Key Points
Elevated IL-2R, IL-1RA, and CXCL9 are associated with shorter event-free survival in newly diagnosed FL, treated with chemoimmunotherapy.
Increased serum IL-12 and IL-1RA is associated with shorter event-free survival in patients who were observed or treated with rituximab alone.
Abstract
Serum cytokines and chemokines may reflect tumor biology and host response in follicular lymphoma (FL). To determine whether the addition of these biological factors may further refine prognostication, 30 cytokines and chemokines were measured in pretreatment serum specimens from newly diagnosed FL patients (n = 209) and from 400 matched controls. Cytokine levels were correlated with clinical outcome in patients who were observed or received single agent rituximab, or those who received chemotherapy. Correlations with outcome in chemotherapy treated patients were further examined in a separate cohort of 183 South West Oncology Group (SWOG) patients and all patients were then included in a meta-analysis. Six cytokines were associated with outcome in the Molecular Epidemiology Resource (MER) after adjusting for the FL international prognostic index. In patients who were observed or treated with rituximab alone, increased serum IL-12 and interleukin 1 receptor antagonist (IL-1RA) (P = .005 and .02) were associated with a shorter event-free survival. In patients receiving chemotherapy, hepatocyte growth factor, IL-8, IL-1RA, and CXCL9 (P = .015, .048, .004, and .0005) predicted a shorter EFS. When the MER chemotherapy treated patients and SWOG patients were combined in a meta-analysis, IL-2R, IL-1RA, and CXCL9 (P = .013, .042, and .0012) were associated with a poor EFS.
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma in the United States.1 While classified as an indolent B-cell lymphoma, the clinical course exhibits a spectrum based on the FL international prognostic index (FLIPI) score with a remarkable tendency for patients to relapse.2 The overall survival rate of patients with FL was previous reported as 75% at 5 years, and ranged from 71% at 10 years for patients with a FLIPI score between 0 to 1, and 36% for those with a FLIPI score of >3. Currently, the median survival of all newly diagnosed patients is approximately 9 years.3 Although the overall survival appears to have improved with advances in chemoimmunotherapy, the median event free survival (EFS) remains around 2 years for patients with advanced disease.4
As a prognostic score, the FLIPI has several limitations. It focuses on clinical factors and does not take into account biological factors such as the tumor microenvironment and the host response. Indeed, some studies have found better prognostication of the very high-risk group with the international prognostic index (IPI) rather than with the FLIPI.5,6 The FLIPI may potentially be further improved, particularly in the high-risk categories, if biological data are added. Gene expression profiling studies on pretreatment specimens from patients with FL have highlighted the prognostic importance of tumor biology in FL by identifying patient subgroups based on the immune composition of the tumor.7 It would be extremely helpful therefore, if biological parameters that are easily accessible in blood and may reflect lymphoma biology, could be used in prognostic models. With some patients with FL surviving <1 year and others >20 years, additional prognostic indicators are clearly needed to refine risk adapted therapy.
Serum cytokines and chemokines are proteins secreted by cells that play a vital role in physiological and pathological immune pathways and have been studied as markers of biological activity in FL.8-11 They play a vital role in cellular differentiation, suppression, expansion, tumorigenesis, and host response to malignancy.12-16 Increased levels of these immunologic receptors and ligands may be reflective of the activity of malignant cells, the tumor microenvironment, as well as the systemic host immune response. The role of cytokines and chemokines in predicting the development of non-Hodgkin lymphoma has been previously reported.8,17 However, most data regarding diagnostic and prognostic significance of serum cytokines in lymphoma has focused on one or two preselected cytokines. These studies have typically been reported in a variety of lymphoma subtypes and have often evaluated small numbers of patients and samples. We conducted this study using multiple cytokines in a large cohort of patients to identify specific cytokine markers that may be associated with the underlying biological makeup of the patient or the microenvironment driving tumor growth, and further examined results in a second patient population of treated patients. The goal of our study was to assess which serum cytokines from a large panel of available cytokines have predictive value independent of FLIPI, and thus, highlight their potential for incorporation into a risk-stratified approach.
Materials and methods
Study populations
Molecular Epidemiology Resource (MER) cohort.
Newly diagnosed patients with FL were prospectively enrolled in the University of Iowa and Mayo Clinic Specialized Programs of Research Excellence (SPORE) MER after providing written informed consent, in accordance with the Declaration of Helsinki. Institutional review boards from both institutions approved the protocol for human subjects. Details on the cohort have been previously reported.18 Patients selected for this analysis had newly diagnosed grades 1 to 3a FL, were enrolled in the MER between September 2002 and February 2008, and had an available pretreatment serum sample (Table 1). Patients were excluded if they had prior diagnoses of lymphoma, leukemia, or HIV infection. The diagnosis was confirmed by the study hematopathologists (W.R.M. and S.S.). The choice of initial therapy and clinical management, as well as the schedule of laboratory/radiologic tests and follow up was done per the treating physician. All patients were prospectively contacted for outcome (progression, retreatment, and death) every 6 months for the first 3 years and annually thereafter; all reported events were confirmed by medical record review.
Patient characteristics for MER cohort
| . | All FL . | OBS/rituximab monotherapy . | Chemotherapy treated . |
|---|---|---|---|
| . | (N = 209) . | (N = 103) . | (N = 81) . |
| Age, median (range) | 59 (23-93) | 61 (23-84) | 56 (28-93) |
| Male | 112 (54%) | 51 (50%) | 44 (54%) |
| Age >60 | 93 (45%) | 53 (51%) | 28 (35%) |
| PS 2+ | 4 (2%) | 0 (0%) | 4 (5%) |
| Ann Arbor stage III/IV | 147 (71%) | 75 (74%) | 67 (83%) |
| 2+ Extranodal sites | 6 (3%) | 0 (0%) | 5 (6%) |
| LDH > ULN | 36 (18%) | 18 (18%) | 15 (19%) |
| B symptoms | 13 (6%) | 4 (4%) | 9 (11%) |
| BM involvement | 86 (41%) | 42 (41%) | 41 (50%) |
| HGB <12 | 14 (7%) | 7 (7%) | 7 (9%) |
| >4 Nodal areas | 76 (37%) | 39 (38%) | 35 (43%) |
| FLIPI | |||
| 0-1 | 91 (43%) | 39 (38%) | 31 (38%) |
| 2 | 65 (31%) | 34 (33%) | 29 (36%) |
| 3-5 | 53 (26%) | 30 (29%) | 21 (26%) |
| Initial therapy | |||
| Observation | 73 (35%) | 73 (71%) | 0 |
| Rituximab monotherapy | 30 (14%) | 30 (29%) | 0 |
| Alkylator-based chemotherapy | 46 (22%) | 0 | 46 (57%) |
| Anthracyline-based chemotherapy | 35 (17%) | 0 | 35 (43%) |
| Other therapy | 24 (12%) | 0 | 0 |
| Grade | |||
| 1-2 | 180 (86%) | 100 (97%) | 56 (69%) |
| 3a | 29 (14%) | 3 (3%) | 25 (31%) |
| Events | 120 (57%) | 77 (75%) | 38 (47%) |
| Deaths | 23 (11%) | 12 (12%) | 9 (11%) |
| . | All FL . | OBS/rituximab monotherapy . | Chemotherapy treated . |
|---|---|---|---|
| . | (N = 209) . | (N = 103) . | (N = 81) . |
| Age, median (range) | 59 (23-93) | 61 (23-84) | 56 (28-93) |
| Male | 112 (54%) | 51 (50%) | 44 (54%) |
| Age >60 | 93 (45%) | 53 (51%) | 28 (35%) |
| PS 2+ | 4 (2%) | 0 (0%) | 4 (5%) |
| Ann Arbor stage III/IV | 147 (71%) | 75 (74%) | 67 (83%) |
| 2+ Extranodal sites | 6 (3%) | 0 (0%) | 5 (6%) |
| LDH > ULN | 36 (18%) | 18 (18%) | 15 (19%) |
| B symptoms | 13 (6%) | 4 (4%) | 9 (11%) |
| BM involvement | 86 (41%) | 42 (41%) | 41 (50%) |
| HGB <12 | 14 (7%) | 7 (7%) | 7 (9%) |
| >4 Nodal areas | 76 (37%) | 39 (38%) | 35 (43%) |
| FLIPI | |||
| 0-1 | 91 (43%) | 39 (38%) | 31 (38%) |
| 2 | 65 (31%) | 34 (33%) | 29 (36%) |
| 3-5 | 53 (26%) | 30 (29%) | 21 (26%) |
| Initial therapy | |||
| Observation | 73 (35%) | 73 (71%) | 0 |
| Rituximab monotherapy | 30 (14%) | 30 (29%) | 0 |
| Alkylator-based chemotherapy | 46 (22%) | 0 | 46 (57%) |
| Anthracyline-based chemotherapy | 35 (17%) | 0 | 35 (43%) |
| Other therapy | 24 (12%) | 0 | 0 |
| Grade | |||
| 1-2 | 180 (86%) | 100 (97%) | 56 (69%) |
| 3a | 29 (14%) | 3 (3%) | 25 (31%) |
| Events | 120 (57%) | 77 (75%) | 38 (47%) |
| Deaths | 23 (11%) | 12 (12%) | 9 (11%) |
BM, bone marrow; LDH, lactate dehydrogenase; OBS, observation; ULN, upper limit of normal.
Controls.
As a reference population to define cytokine ranges, we used clinic-based controls from an ongoing case-control study of lymphoma.19 Controls were selected in a random fashion from patients visiting the Mayo Clinic Department of Medicine for a prescheduled general medical examination, with the following eligibility requirements: age 20 years or older and with a residence in Minnesota, Iowa, or Wisconsin. Controls were matched to the patients by 5-year age group, gender, and distance from Rochester, MN. All controls were enrolled over the same time frame as the patients and provided a serum specimen for cytokine analysis.
South West Oncology Group (SWOG) cohort.
A second cohort of chemotherapy treated patients was assembled from patients with available pretreatment serum from 3 SWOG clinical trials: S9800 (N = 27, a phase 2 trial of CHOP, followed by rituximab for treatment of newly diagnosed FL), S9911 (N = 29, CHOP followed by 131I tositumomab), and S0016 (N = 127, CHOP + rituximab vs CHOP + 131I tositumomab).20-22 The schedule of laboratory and radiologic tests and follow up was per protocol. This chemotherapy treated SWOG cohort was then combined with the MER cohort in a meta-analysis to verify associations with patient outcome (see supplemental Figure 1, available on the Blood Web site).
Measurement of serum cytokines
To define the complex and interdependent interactions between cytokines, chemokines, and growth factors that may control lymphoma cell growth and normal immune function, serum samples were subjected to multiplex enzyme-linked immunosorbent assay (Invitrogen, Camarillo, CA) to measure 30 serum cytokines in pretreatment blood samples. Cytokines associated with T-cell function, immune regulation, and cell migration was included in the assay. Luminex 200 System, version 1.7 was used for reading plates. MasterPlex QT 1.0 system (MiraiBio) was used to analyze data. Cytokines included the following: epidermal growth factor, eotaxin, basic fibroblast growth factor, granulocyte macrophage–colony stimulating factor, hepatocyte growth factor (HGF), IFN-α, IFN-γ, interleukin 1 receptor antagonist (IL-1RA), IL-1β, IL-2, IL-2R, IL-4 to IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, inducible protein-10 (IP10/CXCL10), monocyte chemotactic protein 1, monokine induced by interferon γ (MIG/CXCL9), MIP-1α (CCL3), MIP1β (CCL4), regulated on activation normal T-cell expressed and secreted protein, tumor necrosis factor-α, and vascular endothelial growth factor (VEGF). Cases and controls were randomized across plates. Internal control serum was included in all assays to control for interassay variation. Separate control specimens were used for each cohort.
Statistical analysis
Cytokines were analyzed individually and compared between patients with FL and the clinic-based controls. During initial quality control, 11 cytokines with more than 50% of samples with values outside the limit of detection of the assay in either patients or controls were excluded from further analysis. For these 11 cytokines, the values were typically undetectably low. Due to poor reliability at the low end of the range, data from cytokines with a majority of data below detection were omitted from further analysis to avoid concerns with data accuracy. The 95th percentile of the control population was then used to determine the upper limit of normal for each cytokine and a dichotomous elevated vs normal variable was used for analysis in the FL patients.23,24 Cytokines that passed quality control and were elevated in >10% of patients had evidence of higher concentrations in cases than controls (P = .01), and were thus considered biologically relevant in FL. These cytokines were then assessed for association with clinical outcome. Correlation between cytokines was performed using Spearman correlation coefficients on continuous cytokine measurements. EFS in the MER cohort was defined as time from diagnosis to relapse/progression, transformation, retreatment, or death due to any cause. Serum was collected at the time of diagnosis prior to treatment. EFS in the SWOG trials was defined as the time from registration to the first observation of progression or death, as a result of any cause. Serum was collected at time of study registration. Patients were censored at the time of their last follow up if they did not encounter an event or death. Cox proportional hazards models and Kaplan-Meier curves were used to evaluate the association between individual cytokines and EFS. A meta-analysis approach was used to combine the results from the SPORE chemotherapy treated and SWOG cohorts.25 Due to the small number of cytokines (n = 6) that were assessed for outcome, no formal adjustment for multiple comparisons was performed and the nominal P values are reported in the study and tables.
Results
A total of 209 patients with grades 1 to 3a FL and available pretreatment serum were analyzed. Median age at diagnosis was 59 years (range, 23-93) and 54% were male (Table 1). The majority (71%) of patients had advanced stage disease. FLIPI scores were 0 to 1 in 43%, 2 in 31%, and 3 to 5 in 26% of patients. At a median follow-up of 73 months (range, 13-110), 120 patients (71%) had an event and 23 patients (11%) had died. In the initial analysis, all MER patients were included.
Analysis by treatment subgroup
Because serum cytokines may differ in patients with a low or high disease burden, and in asymptomatic vs symptomatic patients, we subsequently analyzed initially observed/rituximab-treated patients and chemotherapy treated patients as separate cohorts within the MER group. There were no significant differences in clinical characteristics or outcome between patients observed and treated with single agent rituximab, and these groups were therefore combined into a low disease burden group that did not require chemotherapy at diagnosis. The observed/rituximab group contained 103 patients who were initially managed with rituximab monotherapy (n = 30) or observation (watchful waiting, n = 73). The chemotherapy group contained 81 patients who were initially managed with an alkylator-based (CVP [n = 17], R-CVP [n = 28], other [n = 1]) or anthracycline-containing regimen (R-CHOP [n = 34], other [n = 1]). The patients not included were initially managed with radiation only for limited stage disease (n = 21) or other chemotherapy (n = 4).
Cytokines in all FL patients.
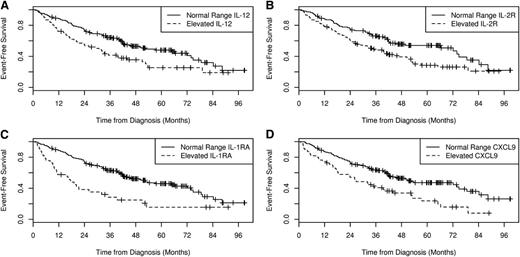
Eleven of the original 30 cytokines (fibroblast growth factor-2, granulocyte macrophage–colony stimulating factor, IFN-g, IL-1β, IL-5 to IL-7, IL-15, IL-17, tumor necrosis factor-α, and VEGF) were excluded for quality control as more than 50% of cases or controls were below the limits of detection; regulated on activation, normal T-cell expressed and secreted protein was above detection in most cases and controls, and was also excluded from further analysis. Normal values were based on the 95th percentile using the results from the 400 normal (noncancer) controls run simultaneously with the FL cases.8 Among the controls, none of the 30 cytokines studied correlated with age, sex, or body mass index as reported previously by our group. Of the remaining 18 cytokines, only 6 were elevated in 10% or more of FL patients and thus considered biologically relevant: HGF (10%), IL-8 (10%), IL-1RA (16%), CXCL9 (26%), IL-12 (29%), and IL-2R (48%) (Table 2). Elevated serum levels of IL-1RA (hazard ratio [HR] = 2.39; 95% CI, 1.56-3.65, P < .00085), CXCL9 (HR = 1.87; 95% CI, 1.29-2.72, P = .0010), IL-2R (HR = 1.71; 95% CI, 1.19-2.44, P = .0035), and IL-12 (HR = 1.69; 95% CI, 1.17-2.44, P = .0050) were all associated with inferior EFS (Table 2; Figure 1). These associations remained significant after adjusting for FLIPI and initial treatment (chemotherapy vs no chemotherapy): IL-1RA (HR = 2.12; 95% CI, 1.36-3.31, P = .00085), CXCL9 (HR = 1.84; 95% CI, 1.22-2.80, P = .0039), IL-2R (HR = 1.54; 95% CI, 1.05-2.25, P = .028), and IL-12 (HR = 1.53; 95% CI, 1.04-2.26, P = .032) (Table 2).
Cytokines and outcome in the MER cohort
| . | All patients (N = 209) . | Initially managed with watchful waiting or rituximab monotherapy (N = 103) . | Initially managed with alkylator or anthracycline based chemotherapy (N = 81) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | % Elevated . | EFS HR (95% CI) . | P . | % Elevated . | EFS HR (95% CI) . | P . | % Elevated . | EFS HR (95% CI) . | P . |
| HGF | 10 | 1.20 (0.70-2.06) | .50 | 13 | 0.56 (0.27-1.17) | .12 | 10 | 2.85 (1.18-6.90) | .020 |
| HGF (FLIPI Adjusted)* | 0.88 (0.50-1.55) | .66 | 0.59 (0.28-1.24) | .16 | 3.03 (1.24-7.41) | .015 | |||
| IL-8 | 10 | 1.53 (0.93-2.52) | .097 | 15 | 0.84 (0.45-1.57) | .58 | 7 | 2.44 (0.86-6.93) | .094 |
| IL-8 (FLIPI Adjusted)* | 0.96 (0.56-1.66) | .89 | 0.89 (0.47-1.69) | .78 | 3.19 (1.01-10.05) | .048 | |||
| IL-12 | 29 | 1.69 (1.17-2.44) | .005 | 32 | 1.90 (1.19-3.03) | .0069 | 32 | 1.15 (0.59-2.25) | .68 |
| IL-12 (FLIPI Adjusted)* | 1.53 (1.04-2.26) | .032 | 1.96 (1.23-3.15) | .0050 | 0.77 (0.35-1.69) | .52 | |||
| IL-2R | 48 | 1.71 (1.19-2.44) | .0035 | 46 | 1.46 (0.93-2.29) | .096 | 59 | 2.16 (1.07-4.37) | .031 |
| IL-2R (FLIPI Adjusted)* | 1.54 (1.05-2.25) | .028 | 1.55 (0.97-2.46) | .065 | 1.79 (0.83-3.86) | .14 | |||
| IL-1RA | 16 | 2.39 (1.56-3.65) | .00006 | 17 | 1.98 (1.12-3.50) | .018 | 19 | 2.62 (1.26-5.46) | .010 |
| IL-1RA (FLIPI Adjusted)* | 2.12 (1.36-3.31) | .00085 | 1.97 (1.11-3.48) | .021 | 2.96 (1.40-6.26) | .0045 | |||
| CXCL9 | 26 | 1.87 (1.29-2.72) | .0010 | 21 | 1.41 (0.84-2.37 | .20 | 38 | 3.43 (1.78-6.63) | .00024 |
| CXCL9 (FLIPI Adjusted)* | 1.84 (1.22-2.80) | .0039 | 1.40 (0.82-2.41) | .22 | 3.96 (1.82-8.59) | .00050 | |||
| . | All patients (N = 209) . | Initially managed with watchful waiting or rituximab monotherapy (N = 103) . | Initially managed with alkylator or anthracycline based chemotherapy (N = 81) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | % Elevated . | EFS HR (95% CI) . | P . | % Elevated . | EFS HR (95% CI) . | P . | % Elevated . | EFS HR (95% CI) . | P . |
| HGF | 10 | 1.20 (0.70-2.06) | .50 | 13 | 0.56 (0.27-1.17) | .12 | 10 | 2.85 (1.18-6.90) | .020 |
| HGF (FLIPI Adjusted)* | 0.88 (0.50-1.55) | .66 | 0.59 (0.28-1.24) | .16 | 3.03 (1.24-7.41) | .015 | |||
| IL-8 | 10 | 1.53 (0.93-2.52) | .097 | 15 | 0.84 (0.45-1.57) | .58 | 7 | 2.44 (0.86-6.93) | .094 |
| IL-8 (FLIPI Adjusted)* | 0.96 (0.56-1.66) | .89 | 0.89 (0.47-1.69) | .78 | 3.19 (1.01-10.05) | .048 | |||
| IL-12 | 29 | 1.69 (1.17-2.44) | .005 | 32 | 1.90 (1.19-3.03) | .0069 | 32 | 1.15 (0.59-2.25) | .68 |
| IL-12 (FLIPI Adjusted)* | 1.53 (1.04-2.26) | .032 | 1.96 (1.23-3.15) | .0050 | 0.77 (0.35-1.69) | .52 | |||
| IL-2R | 48 | 1.71 (1.19-2.44) | .0035 | 46 | 1.46 (0.93-2.29) | .096 | 59 | 2.16 (1.07-4.37) | .031 |
| IL-2R (FLIPI Adjusted)* | 1.54 (1.05-2.25) | .028 | 1.55 (0.97-2.46) | .065 | 1.79 (0.83-3.86) | .14 | |||
| IL-1RA | 16 | 2.39 (1.56-3.65) | .00006 | 17 | 1.98 (1.12-3.50) | .018 | 19 | 2.62 (1.26-5.46) | .010 |
| IL-1RA (FLIPI Adjusted)* | 2.12 (1.36-3.31) | .00085 | 1.97 (1.11-3.48) | .021 | 2.96 (1.40-6.26) | .0045 | |||
| CXCL9 | 26 | 1.87 (1.29-2.72) | .0010 | 21 | 1.41 (0.84-2.37 | .20 | 38 | 3.43 (1.78-6.63) | .00024 |
| CXCL9 (FLIPI Adjusted)* | 1.84 (1.22-2.80) | .0039 | 1.40 (0.82-2.41) | .22 | 3.96 (1.82-8.59) | .00050 | |||
Adjusted for FLIPI and initial therapy.
EFS by cytokine in all MER patients. The EFS by serum IL-12 (A), serum IL-2R (B), IL-1RA (C), and CXCL9 (D) levels for all patients in the MER cohort.
EFS by cytokine in all MER patients. The EFS by serum IL-12 (A), serum IL-2R (B), IL-1RA (C), and CXCL9 (D) levels for all patients in the MER cohort.
Observation/rituximab cohort.
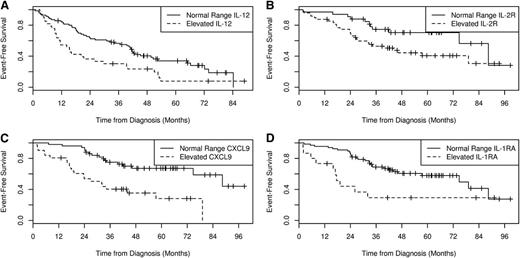
Next, we examined these 6 cytokines in the subset of 103 MER patients with less aggressive disease who were initially managed with watchful waiting or rituximab monotherapy. HGF (13%), IL-8 (15%), IL-1RA (17%), and IL-12 (32%) were more frequently elevated in these patients compared with controls (Table 2). However, only IL-12 (HR = 1.90; 95% CI, 1.19-3.03, P = .0069) and IL-1RA (HR = 1.98; 95% CI, 1.12-3.50, P = .018) were associated with EFS (Figure 2). IL-2R (46%) and CXCL9 (21%) were elevated in this subset but were not associated with EFS (P = .20). After adjusting for the FLIPI and initial treatment (rituximab monotherapy vs observation), both IL-12 (HR = 1.96; 95% CI, 1.23-3.15, P = .0050) and IL-1RA (HR = 1.97; 95% CI, 1.11-3.48, P = .021) remained significantly associated with EFS (Table 2).
EFS by cytokine in MER subgroups. The EFS by serum IL-12 levels in patients in the MER cohort who were observed or received rituximab monotherapy (A). The EFS by serum IL-2R (B), CXCL9 (C), and IL-1RA (D) levels in patients in the MER cohort who were treated with chemotherapy.
EFS by cytokine in MER subgroups. The EFS by serum IL-12 levels in patients in the MER cohort who were observed or received rituximab monotherapy (A). The EFS by serum IL-2R (B), CXCL9 (C), and IL-1RA (D) levels in patients in the MER cohort who were treated with chemotherapy.
Chemotherapy cohort.
We then examined these 6 cytokines in the subset of 81 MER patients with aggressive disease who were initially managed with alkylator- or anthracycline-containing chemotherapy (eg, R-CVP or R-CHOP). The most commonly elevated cytokines were IL-2R (59%), CXCL9 (38%), and IL-12 (32%). CXCL9 (HR = 3.43; 95% CI, 1.78-6.63, P < .001), IL-1RA (HR = 2.62; 95% CI, 1.26-5.46, P = .010), HGF (HR = 2.85; 95% CI, 1.18-6.90), and IL-2R (HR = 2.16; 95% CI, 1.07-4.37) were associated with EFS in univariate analysis (Table 2; Figure 2). After adjusting for FLIPI and treatment (anthracycline, yes vs no), the associations between cytokine and EFS strengthened for CXCL9 (HR = 3.96; 95% CI, 1.82-8.59, P < .001), IL-1RA (HR = 2.96; 95% CI, 1.40-6.26, P = .0045), HGF (HR = 3.03; 95% CI, 1.24-7.41, P = .015), and IL-8 (HR = 3.19; 95% CI, 1.01-10.05, P = .048), whereas IL-2R was no longer significant (HR = 1.79; 95% CI, 0.83-3.86, P = .14) (Table 2).
Additional cohort and meta-analysis
These 6 cytokines were then assessed in 183 chemotherapy treated patients from 3 SWOG clinical trials. The median age of this cohort was 53 years across the 3 trials (range, 24-84). A total of 29% of patients in this cohort were >60 years old and 55% were male, 98% had stage III/IV disease, 24% had elevated lactate dehydrogenase, and 15% had IPI scores of 3 to 5 (FLIPI was not available). The only cytokine reaching statistical significance in the SWOG cohort was IL-2R (84% elevated, IPI adjusted HR = 2.41; 95% CI, 1.04-5.58, P = .04). CXCL9 (49% elevated, IPI adjusted HR = 1.50; 95% CI, 0.93-2.43, P = .10) was also associated with poor EFS, although it did not reach statistical significance (Table 3). In a meta-analysis combining the MER chemotherapy and SWOG cohorts, CXCL9 (HR = 1.96; 95% CI, 1.30-2.95, P = .0012), IL-2R (HR = 2.05; 95% CI, 1.16-3.61, P = .013), and IL-1RA (HR = 1.57; 95% CI, 1.02-2.42, P = .042) were significantly associated with EFS at P < .05 (Table 3).
Cytokines and outcome in the SWOG cohort and MER/SWOG meta-analysis
| . | SWOG cohort (N = 183) . | Meta-analysis on chemotherapy treated patients (total N = 264) . | |||
|---|---|---|---|---|---|
| . | % Elevated . | EFS HR* (95% CI) . | P . | EFS HR (95% CI) . | P . |
| HGF | 18 | 0.80 (0.44-1.45) | .46 | 1.21 (0.73-1.98) | .46 |
| IL-8 | 27 | 1.27 (0.79-2.04) | .32 | 1.45 (0.94-2.25) | .096 |
| IL-12 | 52 | 1.17 (0.74-1.83) | .50 | 1.05 (0.71-1.56) | .79 |
| IL-2R | 84 | 2.41 (1.04-5.58) | .04 | 2.05 (1.16-3.61) | .013 |
| IL-1RA | 21 | 1.14 (0.67-1.94) | .62 | 1.57 (1.02-2.42) | .042 |
| CXCL9 | 49 | 1.50 (0.93-2.43) | .10 | 1.96 (1.30-2.95) | .0012 |
| . | SWOG cohort (N = 183) . | Meta-analysis on chemotherapy treated patients (total N = 264) . | |||
|---|---|---|---|---|---|
| . | % Elevated . | EFS HR* (95% CI) . | P . | EFS HR (95% CI) . | P . |
| HGF | 18 | 0.80 (0.44-1.45) | .46 | 1.21 (0.73-1.98) | .46 |
| IL-8 | 27 | 1.27 (0.79-2.04) | .32 | 1.45 (0.94-2.25) | .096 |
| IL-12 | 52 | 1.17 (0.74-1.83) | .50 | 1.05 (0.71-1.56) | .79 |
| IL-2R | 84 | 2.41 (1.04-5.58) | .04 | 2.05 (1.16-3.61) | .013 |
| IL-1RA | 21 | 1.14 (0.67-1.94) | .62 | 1.57 (1.02-2.42) | .042 |
| CXCL9 | 49 | 1.50 (0.93-2.43) | .10 | 1.96 (1.30-2.95) | .0012 |
Adjusted for IPI and initial therapy (S9800 vs S9911 vs S0016).
Discussion
FL comprises a diverse group of biological entities with a treatment spectrum ranging from uneventful observation lasting years, to up front aggressive chemoimmunotherapy and even stem cell transplantation.26 Further pretreatment characterization of aggressiveness of disease, in addition to FLIPI and pathological grade, will be helpful for making treatment decisions and designing clinical trials.
Standard prognostic variables at the time of diagnosis, such as FLIPI and histologic grade, reasonably predict patient outcome and aid in risk adaptive treatment better than previous models, such as the Italian Lymphoma Intergroup index.2,27-29 However, these parameters do not address the underlying microenvironment and bi-directional cross-talk between FL B-cells and stromal cells that may herald aggressive disease biology in advance of clinical presentation and deterioration.30
Cytokines present within the tumor microenvironment appear to contribute to growth and survival of malignant cells and also ensures a continued inflammatory milieu recruiting reactive cells to lymphoma tissue.31-33 Elevations in various cytokines have been reported as markers of aggressive lymphoma.34,35 Some of these cytokines may represent interdependent cascades with synergistic effects and others may be downstream of one principal cytokine.
Several banked serum studies have provided evidence linking serum cytokine levels, such as TGF-β and VEGF to the development of non-Hodgkin lymphoma.17,31,36,37 Our group has shown that pretreatment circulating cytokines are elevated in both diffuse large B-cell lymphoma and FL.8,24 The elevation of serum cytokines results in the activation of both the malignant cell as well as the tumor microenvironment. IL-4 has been reported to be elevated in FL with downstream phosphorylation of Erk and activation of Mek.38 Gene expression profiling has made inroads by demonstrating activation of IL-4–dependent activation of STAT6 in FL B cells and T-helper cells in the tumor microenvironment indicating an interdependent axis.39 IL-10 and Leptin have been associated with FL development and result in immune suppression.31 Furthermore, T-cells infiltrating FL have reduced signaling for some cytokines such as IL-4, IL-10, and IL-21 due to persistently elevated levels.40-42
In this study, we report that an abnormal pretreatment serum cytokine profile, specifically for IL-2R, IL-12, CXCL9, and IL-1RA predict a shortened EFS independent of FLIPI in the MER cohort. In the meta-analysis, CXCL9, IL-1RA, and IL-2R remained significant. The prognostic importance of increased IL-12 and IL-1RA levels may well be to identify FL patients with a low burden of disease who may benefit from early initiation of chemoimmunotherapy rather than being observed or treated with rituximab alone. Similarly, increased serum levels of CXCL9, IL-1RA, and IL-2R in patients requiring chemoimmunotherapy may identify a subgroup of patients at increased risk for disease progression.
Each of the cytokines associated with outcome in this study has a biological function that may explain this association. IL-1RA interacts with MyD88, which is an intracellular adapter protein that triggers a signaling cascade to enhance expression of inflammatory genes.43 MyD88 has gained recent interest as a therapeutic target due to its striking abundance in low grade lymphomas, such as Waldenström macroglobulinemia, and in about a third of cases of diffuse large B-cell lymphoma.44,45
Soluble IL-2Rα enhances the function of IL-2 favoring T-cell differentiation to Treg cells, which inhibit CD8+ T cells and thus promotes FL growth and worsens prognosis.9 It has been proposed as a surrogate marker for tumor progression in FL and predicts reduced survival.9,46 Elevated soluble IL-2R expression has also been reported in several infectious and autoimmune conditions, which are known risk factors for lymphoma development and correlates with tumor bulk in intestinal lymphomas.47-50
CXCL9 is an inflammatory chemokine released by perivascular macrophages in response to IFN-γ and causes the accumulation of CD8+ T cells in lymphoma.51 It has been proposed as a diagnostic marker, along with CXCL10, in lymphoma-associated hemophagocytic syndrome.52 It is a ligand for CXCL3 and an autocrine loop that promotes marginal zone lymphoma growth as previously described.53 IL-12 signaling induces CXCL9 secretion and has a significant role in Th1 cell development, but this has been associated with T-cell exhaustion and a poor outcome in FL.54
Exploiting cytokines or chemokines as a therapeutic option is an interesting avenue of ongoing research and their role in the future of FL may not be limited to diagnostic and prognostic markers. Several groups have studied cytokines, such as IFN-α, IL-2, IL-12, and IL-21, as interventional targets in FL with mixed results.55-58 Clearly, the role of cytokines in lymphoma biology is complex. The intertwined circuits of cytokines and different helper and effector cells secreting some of the elevated cytokines may represent an immune system response against the tumor and vice versa. The strengths of our study include the initial evaluation of 30 cytokines relevant to lymphomagenesis. Although these assays can lack standard control values and plate variation can be a concern, our study used 400 controls to determine a normal range; we focused our analyses only on cytokines that were detectable in both cases and controls and showed a significant elevation in FL. We also examined the particular trends in 2 specific subgroups of FL at diagnosis, ie, a less aggressive (observed/rituximab) and a more aggressive one treated by chemotherapy (both the MER and SWOG cohort). To our knowledge, such a large pretreatment sample of exclusive FL patients with controls has not been previously reported for cytokine trends.
In conclusion, IL-12 and IL-1RA are predictive of outcome in FL patients who are initially observed or treated with single agent rituximab. IL-2R, CXCL9, and IL-1RA are prognostic factors in patients with more aggressive disease requiring chemoimmunotherapy. These cytokine, chemokine, and receptor levels are independent of the FLIPI score, and therefore provide additional prognostic information and biological insight beyond that provided by clinical factors. Incorporation of these cytokines into future prognostic models should be considered.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health, National Cancer Institute (CA97274), the Lymphoma Research Foundation, the Leukemia & Lymphoma Society, and the Henry J. Predolin Foundation. It was also supported in part by the Public Health Service Cooperative Agreement grant numbers awarded to the SWOG cancer research cooperative group by the National Institutes of Health, National Cancer Institute (DHHS: CA32102, CA38926, CA11083, and CA20319) and by a grant from GlaxoSmithKline to SWOG.
Authorship
Contribution: M.A.M. wrote the manuscript; M.J.M. performed statistical analysis, and created tables and graphs; S.C.Z. performed research and reviewed the manuscript; S.L.S. assisted in statistical analysis; T.H. reviewed the manuscript; W.R.M. reviewed pathological slides; B.K.L. reviewed the manuscript and performed SPORE grant research; S.S. reviewed pathological slides; T.W. reviewed the manuscript; J.W.F., O.P., and M.L. reviewed the manuscript for SWOG; J.R.C. assisted in statistical analysis; A.N. performed research and reviewed the manuscript; and S.M.A. created the concept, performed research, and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no completing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.