM Z-VAD or Q-VD (pan-caspase inhibitors) in the presence or absence of STS or B-PAC-1 for 24 hours and the percentage of apoptosis was measured (C). Representative immunoblot of samples (n = 2) treated with Z-VAD and Q-VD for 24 hours in the presence and absence of B-PAC-1 and ABT-199 (a Bcl-2 antagonist as a positive control) and 0-hour sample (right after isolation of CLL lymphocytes from blood) (D). Reversibility of B-PAC-1–induced apoptosis (E). CLL lymphocytes were incubated with PAC-1a or B-PAC-1 and either not washed or washed after 5 hours (E) and viability (% AnnexinV/PI− cells) was measured. Working model of B-PAC-1 mechanism of action (F). PAC-1a, negative control.
Mechanism of B-PAC-1–mediated apoptosis. Role of exogenous zinc ions in B-PAC-1–mediated apoptosis in CLL lymphocytes (A). Cells were treated with 100 µM exogenous zinc ions in the presence or absence of PAC-1a, ABT199, STS, or B-PAC-1 at indicated concentrations for 24 hours. Titration of zinc concentrations (B). ZnSO4 at 10 µM, 50 µM, and 100 µM was added to CLL lymphocytes in the presence of B-PAC-1 at 0 hours (□) or after 5 hours of B-PAC-1 incubation (▪). Role of caspase inhibitors in B-PAC-1–induced apoptosis (C-D). Cells were treated with 50 µM Z-VAD or Q-VD (pan-caspase inhibitors) in the presence or absence of STS or B-PAC-1 for 24 hours and the percentage of apoptosis was measured (C). Representative immunoblot of samples (n = 2) treated with Z-VAD and Q-VD for 24 hours in the presence and absence of B-PAC-1 and ABT-199 (a Bcl-2 antagonist as a positive control) and 0-hour sample (right after isolation of CLL lymphocytes from blood) (D). Reversibility of B-PAC-1–induced apoptosis (E). CLL lymphocytes were incubated with PAC-1a or B-PAC-1 and either not washed or washed after 5 hours (E) and viability (% AnnexinV/PI− cells) was measured. Working model of B-PAC-1 mechanism of action (F). PAC-1a, negative control.
To confirm that the apoptosis induced by B-PAC-1 was due to chelation of intracellular caspase-associated zinc ions and not due to chelation of zinc ions in the extracellular cell culture media, exogenous zinc was added to CLL lymphocytes after the lymphocytes had been incubated with B-PAC-1 for 5 hours to ensure B-PAC-1 cell permeabilization. The addition of zinc ions led to a similar extent of apoptosis when added simultaneously with B-PAC-1 or 5 hours post-B-PAC-1 incubation; moreover, the cytotoxicity was zinc ion concentration dependent regardless of when the zinc was added (Figure 6B).
To test whether B-PAC-1–induced apoptosis is prevented by caspase inhibitors, pan-caspase inhibitors Z-Val-Ala-Asp-(OMe)-fluoromethylketone (Z-VAD) and Quinoline-Val-Asp-CH2-O-Ph (Q-VD) were used. The inhibitors significantly but partially recovered the viability of CLL lymphocytes from 15% in the presence of B-PAC-1 alone to 41% with Z-VAD (P < .039) and to 65% with Q-VD (P < .009; Figure 6C). To determine whether these pan-caspase inhibitors blocked caspase activity, we analyzed PARP cleavage (an indirect measurement of caspase activity) in time-matched untreated, DMSO-treated, ABT-199–treated (positive control), and B-PAC-1–treated CLL cells from 2 samples. Data from 1 representative patient sample is presented in Figure 6D. Compared with the 0-hour sample, at 24 hours there was spontaneous (endogenous) cell death and expression of cleaved PARP protein in untreated or DMSO-only treated samples. Z-VAD partially and Q-VD completely reversed detection of cleaved PARP. ABT-199 and B-PAC-1 resulted in an intense band of cleaved PARP. Again, this cleavage was blocked completely with Q-VD and partially by Z-VAD. Based on these data, it is clear that Z-VAD partially and Q-VD completely inhibited caspase activity measured as PARP cleavage and B-PAC-1–induced cell death is in part caspase-dependent.
To examine whether B-PAC-1–mediated apoptosis is reversible, CLL lymphocytes were incubated with B-PAC-1 for 5 hours, either washed or not washed, and incubated for an additional 18 hours. CLL lymphocytes washed of B-PAC-1 incubation had 96% viability, which was significantly more than continued B-PAC-1 incubation (21% viability, P < .0016; Figure 6E). Figure 6F provides the working model for B-PAC-1–induced apoptotic pathways.
Combination of B-PAC-1 and Smac mimetics
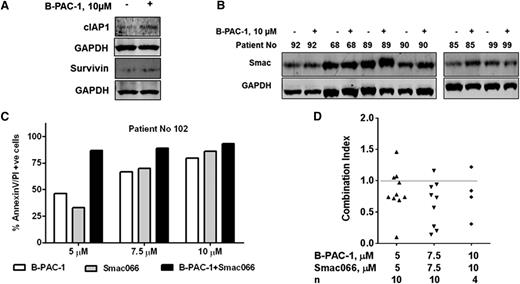
IAPs are potential inhibitors of caspases, and smac mimetics, which are IAP antagonists, reverse this inhibition.23-25 To determine the association between B-PAC-1–induced apoptosis and IAP expression in CLL, we first analyzed the endogenous mRNA expression levels of all IAPs. The mRNAs of NAIP, cIAP1, cIAP2, XIAP, and BRUCE were present in all samples (n = 12; Table 2). B-PAC-1 treatment significantly increased cIAP1 (P = .0097) and survivin (P = .0074) protein levels (n = 11; Figure 7A, supplemental Figure 10) but did not significantly affect cIAP2, Xiap, or Naip1 expression levels. Increase in survivin levels correlated with resistance to B-PAC-1–induced apoptosis in CLL cells (P = .0258; r = 0.729; data not shown). B-PAC-1 treatment significantly increased Smac protein levels (P = .0075; Figure 7B, supplemental Figure 11). These data imply that B-PAC-1–mediated caspase activation is encountered by the high levels of IAPs, and therefore suppressing the functional IAPs with addition of smac mimetic may enhance apoptosis in CLL lymphocytes. Smac066 is a monomeric smac mimetic26 with anticancer effect on MDA-MB231, HL-60, PC-3, and CLL cells.27,28 B-PAC-1 and Smac066 in combination increased apoptosis in CLL lymphocytes more than either agent alone (Figure 7C). Furthermore, the combination index analysis of B-PAC-1 and Smac066 (n = 10) showed synergy between the 2 agents (synergy defined as a combination index of <1; Figure 7D). In contrast to these data in malignant CLL cells, single drug or combination had significantly lower apoptosis in PBMCs from healthy donors (P = .0008, supplemental Figure 12).
Expression of IAP mRNAs in CLL lymphocytes
| Function41 . | Gene name . | Protein name . | Positive samples/Total samples . |
|---|---|---|---|
| Antiapoptotic | BIRC1 | NAIP (neuronal apoptosis inhibitory protein) | 12/12 |
| Antiapoptotic | BIRC2 | cIAP1 (cellular inhibitor of apoptosis 1) | 12/12 |
| Antiapoptotic | BIRC3 | cIAP2 (cellular inhibitor of apoptosis 2) | 12/12 |
| Antiapoptotic | BIRC4 | XIAP (X chromosome-binding IAP) | 12/12 |
| Antiapoptotic | BIRC5 | Survivin | 10/12 (low expression) |
| Antiapoptotic | BIRC6 | Bruce (Apollon) | 12/12 |
| Antiapoptotic | BIRC7 | ML-IAP/KIAP (livin) | 1/12 |
| Antiapoptotic | BIRC8 | Ts-IAP/ILP2 (testis-specific IAP) | 1/12 |
| Function41 . | Gene name . | Protein name . | Positive samples/Total samples . |
|---|---|---|---|
| Antiapoptotic | BIRC1 | NAIP (neuronal apoptosis inhibitory protein) | 12/12 |
| Antiapoptotic | BIRC2 | cIAP1 (cellular inhibitor of apoptosis 1) | 12/12 |
| Antiapoptotic | BIRC3 | cIAP2 (cellular inhibitor of apoptosis 2) | 12/12 |
| Antiapoptotic | BIRC4 | XIAP (X chromosome-binding IAP) | 12/12 |
| Antiapoptotic | BIRC5 | Survivin | 10/12 (low expression) |
| Antiapoptotic | BIRC6 | Bruce (Apollon) | 12/12 |
| Antiapoptotic | BIRC7 | ML-IAP/KIAP (livin) | 1/12 |
| Antiapoptotic | BIRC8 | Ts-IAP/ILP2 (testis-specific IAP) | 1/12 |
Comprehensive analyses of IAP mRNAs using an RT-PCR array (microfluidic card; Applied Biosciences) were carried out in leukemic lymphocytes from 12 CLL patients.
Mechanism-based combination of B-PAC-1. Effect of B-PAC-1 treatment on IAP expression (A). CLL lymphocytes were treated with or without 10 µM B-PAC-1 for 24 hours and cIAP1 and survivin protein levels were analyzed. Effect of B-PAC-1 treatment on Smac protein expression (B). CLL cells were treated with or without 10 µM B-PAC-1 for 24 hours, and smac protein was immunoblotted. Quantitation of IAP family proteins and smac protein are shown as supplemental Figures 10-11, respectively. Mechanistic combination of B-PAC-1 and Smac-mimetic (Smac066) (C-D). CLL lymphocytes were incubated with B-PAC-1, Smac066, or a combination of both at indicated concentrations for 24 hours. Apoptosis was measured by Annexin V/PI staining assay; time-matched DMSO-treated cells served as control. Data from one patient are shown in panel C. Combination index of B-PAC-1 and Smac066 in 10 patient samples are plotted (D). CLL cells were incubated with indicated concentrations of each drug and their combination for 24 hours, and combination index using the percentage of apoptosis was calculated. CI, combination index (a CI of <1 indicates synergy; calculated using CompuSyn computer software).
Mechanism-based combination of B-PAC-1. Effect of B-PAC-1 treatment on IAP expression (A). CLL lymphocytes were treated with or without 10 µM B-PAC-1 for 24 hours and cIAP1 and survivin protein levels were analyzed. Effect of B-PAC-1 treatment on Smac protein expression (B). CLL cells were treated with or without 10 µM B-PAC-1 for 24 hours, and smac protein was immunoblotted. Quantitation of IAP family proteins and smac protein are shown as supplemental Figures 10-11, respectively. Mechanistic combination of B-PAC-1 and Smac-mimetic (Smac066) (C-D). CLL lymphocytes were incubated with B-PAC-1, Smac066, or a combination of both at indicated concentrations for 24 hours. Apoptosis was measured by Annexin V/PI staining assay; time-matched DMSO-treated cells served as control. Data from one patient are shown in panel C. Combination index of B-PAC-1 and Smac066 in 10 patient samples are plotted (D). CLL cells were incubated with indicated concentrations of each drug and their combination for 24 hours, and combination index using the percentage of apoptosis was calculated. CI, combination index (a CI of <1 indicates synergy; calculated using CompuSyn computer software).
Discussion
Deregulation of the apoptosis network in CLL contributes to both disease pathogenesis and development of chemoresistance. BCR kinases, Bcl-2 family antiapoptotic proteins, and IAP family proteins have emerged as potential pathways to target. Clinical results with agents that inhibit these apical targets have been encouraging29 ; however, their efficacy depends on the level of downstream survival proteins in malignant cells. Here, we present a new approach of targeting CLL cells through terminal procaspases that is independent of prosurvival proteins. Malignant cells in multitude of neoplasms such as melanoma,30 neuroblastomas,31 colon cancer,32 lymphoma,33 and acute myelogenous leukemia34 express higher levels of procaspase-3 than do the respective normal cells, indicating that this executioner zymogen is a relevant therapeutic target.
The present study demonstrates that B-PAC-1, a small-molecule executioner procaspase-activating compound, activates cell death machinery in primary CLL cells. This is the first demonstration of this approach for killing CLL malignant lymphocytes. Consistent with our hypothesis, CLL lymphocytes express higher levels of procaspases than PBMCs do, suggesting the functional relevance of the given approach (Figure 1). In harmony, B-PAC-1 induced dose- and time-dependent apoptosis in the majority of the patient samples tested (Figure 2) and exhibited a therapeutic index that spared normal lymphocytes (Figure 2G). Although procaspase expressions were not different between CLL and normal B cells, the mechanism of differential cytotoxicity to normal B cells vs malignant CLL cells remains unknown. As expected due to terminal status of these procaspases in cell death, B-PAC-1–induced apoptosis was independent of most prognostic markers of CLL (Figure 4), which needs to be tested clinically. Interestingly, β2-microglobulin levels in plasma, a prognostic marker in CLL, impacted B-PAC-1–mediated cell death. Intracellular levels and the role of β2-microglobulin in modulating B-PAC-1 cytotoxicity need to be investigated.
In CLL treatment, standard-of-care DNA-damaging agents act through p53-dependent mechanisms, and targeted therapies work through p53-independent pathways. However, high levels of antiapoptotic proteins such as Mcl-1, Bcl-2, and Bcl-xL have impeded the success of these agents in the clinic. Our data evidenced that the target of B-PAC-1 is exclusively downstream of the mitochondria and requires neither Bax/Bak nor caspase-8, suggesting a new approach of bypassing the resistant mechanisms (Figures 5B-D, 6F).
The initiator procaspase-8 and -9 are in monomer forms as zymogens and are activated by dimerization followed by further reinforcement through cleavage and maturation through catalytic regions. In contrast, executioner (also called effector) procaspases (procaspase-3, -6, and -7) reside in cells in dimeric forms as latent zymogens and are activated through initiator caspases or through activated effectors.3 Executioner caspases have another level of regulation, in which zinc has been shown to be colocalizing with procaspases.4,35 Moreover, it has been shown that zinc inhibits procaspase-3 activation and that removal of zinc allows for activation of executioner caspases.12 Our studies consistently showed that the loss of caspase-8 (Figure 5B) did not abrogate B-PAC-1–induced apoptosis; however, knockdown of procaspase-3 and procaspase-7 (Figure 5C) prevented apoptosis, supporting the notion that B-PAC-1 acts primarily on procaspase-3 and procaspase-7 via zinc ion chelation. Consistent with these data, addition of exogenous zinc ions reversed B-PAC-1–induced apoptosis (Figure 6). The activated executioner caspases in turn dismantle cells by cleaving critical proteins and initiating caspase-cascade leading to cell demise.
We have not tested direct involvement of caspases-9 activation through B-PAC-1; however, several observations support the notion that B-PAC-1 treatment did not activate procaspase-9 directly. First, in prior reports (Putinski et al16 and Wang et al17 ), activation of procaspase-3 before procaspase-9 was observed by the parent compound PAC-1. Second, although zinc ion binding sites have been identified on procaspase-9,36 zymogen procaspase-9 exists as a monomer, and activation by zinc ion chelation requires dimerization. Third, the activity of Bcl-2 antagonists (ABT-199), which act through the mitochondrial and caspase-9 activation pathway, was not impeded by the addition of exogenous zinc ions, suggesting that the activation of caspase-9 is due to the inhibition of Bcl-229 but not through the removal of labile zinc ions (Figure 6).
In contrast to apoptotic procaspases, B-PAC-1 had no significant effect on the level of nonapoptotic procaspase (α isoform of caspase-1) in CLL cells (supplemental Figure 6). Similarly, the B-PAC-1 zinc action was specific to procaspases as activities of other zinc-dependent enzymes such as carboxypeptidase A and HDAC were not modulated by B-PAC-1 (supplemental Figure 2). This difference may be due to labile vs tight binding of zinc to these enzymes.
Experiments with pancaspase inhibitors suggested that B-PAC-1–mediated cell death was in part due to caspase-dependent apoptosis and blocking caspase activity (measured as PARP cleavage inhibition) reversed the percentage of CLL cells undergoing apoptosis. However, treatment with Q-VD resulted in complete recovery of PARP cleavage but partial recovery of a percentage of apoptosis in CLL cells treated with B-PAC-1 (Figure 6D). This discrepancy might be due to caspase-independent apoptosis by B-PAC-1 or due to differences in the sensitivity of an Annexin V/PI positivity assay for apoptosis (more sensitive) vs immunoblot technique for PARP cleavage (semiquantitative). Along the same line, DMSO-treated and Q-VD–treated cells had nearly similar apoptosis but had different cleaved PARP status (Figure 6D).
Bcl-2 family antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 are expressed intrinsically at high levels in CLL cells37 and further induced by the extrinsic microenvironment. In CLL cells, the bone marrow microenvironment upregulates Mcl-1,19,21 nurse-like cells representing the lymph node microenvironment elevate Bcl-2A1 levels,38 and CD40 ligation raises Bcl-2A1 and Bcl-xL levels.39 Several IAP members that are endogenously high in CLL cells40 are also upregulated by the microenvironment.41 Our evaluation of B-PAC-1 activity with various microenvironmental factors revealed that bone marrow stromal cells (NKTert), IL-6 (cytokine), and anti-IgM (BCR activation) did not modulate B-PAC-1–induced apoptosis (Figure 3A-B). This finding goes in line with the notion that the protective microenvironment that induces Mcl-1,19 IAPs,28 AKT, and extracellular signal-regulated kinase (ERK)21,42 does not interfere with the course of B-PAC-1–induced apoptosis as its target is at the terminus.
Another mechanism of regulation for intracellular caspases is their repossession by the baculoviral IAP repeat (BIR) domains of IAPs.2,43 This sequestration is effectively disrupted by the Smac/Diablo protein. B-PAC-1 treatment significantly increased cIAP1 and survivin levels (Figure 7A, supplemental Figure 10). Survivin levels correlated with resistance to B-PAC-1–induced apoptosis, supporting previous findings that CLL lymphocytes express higher levels of cIAP1 and survivin than normal lymphocytes and higher levels of these proteins correlate with worse treatment outcomes.43
The augmentation of survivin and cIAP1 protein levels suggested that a smac mimetic may be a rational addition to B-PAC-1. As postulated, combination enhanced apoptosis (Figure 7C), suggesting a dual effect: activation of zymogen procaspases via zinc ion chelation (B-PAC-1 action) and displacement of IAP-sequestered caspases (Smac066 mechanism). Consistent with these statements, synergistic interactions were observed at ≥5 µM drug combinations (Figure 7D).
In terms of therapeutic index, B-PAC-1–induced significant apoptosis in CLL at 10 µM (Figure 2A). In addition, B-PAC-1–induced cytotoxicity to PBMCs of healthy donors was less compared with peripheral blood lymphocytes of patients (Figure 2G). Collectively, these data suggest a therapeutic index for B-PAC-1 and point toward in vivo testing of this agent.
In summary, our study demonstrated the utility of a small-molecule activator of procaspases in CLL. The therapeutic index and plasma levels of PAC-1 suggest the feasibility of this approach. Our mechanistic studies illustrated that B-PAC-1 activates executioner caspases by removing labile zinc ions and can be synergistically combined with smac mimetics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are thankful to Ms Sarah Bronson for critically editing the manuscript and Paul Hergenrother for his intellectual interactions and suggestions.
This work was supported in part by grant CLL PO1 CA81534 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; a CLL Global Research Foundation Alliance grant; and a sponsored research agreement from Vanquish Oncology. The work is also supported by Cancer Center Support Grant P30CA016672.
K.B., W.G.W., and V.G. are members of the CLL Consortium.
Authorship
Contribution: V.P. designed research, performed experiments, analyzed results, and wrote the manuscript; K.B. directed V.P., designed experiments, and wrote and reviewed the manuscript; M.J.K. and W.G.W. identified patients to obtain peripheral blood samples, provided clinical and patient-related input, and reviewed the manuscript; and V.G. conceptualized and supervised the research, obtained funding, analyzed data, and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: V.G. received research funding from Vanquish Oncology. The remaining authors declare no competing financial interests.
Correspondence: Varsha Gandhi, Department of Experimental Therapeutics, The University of Texas MD Anderson Cancer Center, Unit 1950, PO Box 301429, Houston, TX 77230-1429; e-mail: vgandhi@mdanderson.org.