Key Points
The production of NO by platelets and its possible role are controversial.
We visualize NO formed by single platelets adhering to collagen under flow conditions and show that it depends on Ca++ and modulates adhesion.
Abstract
Nitric oxide (NO) exerts vasodilatatory, antiplatelet, antioxidant, and antiproliferative effects. Endothelium-derived NO has been shown to be of crucial importance in cardiovascular protection, whereas evidence that NO is synthesized by platelets and regulates platelet function is still controversial. By using a sensitive and specific fluorescent probe, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM), we visualized NO production in individual platelets undergoing adhesion on a collagen substrate under flow conditions. NO production, monitored in real time, was dependent on the shear rates applied, increasing with the raising of the shear rates. Furthermore, NO production increased in the presence of l-arginine (nitric-oxide synthase [NOS] substrate), and it decreased in the presence of L-NG-monomethyl arginine (L-NMMA) (NOS inhibitor) but not of D-NG-monomethyl arginine (D-NMMA) (L-NMMA–inactive enantiomer). Platelet deposition, measured with mepacrine-labeled platelets, was inversely related to NO production. A correlation was evident between Ca++ elevation and NO production, suggesting that platelet NO formation is triggered by intracytoplasmic Ca++ elevation. Simultaneous measurement of NO and Ca++ indicated that NO production in individual platelets is preceded by Ca++ elevations, with a lag phase of 33 ± 9.5 s. Our studies provide the first direct demonstration of platelet NO production triggered by the interaction with an activating surface under flow and suggest that intraplatelet Ca++ elevation elicits the production of NO which, in turn, modulates thrombus size.
Introduction
Nitric oxide (NO) is involved in many biological processes, but in particular it plays an important role in the cardiovascular system, where it displays vasodilatory, antithrombotic, and antiatherosclerotic effects.1-3 NO is synthesized from l-arginine by the family of enzymes known as nitric-oxide synthases (NOS). There are 3 isoforms of NOS: NOS1 (neuronal NOS [nNOS]), NOS2 (inducible NOS [iNOS]), and NOS3 (endothelial NOS [eNOS]).4 nNOS and eNOS are constitutively expressed in cells and their enzymatic activities are regulated by intracellular Ca++ levels and by protein phosphorylation,5 whereas iNOS is considered a Ca++-independent enzyme normally not expressed in resting cells and induced by inflammatory stimuli.1,4
Of the 3 NOS isoforms identified to date, 2 have been reported to be expressed in platelets, namely the endothelial (eNOS) and the inducible (iNOS) types.4,6-8 NO inhibits platelet adhesion to the vascular endothelium and platelet aggregation through the activation of soluble guanylyl cyclase and the consequent increase in intraplatelet cGMP.9,10 It has been reported that platelet-derived NO plays a significant role in modulating platelet-thrombus formation in vivo.11-15 Therefore, the generation of NO by platelets may act as a negative feedback mechanism to limit platelet activation after an aggregating stimulus and to reduce platelet recruitment to a growing thrombus.11-13
However, because of the evanescent nature of NO and the very small amounts produced by platelets, estimated at about 5 × 10−17 mol/platelet (determined by microelectrode in platelet-rich plasma [PRP] stimulated for 2 minutes with 5 μM ADP),16 several studies have questioned the pathophysiologic role of platelet-derived NO in regulating platelet activation and in vivo thrombus formation,17-19 with a few even negating the presence of eNOS in platelets.20
NO is a small free radical very much diffusible in the aqueous environment of blood and across cellular membranes, but it is highly reactive and has a very short half-life in the circulation, in the order of a few seconds; therefore it acts mainly as an autoacoid exerting its biological effects on the cells that have produced it or in their immediate proximity.21 Therefore, the systemic measurement of NO (eg, in biological fluids) is not only difficult but also insufficiently informative.22,23
Recently, the assessment of NO production by single cells, including blood cells, has been made possible by the use of the plasma membrane–penetrating fluorescent dye 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM). All blood cells form NO intracellularly, with monocytes and neutrophils generating the strongest fluorescence signals, followed by lymphocytes and red blood cells, and then by platelets.24 Because erythrocytes are the most numerous blood cell population, it was suggested that red blood cell–derived NO plays a preeminent role in cardiovascular physiology, and the observation that in patients with coronary heart disease erythrocyte-related NO fluorescence is impaired, was reported as a confirmation of its pathophysiologic relevance.24 On the other hand, erythrocyte hemoglobin (Hb) represents an important scavenger of NO.2 Moreover, studies with erythrocytes were carried out under static conditions, without consideration of the intercellular interactions and the potential pathophysiologic role of platelet-produced NO under flow.24
The formation of NO inside individual activated platelets under flow conditions and in parallel its possible regulatory role in platelet activation and thrombus formation have not been studied thus far.
The aim of our study was to visualize NO production in single platelets undergoing adhesion to an activating surface under flow conditions and to compare these events with intracellular Ca++ elevations, and with platelet deposition, by using highly sensitive and specific fluorescent probes for NO and intraplatelet Ca++, and by use of a video imaging system.
We found that the interaction of platelets with fibrillar collagen type I under flow induces platelet NO synthesis, which is enhanced by the addition of NOS substrate (l-arginine), is greatly decreased by a NOS inhibitor (L-NMMA), and is influenced by the shear rate conditions. Correlation of intraplatelet NO and Ca++ rises with platelet adhesion show that platelet NO formation follows intraplatelet Ca++ increase induced by the interaction with collagen under flow conditions, and in turn it inhibits platelet activation.
Materials and methods
NO-fluorometric assay
Platelet-rich plasma (PRP) prepared as described25 was incubated for 30 minutes at 37°C with DAF-AM (30 μM) (Molecular Probes, Eugene, OR), centrifuged at 2500g for 10 minutes at room temperature, and resuspended in Tyrode’s buffer pH 7.4, without Ca++ and Mg++. DAF-FM–loaded washed platelets were preincubated with the NOS substrate l-arginine (100 µM); the NOS antagonists N5-1-iminoethyl-L-Ornitine (L-NIO; Sigma/Merck, St. Louis, MO; 50 µM) or L-NG-monomethyl arginine (L-NMMA; Sigma/Merck; 100 µM), which are, respectively, irreversible and reversible; the inactive stereoisomer of L-NMMA, D-NG-monomethyl arginine (D-NMMA; Sigma/Merck; 100 µM); with 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5,-tetramethyl-1H-imidazolyl-1-oxy-3-oxidepotassium salt (carboxyPTIO; Sigma/Merck; 100 µM), an NO scavenger; with the intracellular Ca++ chelator 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM; Sigma/Merck; 100 µM); or with the extracellular Ca++ chelator ethylene-bis(oxyethylenenitrilo)tetraacetic acid (EGTA; Sigma/Merck; 5 mM) for 10 minutes at 37°C. They were then challenged with collagen (fibrillar Type I from equine tendon; Mascia Brunelli, Milan, Italy; 50 µg/mL). In some experiments, DAF-FM–loaded platelets were incubated with the NO-donor SNAP (100 µM) or with H2O2 (100 µM) to assess the specificity of DAF-FM fluorescence (see supplemental Methods available on the Blood Web site).
Preparation of blood samples for flow experiments
PRP was incubated for 30 minutes at 37°C with 30 μM DAF-FM or for 20 minutes at 37°C with 8 μM FLUO 3-acetoxymethyl ester (FLUO 3-AM; Molecular Probes).26,27 For confocal microscopy experiments, platelets were incubated simultaneously with DAF-FM and with the fluorescent calcium probe X-Rhod1-AM (Molecular Probes) 8 μM for 30 minutes at 37°C. Subsequently, reconstituted blood was prepared as described27 (see supplemental Methods).
Platelets from eNOS−/− male (JaxMice, Bar Harbor, ME) or wild-type C57BL/6 mice (Charles River, Lecco, Italy) were also used. Blood sampling and platelet preparation were previously reported28 (see supplemental Methods).
Perfusion experiments
Glass coverslips coated with suspensions of acid-insoluble fibrillar type I collagen from bovine Achilles tendon (Sigma/Merck) in 0.5 M acetic acid (pH 2.8) were prepared as previously described27-31 (see supplemental Methods). Perfusion experiments were performed at a wall shear rate of 3000, 1500, 400, and 250 s−1 at the inlet2,31-34 (see supplemental Methods).
Measurement of NO formation and Ca++ mobilization
DAF-FM or FLUO 3-AM–loaded platelets in reconstituted whole blood were perfused over collagen-coated coverslips at different shear rates, and surface-interacting platelets were monitored in real time for 3 minutes. Images were obtained every 0.04 seconds and the percentage of platelets showing increased fluorescence was calculated at 3 minutes, as reported27 (see supplemental Methods). In selected experiments, platelets from the same donor were incubated with either the fluorescent indicator FLUO 3-AM (8 µM) or with DAF-FM (30 µM), and experiments were run in parallel. Fluorescences of surface-interacting platelets at 250 and 3000 s−1 were monitored in real time for the first 30 seconds. For the simultaneous measurement of NO and Ca++ mobilization in platelets loaded with both DAF-FM and X-Rhod1-AM, the flow chamber was placed in an inverted fluorescence microscope equipped with a confocal module (UltraVIEW, Perkin Elmer, Milan, Italy) and images were simultaneously obtained in real time (see supplemental Methods) using the Andor TECHNOLOGY iQ software package.
Measurement of platelet adhesion
After 3 minutes of perfusion, blood cell suspensions were treated with the fluorescent dye mepacrine (8 µM), single-frame images were captured from videotapes after 1 minute from mepacrine addition, and the area occupied by platelets in each microscopic field was measured using the MicroImage software (Casti Imaging, Venice, Italy).
Statistical analysis
Each experiment was performed in triplicate and data are shown as means ± 95% confidence intervals (CI). One-way analysis of variance was applied to assess the effects of antibodies and inhibitors on platelet adhesion/fluorescence. All analyses were performed in MATLAB 6.3 using Stats Toolbox.
Results
Increase of DAF fluorescence: specificity for NO in platelets
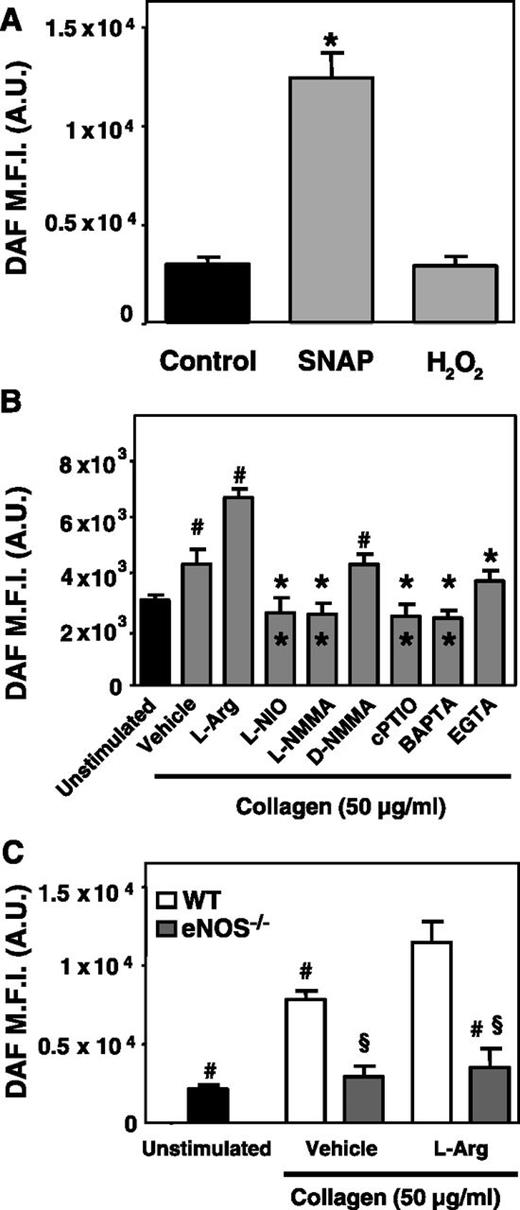
DAF-FM platelet fluorescence was enhanced when platelets in suspension were incubated with the NO-donor SNAP, but not with H2O2, indicating that fluorescence was specific for NO and not induced by other oxygen radicals (Figure 1A).
DAF fluorescence intensity in fluorimetric assay: specificity for NO in human and mice platelets. (A) DAF-FM–loaded washed platelets in suspension were incubated with either SNAP (100 µM) or H2O2 (100 µM) (*P < .01 vs control and H2O2). (B) DAF-FM–loaded platelets were stimulated with collagen (50 µg/mL) after preincubation with vehicle (control), l-arginine, L-NIO, L-NMMA, D-NMMA, cPTIO, BAPTA, or EGTA, and the DAF-FM fluorescence intensity was recorded (#P < .005 vs unstimulated; *outside the column P < .0001 vs l-Arg; *inside the column P < .005 vs vehicle). (C) DAF-FM–loaded washed platelets from wild-type (white columns) or eNOS−/− (gray columns) mice were stimulated with collagen after preincubation with vehicle (control) or with the eNOS substrate l-Arg, 100 μM (#P < .001 vs l-Arg–treated wild-type; §P < .001 vs wild-type).
DAF fluorescence intensity in fluorimetric assay: specificity for NO in human and mice platelets. (A) DAF-FM–loaded washed platelets in suspension were incubated with either SNAP (100 µM) or H2O2 (100 µM) (*P < .01 vs control and H2O2). (B) DAF-FM–loaded platelets were stimulated with collagen (50 µg/mL) after preincubation with vehicle (control), l-arginine, L-NIO, L-NMMA, D-NMMA, cPTIO, BAPTA, or EGTA, and the DAF-FM fluorescence intensity was recorded (#P < .005 vs unstimulated; *outside the column P < .0001 vs l-Arg; *inside the column P < .005 vs vehicle). (C) DAF-FM–loaded washed platelets from wild-type (white columns) or eNOS−/− (gray columns) mice were stimulated with collagen after preincubation with vehicle (control) or with the eNOS substrate l-Arg, 100 μM (#P < .001 vs l-Arg–treated wild-type; §P < .001 vs wild-type).
When DAF-FM–loaded washed platelets in suspension were stimulated with collagen, DAF fluorescence increased compared with unstimulated platelets. Preincubation with l-arginine lead to a further increase in DAF fluorescence, which instead was completely inhibited by preincubation with L-NIO, L-NAME, or carboxyPTIO, but not with the inactive stereoisomer D-NMMA35 (Figure 1B). Preincubation with both the extracellular Ca++-chelator, EGTA, and with the intracellular Ca++-chelator, BAPTA, reduced collagen-induced increase of platelet DAF fluorescence, although inhibition was complete only with BAPTA, suggesting that the release of Ca++ from intraplatelet stores plays a preeminent role in eNOS activation (Figure 1B). Moreover, a sustained increase of DAF fluorescence was observed when platelets were activated with 10 μM ADP or 1 μM U46619, and this was abolished by preincubation with L-NMMA and unaffected by D-NMMA. Therefore, soluble stimuli also increase NO production by individual platelets (supplemental Figure 1). Collagen-induced increase of intraplatelet DAF-FM fluorescence was also tested with mouse platelets; although fluorescence increased after collagen stimulation of platelets from wild-type mice, it did not increase in platelets from eNOS−/− mice, further confirming the specificity of the DAF-FM–detected fluorescence signal (Figure 1C). Collagen-induced platelet NO synthesis was unaffected by preincubation with aspirin or a P2Y12 antagonist (supplemental Figure 2).
Visualization of platelet NO production under flow
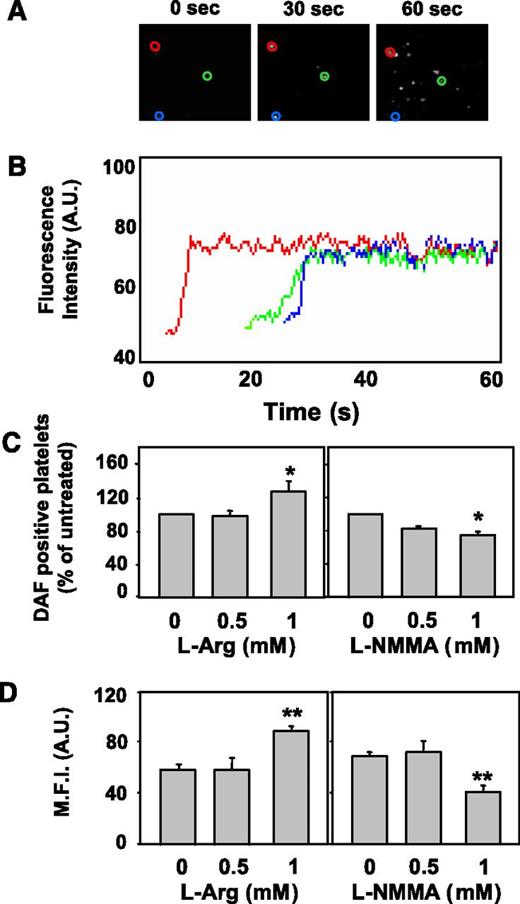
DAF-loaded platelets flowing in reconstituted blood and interacting with immobilized type I collagen (shear rate 3000 s−1) showed an increase of fluorescence, demonstrating NO production, during the first 60 seconds of perfusion (Figure 2A). The response is characterized by a fast increase in fluorescence reaching a plateau in a few seconds (Figure 2B) and then persisting for the whole observation period, because of the covalent binding of NO to the fluorochrome forming a benzotriazolic derivative. After 3 minutes of perfusion, the number of adhering platelets showing increased fluorescence (ie, producing NO) was 64.4 ± 16.4 platelets per field (n = 90). Preincubation with l-Arg (1 mM) increased NO-positive platelets by 28.5 ± 1.5% (P < .01 vs control; Figure 2C, left panel), whereas preincubation with L-NMMA caused a concentration-dependent inhibition of platelet NO elevation. Significant inhibition was attained at 1 mM, which induced a 25 ± 3.5% decrease (P < .01 vs control) in the percentage of NO-positive platelets (Figure 2C, right panel). Preincubation with l-Arg (1 mM) increased platelet mean fluorescence index (MFI) (from 59 ± 6 to 85 ± 4, +44%, P < .001; Figure 2D, left panel), whereas preincubation with L-NMMA (1 mM) reduced MFI (from 72 ± 3 to 39 ± 4, −46%, P < .001; Figure 2D, right panel). Preincubation with D-NMMA did not modify either the percentage of NO-positive platelets or MFI (data not shown). The inhibitory effect of L-NMMA on MFI under flow was stronger than that on NO-positive platelets, in agreement with results previously reported with red blood cells.24 The degree of inhibition is probably maximal considering the background fluorescence possibly generated by the formation of unspecific fluorescent adducts36 or by the presence of fluorescent impurities in the DAF-FM solution.24
Visualization of intracellular NO production in platelets adhering to type I collagen under flow. DAF-FM–loaded platelets (5 × 107/mL) and washed erythrocytes (hematocrit 42%-45%) were suspended in plasma and HEPES-Tyrode buffer containing 2 mM CaCl2/MgCl2 (1:1 vol/vol). The cell suspensions were perfused for 3 minutes over fibrillar type I collagen at the wall shear rate of 3000 s−1 and fluorescence intensity within surface-interacting platelets was monitored for 60 seconds. (A) Representative single-frame images demonstrating fluorescence increases in the first 60 seconds of perfusion. (B) Surface-interacting platelets were monitored in real time during the first 60 seconds. The traces, obtained with the automatic Casti Imaging procedure, are representative curves of fluorescence intensity of 3 single platelets (representative example of 3 experiments). (C) The number of platelets exhibiting a fluorescence increase over a 3-minute period was enumerated in the absence (control) or presence of different concentrations of l-Arg or of L-NMMA, as indicated, and expressed as percent DAF-positive platelets of control (*P < .01 vs untreated). (D) The mean fluorescence intensity (MFI), an index of NO production in DAF-FM–loaded platelets, was quantified for 30 seconds in the absence (control) or presence of different concentrations of l-Arg or of L-NMMA, as indicated (**P < .001 vs untreated).
Visualization of intracellular NO production in platelets adhering to type I collagen under flow. DAF-FM–loaded platelets (5 × 107/mL) and washed erythrocytes (hematocrit 42%-45%) were suspended in plasma and HEPES-Tyrode buffer containing 2 mM CaCl2/MgCl2 (1:1 vol/vol). The cell suspensions were perfused for 3 minutes over fibrillar type I collagen at the wall shear rate of 3000 s−1 and fluorescence intensity within surface-interacting platelets was monitored for 60 seconds. (A) Representative single-frame images demonstrating fluorescence increases in the first 60 seconds of perfusion. (B) Surface-interacting platelets were monitored in real time during the first 60 seconds. The traces, obtained with the automatic Casti Imaging procedure, are representative curves of fluorescence intensity of 3 single platelets (representative example of 3 experiments). (C) The number of platelets exhibiting a fluorescence increase over a 3-minute period was enumerated in the absence (control) or presence of different concentrations of l-Arg or of L-NMMA, as indicated, and expressed as percent DAF-positive platelets of control (*P < .01 vs untreated). (D) The mean fluorescence intensity (MFI), an index of NO production in DAF-FM–loaded platelets, was quantified for 30 seconds in the absence (control) or presence of different concentrations of l-Arg or of L-NMMA, as indicated (**P < .001 vs untreated).
Collagen-induced platelet NO production and calcium elevation depend on shear rate
The number of platelets exhibiting an increase in DAF-FM fluorescence when perfused over a collagen-coated surface at 250 s−1 was significantly lower than that observed at higher shear rates. When comparing a shear rate of 250 s−1 with 3000 s−1, the number of NO-positive platelets increased >fourfold (9 ± 3 positive platelets/0.007 mm2 vs 41 ± 5 positive platelets/0.007 mm2) (Figure 3A). When calculated as MFI, a significant increase of NO fluorescence in platelets was observed only at 3000s−1 compared with a 250 s−1 shear (Figure 3B). Therefore, all subsequent experiments were carried out at 3000 s−1. Indeed, at 250 s−1, DAF-fluorescence was negligible, whereas at 3000 s−1 a strong and sustained NO signal was evident (Figure 3C). Similarly, the increase of Ca++, expressed as fluorescence intensity, was less pronounced at 250 s−1 than at 3000 s−1. At 3000 s−1, sustained calcium signals were observed, which corresponded to full NO production (Figure 3C-D). These results suggest that a certain threshold of calcium signaling is necessary to induce efficient NO production.
Shear rate effects on platelet NO elevation. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized fibrillar collagen type I at different shear rates for 3 minutes. (A) The number of platelets exhibiting an increase in fluorescence after the perfusion of an identical volume of blood (1.41 mL) was enumerated at the indicated shear rate conditions (250, 400, 1500, 3000 s−1) (*P < .01 vs 250 s−1). (B) The MFI, an index of NO production in DAF-FM–loaded platelets, was quantified for 30 seconds at different shear rates, as indicated (**P < .001 vs 250 s−1). (C) Surface-interacting platelets at 250 and 3000 s−1 were monitored in real time for the first 30 seconds after fluorescence increased. Traces, obtained with MATLAB through computational analysis, are representative of fluorescence intensity of 3 single platelets loaded with DAF-FM. (D) FLUO 3-AM–loaded platelets, prepared as described under Methods, were perfused over immobilized fibrillar collagen type I at different shear rates for 3 minutes. Surface-interacting platelets at 250 and 3000 s−1 were monitored in real time for the first 30 seconds. Traces are representative of fluorescence intensity of 3 single platelets loaded with FLUO 3-AM. The time scale indicates only the temporal trend of the fluorescent signals and not its evolution in relation to platelet-collagen interaction.
Shear rate effects on platelet NO elevation. A blood cell suspension, prepared as described in the legend to Figure 2, was perfused over immobilized fibrillar collagen type I at different shear rates for 3 minutes. (A) The number of platelets exhibiting an increase in fluorescence after the perfusion of an identical volume of blood (1.41 mL) was enumerated at the indicated shear rate conditions (250, 400, 1500, 3000 s−1) (*P < .01 vs 250 s−1). (B) The MFI, an index of NO production in DAF-FM–loaded platelets, was quantified for 30 seconds at different shear rates, as indicated (**P < .001 vs 250 s−1). (C) Surface-interacting platelets at 250 and 3000 s−1 were monitored in real time for the first 30 seconds after fluorescence increased. Traces, obtained with MATLAB through computational analysis, are representative of fluorescence intensity of 3 single platelets loaded with DAF-FM. (D) FLUO 3-AM–loaded platelets, prepared as described under Methods, were perfused over immobilized fibrillar collagen type I at different shear rates for 3 minutes. Surface-interacting platelets at 250 and 3000 s−1 were monitored in real time for the first 30 seconds. Traces are representative of fluorescence intensity of 3 single platelets loaded with FLUO 3-AM. The time scale indicates only the temporal trend of the fluorescent signals and not its evolution in relation to platelet-collagen interaction.
Collagen-induced platelet NO and calcium elevation under flow: role of platelet integrin receptors and GPIbα
Preincubation with integrin- and GPIbα-blocking antibodies reduced the number of adhering platelets by 46 ± 1% (anti αIIbβ3), 64 ± 3% (anti α2β1), and 90 ± 6% (anti GPIbα) compared with control (Figure 4A). Similarly, the percentage of Ca++-positive platelets was significantly reduced by all monoclonal antibodies (anti-αIIbβ3 −16 ± 4%, anti-α2β1 −64 ± 2%, anti-GPIbα −79 ± 8%) (Figure 4B). In control conditions, 60% of adhering platelets were NO-positive; upon incubation with different antibodies, NO-positive platelets were significantly reduced (anti-αIIbβ3 −16 ± 10%, anti-α2β1 −42 ± 5%, anti-GPIbα −75 ± 17%) (Figure 4C). These data suggest that platelet NO production is strictly associated with intraplatelet Ca++ rises, which in turn are dependent on different platelet adhesive receptors.27 Preincubation with blocking anti-α2β1 and anti-GPIbα reduced platelet calcium signaling and NO production concomitantly (Figure 4). Instead, although the blockade of αIIbβ3 reduced the percentage of platelets exhibiting Ca++ fluorescence only marginally, it strikingly diminished the total amount of intracellular calcium in single platelets (data not shown). These data are in agreement with our previous results showing that blocking αIIbβ3 obliterated the long-lasting calcium signals (γ-spikes) in platelets adhering to von Willebrand factor under high shear rates27 and further confirm the close relationship between intraplatelet Ca++ increases and NO production. Platelet calcium signaling and NO production were strikingly, and consensually, decreased by GPIbα blockade. However, it must be considered that in the presence of an anti-GPIbα antibody, the number of adhering platelets was very low; therefore the estimation of the percentage of platelets displaying DAF-fluorescence was of somewhat uncertain interpretation.
Platelet adhesion and NO and calcium elevation on immobilized fibrillar type I collagen: role of different integrins and GPIbα. DAF-FM- or FLUO 3-AM–loaded platelets, prepared as described in the legend to Figure 2, were perfused over immobilized fibrillar collagen type I at a shear rate of 3000 s−1 for 3 minutes. When indicated, blood cells were incubated for 10 minutes at 37°C with 100 µg/mL of LJ-CP8, a monoclonal anti-αIIbβ3 antibody that completely blocks fibrinogen and von Willebrand factor binding, or 100 µg/mL of LT-1bI, an anti-GPIbα monoclonal antibody, or 100 µg/mL of an anti-α2β1 monoclonal antibody specific for the α2 integrin.32 Platelets present in each optical field (control: 40-60/field) were enumerated and expressed as percentage of the control (A). The number of FLUO 3-AM–loaded platelets in which at least one [Ca++] elevation was observed, was enumerated over a 3-minute period as a fraction of the percentage of adhering platelets, in the absence (control) or presence of different antibodies, as indicated (B). At the same time, the DAF-FM–loaded platelets exhibiting a fluorescence increase over a 3-minute period were enumerated as a fraction of the percentage of adhering platelets in the absence (control) or in the presence of different antibodies, as indicated (C). Data are the mean ± 95% CIs of at least 3 different experiments. Significant difference from the corresponding control: *P < .05, **P < .01, ***P < .001.
Platelet adhesion and NO and calcium elevation on immobilized fibrillar type I collagen: role of different integrins and GPIbα. DAF-FM- or FLUO 3-AM–loaded platelets, prepared as described in the legend to Figure 2, were perfused over immobilized fibrillar collagen type I at a shear rate of 3000 s−1 for 3 minutes. When indicated, blood cells were incubated for 10 minutes at 37°C with 100 µg/mL of LJ-CP8, a monoclonal anti-αIIbβ3 antibody that completely blocks fibrinogen and von Willebrand factor binding, or 100 µg/mL of LT-1bI, an anti-GPIbα monoclonal antibody, or 100 µg/mL of an anti-α2β1 monoclonal antibody specific for the α2 integrin.32 Platelets present in each optical field (control: 40-60/field) were enumerated and expressed as percentage of the control (A). The number of FLUO 3-AM–loaded platelets in which at least one [Ca++] elevation was observed, was enumerated over a 3-minute period as a fraction of the percentage of adhering platelets, in the absence (control) or presence of different antibodies, as indicated (B). At the same time, the DAF-FM–loaded platelets exhibiting a fluorescence increase over a 3-minute period were enumerated as a fraction of the percentage of adhering platelets in the absence (control) or in the presence of different antibodies, as indicated (C). Data are the mean ± 95% CIs of at least 3 different experiments. Significant difference from the corresponding control: *P < .05, **P < .01, ***P < .001.
Simultaneous measurement of collagen-induced platelet NO production and calcium elevation
Figure 5 and the supplemental video represent an original image of a platelet loaded with both DAF-FM and X-Rhod1-AM. The single cell shows a sequential increase of Ca++ signaling followed by NO production. The analysis of numerous platelets under the same conditions revealed a mean lag phase between the Ca++ and the NO signals of 33.0 ± 9.5 seconds (n = 100). These results show that platelet NO production follows calcium mobilization and that NO production occurs after a delay of several seconds.
Simultaneous measurement of collagen-induced platelet NO production and calcium elevation. X-Rhod1-AM and DAF-FM double-labeled platelets, prepared as described under Methods, were perfused for 3 minutes at 3000 s−1 on a collagen-coated surface and the 2 fluorescence intensities in surface-interacting platelets were monitored for 60 seconds. Representative curves of the fluorescence intensities of single platelets labeled with X-Rhod1-AM and DAF-FM are reported. The analysis of 100 platelets revealed a mean lag phase between the calcium peak and NO signals of 33 ± 9.5 seconds (mean ± 95% CIs).
Simultaneous measurement of collagen-induced platelet NO production and calcium elevation. X-Rhod1-AM and DAF-FM double-labeled platelets, prepared as described under Methods, were perfused for 3 minutes at 3000 s−1 on a collagen-coated surface and the 2 fluorescence intensities in surface-interacting platelets were monitored for 60 seconds. Representative curves of the fluorescence intensities of single platelets labeled with X-Rhod1-AM and DAF-FM are reported. The analysis of 100 platelets revealed a mean lag phase between the calcium peak and NO signals of 33 ± 9.5 seconds (mean ± 95% CIs).
Effect of platelet NO on platelet deposition onto collagen under flow
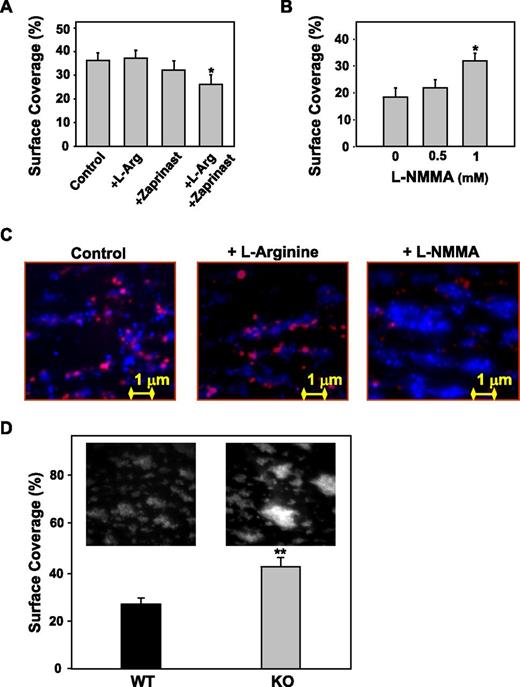
Perfusion of reconstituted blood over collagen at a high shear rate (3000 s−1) leads to platelet deposition (36.3 ± 3.3% surface coverslip area covered by platelets). Preincubation with l-Arg (1 mM) and zaprinast37 (1 µM) significantly reduced the coverslip surface area covered by platelets (–10 ± 2.4%, P < .01; Figure 6A). The total surface covered by platelets was significantly increased after preincubation with L-NMMA (1 mM, +32 ± 2.8%, P < .001 vs control, Figure 6B), as a consequence of enhanced platelet reactivity.
Effect of platelet NO on platelet deposition onto collagen under flow. A human blood-cell suspension prepared as described in the legend to Figure 2 was perfused over immobilized fibrillar collagen type I at the shear rate of 3000 s−1 for 3 minutes. When indicated, blood cells were incubated for 10 minutes at 37°C with l-Arg, with the cGMP phosphodiesterase inhibitor (Zaprinast, 1 µM)37 , with the two combined or with L-NMMA at various concentrations. After 3 minutes (to allow visualization of all adhering platelets), the fluorescent dye mepacrine (8 µM) was added and the percent of surface coverage was calculated. (A) l-arginine in combination with Zaprinast inhibits platelet deposition onto collagen (*P < .01 vs control). (B) L-NMMA increases platelet deposition onto collagen (*P < .01 vs control). (C) Representative single-frame images resulting from the merging of 2 distinct grayscale images obtained at 3 and 4 minutes of perfusion. Red = DAF-FM–loaded platelets after 3 minutes of perfusion; blue = quinacrine-loaded platelets after 4 minutes of perfusion. (D) Whole blood from wild-type or eNOS−/− mice, treated with the fluorescent dye mepacrine for platelet visualization, was perfused over immobilized fibrillar collagen type I at the shear rate of 1500 s−1 for 3 minutes. eNOS−/− mice form larger thrombi and the quantification of the surface area covered by platelets after perfusion demonstrates a significantly larger surface area in the eNOS−/− compared with wild-type mice (*P < .01 vs wild-type).
Effect of platelet NO on platelet deposition onto collagen under flow. A human blood-cell suspension prepared as described in the legend to Figure 2 was perfused over immobilized fibrillar collagen type I at the shear rate of 3000 s−1 for 3 minutes. When indicated, blood cells were incubated for 10 minutes at 37°C with l-Arg, with the cGMP phosphodiesterase inhibitor (Zaprinast, 1 µM)37 , with the two combined or with L-NMMA at various concentrations. After 3 minutes (to allow visualization of all adhering platelets), the fluorescent dye mepacrine (8 µM) was added and the percent of surface coverage was calculated. (A) l-arginine in combination with Zaprinast inhibits platelet deposition onto collagen (*P < .01 vs control). (B) L-NMMA increases platelet deposition onto collagen (*P < .01 vs control). (C) Representative single-frame images resulting from the merging of 2 distinct grayscale images obtained at 3 and 4 minutes of perfusion. Red = DAF-FM–loaded platelets after 3 minutes of perfusion; blue = quinacrine-loaded platelets after 4 minutes of perfusion. (D) Whole blood from wild-type or eNOS−/− mice, treated with the fluorescent dye mepacrine for platelet visualization, was perfused over immobilized fibrillar collagen type I at the shear rate of 1500 s−1 for 3 minutes. eNOS−/− mice form larger thrombi and the quantification of the surface area covered by platelets after perfusion demonstrates a significantly larger surface area in the eNOS−/− compared with wild-type mice (*P < .01 vs wild-type).
In Figure 6C, representative images of the colocalization of NO production and surface coverage demonstrate that in the presence of l-Arg, NO production is increased and surface coverage decreased compared with control, whereas in the presence of NOS inhibitor (L-NMMA), NO production is decreased and surface coverage increased.
Moreover, blood from e-NOS−/− mice formed thrombi covering a significantly larger area (40 ± 3%) than blood from wild-type mice (27 ± 2.6%) when perfused over type I collagen–coated coverslips at a shear rate of 1500 s−1, suggesting an important role of platelet-derived NO in thrombus formation under flow conditions (Figure 6D).
Discussion
It is generally held that the regulation of localized platelet activation is attained by a balance between the generation by endothelial cells of NO and prostacyclin, which inhibit platelet activation, and the release by activated platelets of substances that further promote platelet activation.3
In contrast to this paradigm, several studies have reported that upon stimulation by certain agonists (eg, IGF-1, adenosine diphosphate, insulin, isoproterenol, collagen), platelets also produce NO, a potent inhibitor of platelet activation.13,38,39 The physiologic relevance of platelet-derived NO has been debated, because its amount is low and some authors have even questioned the presence of eNOS in platelets.20 Moreover, it has been reported that low concentrations of NO producing a small cGMP increase have a stimulating effect on platelets in certain conditions.19,40
Until recently, the main tools used to detect NO production have been the measurement of NO degradation products, nitrite plus nitrate, in the supernatant of stimulated cells; the measurement of citrulline production (the other end product of NOS activity) after preincubation with radiolabeled l-arginine; or the direct measurement of NO with a highly sensitive NO-specific electrode. With these methods, it is not possible to define the cellular origin of the NO measured with certainty, and this leaves open the possibility that contamination of platelet preparations by other NO-producing cells, like white or red blood cells, can be responsible for the NO detected. Only recently, NO-sensitive fluorescent probes have been made available, opening up the possibility to visualize NO production in single cells.
In our study we used a cell-permeable, highly specific and highly sensitive fluorescent probe derived from diamminofluorescein (DAF-FM DA), which is essentially nonfluorescent until it reacts with NO to form a fluorescent benzotriazole. DAF-FM DA is cell permeable and passively diffuses across cellular membranes. Once inside cells, it is deacetylated by intracellular esterases to become DAF-FM. The same probe was recently used to visualize NO production by red blood cells.24 In that study, DAF-FM fluorescence was also detected in other blood cells, including monocytes, that showed the highest intracellular fluorescence intensity, neutrophils, and lymphocytes but also platelets.24
However, no assessment was made of the formation of NO by platelets upon contact with an activating surface under flow conditions (ie, in a situation of pathophysiologic relevance) and of the role of platelet-derived NO in regulating platelet deposition.
Here we show that DAF-FM–loaded platelets perfused on fibrillar type I collagen at high shear rates display a rapid increase in fluorescence intensity, which persists as a result of the covalent binding of the probe to form a benzotriazole derivative.
DAF-FM fluorescence was specific for NO compared with other oxygen radicals, because we found that no increase of fluorescence was detected in DAF-loaded platelets after incubation with H2O2, whereas it was clearly enhanced in the presence of a NO donor. Moreover, when DAF-loaded platelets were stimulated with a soluble agonist (collagen), DAF fluorescence increased and its intensity was modulated by preincubation with the NOS substrate l-arginine, the NOS inhibitors L-NIO and L-NMMA (but not the inactive enantiomer D-NMMA36 ), or the cell-permeable NO scavenger carboxyPTIO. Thus, DAF-FM can be used as a fluorescent probe for detecting NO within human platelets and demonstrates a good specificity for NO.
DAF fluorescence was lit up in platelets adhering to immobilized collagen under flow, and the number of DAF-positive platelets and the MFI were dependent on the shear rates applied, increasing with increasing shear rates, and regulated by NO-synthesis modulators, like the NOS substrate l-arginine, which increased DAF-positive platelets, or the competitive eNOS antagonist L-NMMA, which decreased DAF-positive platelets, but not by D-NMMA. Given that a background DAF fluorescence was also present in unstimulated platelets in suspension and that some residual DAF fluorescence was observed in platelets adhering to collagen under flow after incubation with L-NMMA, it is possible that NO came in part from the reduction of inorganic nitrite within platelets.41
We also showed that platelet NO production by individual platelets temporally follows the increase in cytosolic Ca++ concentration, because in the majority of platelets (>90%), fluorescence for NO was preceded by an intracellular Ca++ increase, similarly to what was previously shown in smooth muscle cells42 and endothelial cells.43-46 The lag time between the 2 events in platelets (33 ± 9.5 s) seems to be longer than that reported in vascular endothelial cells stimulated with bradykinin, in which a delay of ∼5 seconds was observed,45 or in human umbilical cord endothelial cells stimulated with histamine,46 or in porcine aortic endothelial cells stimulated with thrombin, where almost no lag phase between the 2 events was observed.47 These apparent discrepancies could be explained considering the different roles played by endothelial-released and platelet-released NO. Platelet-released NO is likely meant to prevent the recruitment of additional platelets to growing thrombi without interfering with the initial adhesion process, and thus its production is required at a later stage.
In contrast, an immediate generation of NO by endothelial cells is appropriate to modulate vascular tone. Indeed, endothelium-derived NO exerts important vasodilator properties, and basal release of NO by the endothelial layer stimulated by shear stress plays a crucial role in the maintenance of basal arterial tone.48 The NO basally released by the endothelium would principally maintain the antithrombotic vascular wall milieu, reducing the expression of adhesive receptors for platelets and circulating inflammatory cells,49 rather than prevent an acute thrombotic deposition. In agreement with this hypothesis, mice with only circulating platelets deficient of eNOS and with a normal endothelium showed a shortened bleeding time upon endothelial damage.39
The preeminent role of platelet- instead of endothelium-derived NO in the antithrombotic activity of dl-nebivolol previously reported12 further suggests that NO generated by platelets upon activation at an arterial damage site is more effective than that generated by endothelium-derived NO in preventing thrombosis, probably because it acts exactly where platelets become activated.12 These different roles would explain why the endothelial NO response to shear must be an immediate and continuous reaction, whereas platelet NO production would occur only “on demand” and when the initial hemostatic process has already taken place.
In our studies, the kinetics of NO production in platelets differed from previous reports, in which NO production was detected immediately after the addition of collagen,16 but the two phenomena are not comparable because in the study by Malinsky the agonist was added in solution to platelet-rich plasma under stirring conditions (ie, in conditions of low shear).16
In fact, it has been demonstrated that platelet-derived NO affects platelet adhesion at intermediate and high shear and that the production of NO in platelets is dominated by receptor-mediated interactions at low-intermediate shear and by mechano-transduction at high shear.9,38,50
In our experimental conditions, platelet adhesion and thrombus formation onto a collagen surface under flow conditions take place in only a few minutes with the contribution and interplay of different receptors like GPIb-V-IX, GPVI, α2β1, the purinergic receptors P2Y1 and P2Y12, and αIIbβ3 which, to a different extent, regulate the global increase of intracytoplasmic Ca++ .35
The increase in cytoplasmic Ca++ promotes the association between NOS and calmodulin, thereby activating the enzyme.51 Previous evidence suggests that activation of the enzyme may also occur independently of intracellular Ca++ because some stimuli can activate the enzyme with no detectable effect on intracellular Ca++.38,50,52 In our experimental conditions, however, with platelet activation triggered by collagen under flow conditions, intracellular Ca++ is the driving pathway leading to NO production. In fact, while chelation of extracellular Ca++ greatly decreased NO production, the chelation of intracellular calcium abolished DAF-FM fluorescence, at least under static conditions. Additional support to this conclusion comes from our studies with platelets labeled with both a Ca++ and a NO indicator, in which we showed that platelet NO production is correlated to the increase in cytosolic Ca++. At low shear rates, a low production of NO was observed subsequent to a moderate increase in intraplatelet Ca++, whereas at higher shear rates, a sustained NO production was observed secondary to a high intensity and long-lasting Ca++ increase, suggesting that a certain threshold of intracytoplasmatic Ca++ rise is required to induce platelet NO production.
Our data also show that the production of NO by platelets triggered by the interaction with immobilized collagen under high shear rate limits further platelet deposition. In fact, the collagen-coated surface covered by platelets was modulated by the NOS activity, being clearly enhanced by the inhibition of NOS and decreased by preincubation with the NOS substrate l-arginine that enhanced platelet-NO fluorescence, as well as a cGMP phosphodiesterase inhibitor. By using mouse models of platelet thrombus formation, contradictory data on the role of eNOS in limiting platelet activation were reported; in fact, although some reports did not observe a thrombotic phenotype in eNOS−/− mice, others described an enhanced thrombotic response.12,17-19,39 Recently it was documented that eNOS plays a role in the resolution of a platelet thrombus upon moderate to strong thrombotic stimulation.53 In our experimental flow conditions on type I collagen, probably mimicking a rather intense thrombotic stimulus, the role of NO in controlling platelet deposition was confirmed by the significant increase of the collagen surface covered by platelets observed with blood from eNOS−/− mice compared with blood from wild-type mice.
In conclusion, we show here for the first time that NO production can be visualized in single platelets undergoing adhesion to collagen at high shear rates, that platelet NO formation is correlated to an intracytoplasmic Ca++ increase, and that NO derived from activated platelets acts as a modulator (at least with a paracrine function) of platelet adhesion and aggregation over thrombogenic surfaces at high shear, thus potentially limiting thrombus formation, and we provide conclusive evidence on the role of platelet NO production in the limitation of thrombus growth.
Indeed, previous reports have shown that platelet NO release may be altered in the setting of disease/inflammation in humans. In particular, low platelet NO production was independently associated with the presence of an acute coronary syndrome, a strongly inflammatory condition—a finding that could not be attributed to concurrent medical therapy or other clinical or coronary risk factors.15 Moreover, impaired platelet-derived NO production has been shown in several conditions associated with enhanced cardiovascular risk.54,55 Recent data in patients with familial myocardial infarction and enhanced platelet reactivity carrying 2 gene mutations affecting the function of platelet-soluble guanylyl cyclase with impaired cGMP formation, confirm the importance of NO-mediated platelet inhibition in the prevention of thrombosis.56 Treatments aimed at restoring and/or enhancing the platelet production of NO may have therapeutic potential.12,57,58
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Sara Orsini for skilled editorial assistance.
This work was supported in part by a grant from the Italian Ministry of University and Scientific Research (MIUR 2012 prot. 2012773NE3) (P.G.) and by grants from the Italian Ministry of Health RF-2010-2316198 (L.D.M.) and RF-2010-231993 (M.M.).
Authorship
Contribution: M.R.C. and G.G. performed experiments, analyzed data, and contributed to writing the manuscript; M.B. and S.M. performed the experiments with mice; E.L. participated in performing flow experiments; E.C.M. contributed to drafting the manuscript; D.D.Z. performed computational imaging analysis; M.M. designed experiments and interpreted data; P.G. and L.D.M. designed the study, supervised research, interpreted data, and wrote the manuscript; and all authors made critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.R.C. is Department of Translational Research, Oncological Pathology Laboratory, IRCCS-CRO Aviano.
Correspondence: Paolo Gresele, Department of Medicine, Division of Internal and Cardiovascular Medicine, University of Perugia, Via E. dal Pozzo, 06126 Perugia, Italy; e-mail: paolo.gresele@unipg.it; and Luigi De Marco, Department of Translational Research, Stem Cells Unit, CRO National Cancer Institute, Via Franco Gallini n° 2, 33081 Aviano (PN) Italy; e-mail: ldemarco@cro.it.
References
Author notes
M.R.C. and G.G. contributed equally to this study.
P.G. and L.D.M. are co–senior authors.


![Figure 4. Platelet adhesion and NO and calcium elevation on immobilized fibrillar type I collagen: role of different integrins and GPIbα. DAF-FM- or FLUO 3-AM–loaded platelets, prepared as described in the legend to Figure 2, were perfused over immobilized fibrillar collagen type I at a shear rate of 3000 s−1 for 3 minutes. When indicated, blood cells were incubated for 10 minutes at 37°C with 100 µg/mL of LJ-CP8, a monoclonal anti-αIIbβ3 antibody that completely blocks fibrinogen and von Willebrand factor binding, or 100 µg/mL of LT-1bI, an anti-GPIbα monoclonal antibody, or 100 µg/mL of an anti-α2β1 monoclonal antibody specific for the α2 integrin.32 Platelets present in each optical field (control: 40-60/field) were enumerated and expressed as percentage of the control (A). The number of FLUO 3-AM–loaded platelets in which at least one [Ca++] elevation was observed, was enumerated over a 3-minute period as a fraction of the percentage of adhering platelets, in the absence (control) or presence of different antibodies, as indicated (B). At the same time, the DAF-FM–loaded platelets exhibiting a fluorescence increase over a 3-minute period were enumerated as a fraction of the percentage of adhering platelets in the absence (control) or in the presence of different antibodies, as indicated (C). Data are the mean ± 95% CIs of at least 3 different experiments. Significant difference from the corresponding control: *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/4/10.1182_blood-2014-06-579474/4/m_697f4.jpeg?Expires=1765989236&Signature=SrC4Wj6JgplpZOlEtceUVCzRNeHTOMhJR~QFpGWJTr-p3xMxiWQmX-blfJuJI93yKJrduD3PhsmtuMmc~B43XzgXKLnq-CD1Sbrf6bvdAGIf4~m1DKWcbS8Cy-TNdF5FTdxGMMz0d-PMxZ4ccFv6v0pcJMEiOB28SO3FrwXG2vR2NMfvp~X4Y~N7-waPHkPhJI699LtWYJPbSpxCRq4ucZQMyDgt67yXkXSzezY7gBwN5Bt~LTRAwEDKBITUxLsptSJSYUEU4sa0stIHgXlSjtBe-nSyW0zITy8bNfDabCZYdhSZj1tS9YpnQvbazkG3RR3yxMXdwSiK1D5KjgHLRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal