Abstract
Chronic graft-versus-host disease (GVHD) remains a common and potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation (HCT). The 2-year cumulative incidence of chronic GVHD requiring systemic treatment is ∼30% to 40% by National Institutes of Health criteria. The risk of chronic GVHD is higher and the duration of treatment is longer after HCT with mobilized blood cells than with marrow cells. Clinical manifestations can impair activities of daily living and often linger for years. Hematology and oncology specialists who refer patients to centers for HCT are often subsequently involved in the management of chronic GVHD when patients return to their care after HCT. Treatment of these patients can be optimized under shared care arrangements that enable referring physicians to manage long-term administration of immunosuppressive medications and supportive care with guidance from transplant center experts. Keys to successful collaborative management include early recognition in making the diagnosis of chronic GVHD, comprehensive evaluation at the onset and periodically during the course of the disease, prompt institution of systemic and topical treatment, appropriate monitoring of the response, calibration of treatment intensity over time in order to avoid overtreatment or undertreatment, and the use of supportive care to prevent complications and disability.
Introduction
The prevalence and severity of chronic graft-versus-host disease (GVHD) have increased during the past 2 decades in association with the increasing use of hematopoietic stem cell transplantation (HCT) for treatment of older age patients, the widespread use of mobilized blood cells instead of marrow for grafting, and improvements in survival during the first several months after allogeneic HCT.1-6
Pathophysiological understanding of chronic GVHD is emerging,7,8 but the long-standing reliance on prednisone described as the mainstay of treatment in Vogelsang’s “How I Treat” review in 2001 has persisted to the present.9 The 2005 National Institutes of Health (NIH) Consensus Conference developed a framework for characterizing the pleomorphic manifestations of chronic GVHD. The consensus project defined minimal criteria for the clinical diagnosis, emphasized differences in the clinical manifestations of chronic and acute GVHD, established criteria for scoring the severity of clinical manifestations in affected organs, and proposed new categories for describing overall disease severity and indications for treatment.10 The consensus project also proposed measures for monitoring disease progression and response to therapy and provided other information for purposes of clinical trials.11-13 In 2014, the NIH Conference was reconvened, and revisions are under consideration to update the recommendations based on available evidence and insights from clinical application of the original recommendations.14-33
Chronic GVHD has a wide range of pleomorphic manifestations, and many complications can emerge from both the disease and its treatment. A dedicated multidisciplinary team approach with relevant expertise is necessary in order to provide the best care for patients with a chronic illness that can have devastating effects on quality of life. Our approach to treatment emphasizes the importance of early recognition in the management of chronic GVHD, with respect to making the initial diagnosis, monitoring the response to initial treatment, and preventing complications and disability. Nuances applicable only to children are not addressed in this review.
Case summary
A 45-year-old man received growth factor–mobilized blood cells from an HLA-matched unrelated male donor after conditioning with 12 Gy total body irradiation and cyclophosphamide for treatment of acute myeloid leukemia with persistent disease. He received methotrexate and tacrolimus for immunosuppression after HCT. He developed acute GVHD of the skin and gut, which resolved after treatment with steroid cream and oral beclomethasone and budesonide. Because malignant cells persisted after HCT, treatment was administered with azacytidine, and immunosuppression with tacrolimus was withdrawn by day 100, 3 months earlier than originally planned.
Malignant cells disappeared, but 7 months after HCT and 2 months after the third cycle of azacytidine, he was diagnosed with severe chronic GVHD (NIH global score). Affected sites included the skin (erythematous rash involving >50% body surface area [BSA]), mouth (ulcers and lichenoid features), fasciae (wrist tightness and leg edema), liver (alanine aminotransferase twice the normal upper limit with normal total serum bilirubin concentration), and eosinophilia (1800 per μL). Forced expiratory volume in the first second (FEV1) was 79% of predicted, and the ratio of FEV1 to forced vital capacity (FVC) was 78% of predicted, representing an absolute 8% decline from the baseline before HCT.
Treatment was started with prednisone at 1 mg/kg per day, and antibiotic prophylaxis was administered to prevent Pneumocystis pneumonia and infection with encapsulated bacteria. Antiviral prophylaxis was continued with acyclovir. Daily intake of vitamin D 1000 IU and calcium 1500 mg was recommended. After 2 weeks, improvement was noted in wrist discomfort, leg edema, and the extent of rash, with resolution of eosinophilia and liver function abnormalities. The dose of prednisone was tapered to reach 80 mg every other day, with continued clinical monitoring and pulmonary function tests (PFTs) at monthly intervals.
During the next 3 months, PFTs improved, but other manifestations showed progressive worsening, with cutaneous sclerosis involving ∼28% of the BSA with a Rodnan modified total score34 of 8, oral ulceration, and decreased wrist mobility. The patient enrolled in a randomized clinical trial comparing imatinib vs rituximab for steroid-refractory sclerotic GVHD. He was randomized to treatment with imatinib (200 mg daily) while continuing treatment with prednisone 80 mg every other day. Dexamethasone oral rinses and clobetasol ointment were used to control oral ulceration.13
The extent and severity of sclerosis remained stable for 3 months. Sclerosis then began to progress, involving ∼50% of BSA with a Rodnan score of 28 after 6 months of treatment with imatinib. The patient met the criteria for crossover according to the study design, and he was treated with 2 cycles of rituximab, 375 mg/m2 per week for 4 weeks per cycle. Due to hypertension and hyperglycemia, the dose of prednisone was gradually tapered to 40 mg every other day within 6 months. The patient subsequently reported improved flexibility, and after 7 months, sclerosis remained stable with a Rodnan score of 26.
Eleven months after the last dose of rituximab, fasciitis progressed with further decrease in range of motion while continuing treatment with prednisone at 40 mg every other day. Extracorporeal photopheresis (ECP)35 (reviewed in Inamoto and Flowers36 ), low-dose interleukin-2 (IL-2),37 and sirolimus were considered as possible further treatment. The patient opted for daily low-dose IL-2, but he had significant local reactions, low-grade fever, and no appreciable improvement in GVHD. Three months after treatment with IL-2 was discontinued, he developed a new erythematous maculopapular rash affecting 50% of BSA. The dose of prednisone was increased to 40 mg per day, and sirolimus was added. The rash subsequently improved, but sclerosis and fasciitis continued to worsen, prompting treatment with ECP for the past 3 months. Efficacy cannot yet be assessed because improvement is often not observed until treatment with ECP has been continued for at least 6 months.35,38
Pathophysiology
Recent laboratory studies have yielded some insights into the pathophysiology of chronic GVHD, and candidate biomarkers that could be used for diagnosis or monitoring have been identified.7,8 The disease likely represents a syndrome in which the respective contributions of inflammation, innate and adaptive cell-mediated immunity, humoral immunity, abnormal immune regulation, and fibrosis vary considerably from 1 patient to the next. The risk of chronic GVHD can be substantially decreased by administration of rabbit anti-thymocyte globulin or alemtuzumab in the conditioning regimen before HCT,39-43 or by administration of high-dose cyclophosphamide on days 3 and 4 after HCT.44,45 These results demonstrate that the pathophysiological mechanisms leading to development of chronic GVHD are set in motion at the time of HCT, even though the manifestations of the disease typically do not become apparent until several months later.
Animal models that replicate many features of the disease have been established, but mechanisms linking inflammation with abnormal immune regulation and mechanisms linking immune-mediated injury with fibrosis have not been well defined. In the absence of a definitive understanding of the pathophysiological mechanisms or clear explanation accounting for the considerable variability of disease manifestations among patients, efforts to develop new treatment have relied on empirical testing of agents approved for other indications where inflammation, abnormal immune regulation, or fibrosis have been implicated as pathogenic mechanisms.
Diagnosis and evaluation
Manifestations of chronic GVHD can resemble autoimmune or other immune-mediated disorders such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency. Manifestations typically appear within the first year after HCT, most often when doses of immunosuppressive medications are weaned. The disease can begin as early as 2 months and as late as 7 years after HCT, although onset at >1 year from HCT occurs in <10% of cases.46 Chronic GVHD should be suspected at the onset of any perturbation in laboratory tests, symptoms, or signs, especially during the first year after HCT. Conversely, not every problem after allogeneic HCT represents chronic GVHD. Other conditions such as eczema, iron overload, hypothyroidism, adrenal insufficiency, infections, or drug effect can be misdiagnosed as chronic GVHD.47
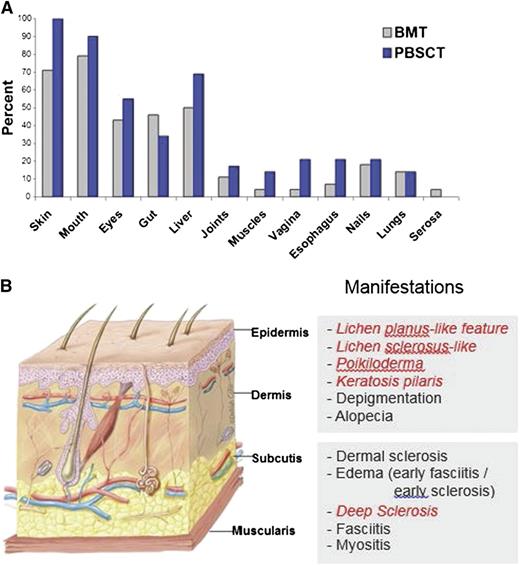
Chronic GVHD generally involves several organs or sites, although manifestations are sometimes restricted to a single organ or site. The disease is characterized by features that differ from the typical dermatitis, enteritis, and cholestatic liver manifestations of acute GVHD (Table 1).10 Patients frequently have erythematous rash, enteritis, or hepatic involvement characterized by transaminase elevation or hyperbilirubinemia at initial presentation and intermittently afterward during the course of the disease. The term “overlap syndrome” has been used to indicate that manifestations typical of acute GVHD are present in a patient with chronic GVHD. These inflammatory manifestations are often transient, disappearing when the intensity of immunosuppression is increased and reappearing when the intensity of immunosuppression is tapered. According to NIH criteria, the diagnosis of chronic GVHD requires at least 1 diagnostic sign or at least 1 distinctive sign confirmed by biopsy, other tests, or by radiography in the same or another organ, and exclusion of other diagnoses (Table 1).10 Manifestations of chronic GVHD have a wide range of severity and impact on quality of life after HCT. Certain manifestations are particularly difficult to manage and require prolonged treatment. These include fasciitis or cutaneous sclerosis, severe ocular sicca, and BOS, occurring in ∼20%,48 12%,3 and about 10%3 of patients, respectively. The most common organs and sites affected by chronic GVHD include the skin, mouth, eyes, gastrointestinal tract, and liver (Figure 1).
Clinical manifestations of chronic GVHD
| Organ or site . | Diagnostic (sufficient for diagnosis) . | Distinctive (insufficient alone for diagnosis) . | Other . | Features seen in both acute and chronic GVHD . |
|---|---|---|---|---|
| Skin | Poikiloderma | Depigmentation | Erythema | |
| Lichen planus-like | Papulosquamous | Maculopapular | ||
| Sclerosis | Pruritus | |||
| Morphea-like | ||||
| Lichen sclerosis-like | ||||
| Nails | Dystrophy | |||
| Onycholysis | ||||
| Nail loss | ||||
| Pterygium unguis | ||||
| Scalp and body hair | Alopecia (scarring or nonscarring) | |||
| Scaling | ||||
| Mouth | Lichen planus-like | Xerostomia | Gingivitis | |
| Mucoceles | Mucositis | |||
| Mucosal atrophy | Erythema | |||
| Pseudomembranes or ulcers* | Pain | |||
| Eyes | New dry, gritty, or painful eyes (sicca) | |||
| Keratoconjunctivitis sicca | ||||
| Punctate keratopathy | ||||
| Genitalia | Lichen planus-like | Erosions* | ||
| Lichen sclerosis-like | Fissures* | |||
| Female: | Ulcers* | |||
| Vagina scarring or stenosis | ||||
| Clitoral or labial agglutination | ||||
| Male: | ||||
| Phimosis | ||||
| Urethral scarring or stenosis | ||||
| GI tract | Esophageal web | Diarrhea | ||
| Esophageal stricture | Anorexia | |||
| Nausea or emesis | ||||
| Failure to thrive | ||||
| Weight loss | ||||
| Liver | Total bilirubin, alkaline phosphatase or ALT >2× ULN | |||
| Lung | Bronchiolitis obliterans diagnosed by biopsy BOS§ | Cryptogenic organizing pneumonia† | ||
| Restrictive lung disease† | ||||
| Muscles, fascia, joints | Fasciitis | Myositis | ||
| Joint stiffness or contractures due to sclerosis | Polymyositis | |||
| Hematopoietic and Immune | Thrombocytopenia | |||
| Eosinophilia | ||||
| Hypo- or hypergammaglobulinemia | ||||
| Autoantibodies | ||||
| Raynaud phenomenon | ||||
| Others | Effusions‡ | |||
| Nephrotic syndrome | ||||
| Myasthenia gravis | ||||
| Peripheral neuropathy |
| Organ or site . | Diagnostic (sufficient for diagnosis) . | Distinctive (insufficient alone for diagnosis) . | Other . | Features seen in both acute and chronic GVHD . |
|---|---|---|---|---|
| Skin | Poikiloderma | Depigmentation | Erythema | |
| Lichen planus-like | Papulosquamous | Maculopapular | ||
| Sclerosis | Pruritus | |||
| Morphea-like | ||||
| Lichen sclerosis-like | ||||
| Nails | Dystrophy | |||
| Onycholysis | ||||
| Nail loss | ||||
| Pterygium unguis | ||||
| Scalp and body hair | Alopecia (scarring or nonscarring) | |||
| Scaling | ||||
| Mouth | Lichen planus-like | Xerostomia | Gingivitis | |
| Mucoceles | Mucositis | |||
| Mucosal atrophy | Erythema | |||
| Pseudomembranes or ulcers* | Pain | |||
| Eyes | New dry, gritty, or painful eyes (sicca) | |||
| Keratoconjunctivitis sicca | ||||
| Punctate keratopathy | ||||
| Genitalia | Lichen planus-like | Erosions* | ||
| Lichen sclerosis-like | Fissures* | |||
| Female: | Ulcers* | |||
| Vagina scarring or stenosis | ||||
| Clitoral or labial agglutination | ||||
| Male: | ||||
| Phimosis | ||||
| Urethral scarring or stenosis | ||||
| GI tract | Esophageal web | Diarrhea | ||
| Esophageal stricture | Anorexia | |||
| Nausea or emesis | ||||
| Failure to thrive | ||||
| Weight loss | ||||
| Liver | Total bilirubin, alkaline phosphatase or ALT >2× ULN | |||
| Lung | Bronchiolitis obliterans diagnosed by biopsy BOS§ | Cryptogenic organizing pneumonia† | ||
| Restrictive lung disease† | ||||
| Muscles, fascia, joints | Fasciitis | Myositis | ||
| Joint stiffness or contractures due to sclerosis | Polymyositis | |||
| Hematopoietic and Immune | Thrombocytopenia | |||
| Eosinophilia | ||||
| Hypo- or hypergammaglobulinemia | ||||
| Autoantibodies | ||||
| Raynaud phenomenon | ||||
| Others | Effusions‡ | |||
| Nephrotic syndrome | ||||
| Myasthenia gravis | ||||
| Peripheral neuropathy |
Modified from Stem Cell Trialists’ Collaborative Group.6
ALT, alanine aminotransferase; BOS, bronchiolitis obliterans syndrome; GI, gastrointestinal; ULN, upper limit of normal.
In all cases infection, drug effect, malignancy, endocrine causes must be excluded as applicable.
These pulmonary manifestations are under investigation or unclassified.
Pericardium, pleural, or ascites.
BOS can be diagnostic for lung chronic GVHD only, if distinctive feature present in another site.
The frequency of involvement by chronic GVHD varies across organs and sites and is higher after HCT with mobilized blood cells as compared with marrow. (A) The most frequently involved organs and sites are the skin, mouth, eyes, gastrointestinal tract, and liver.3 (B) Chronic GVHD can affect all layers of the skin. Photographs of each manifestation in italic may be found in the supplemental Data, available on the Blood Web site. Artwork by Delilah Cohn, MFA, CMI, used with permission.
The frequency of involvement by chronic GVHD varies across organs and sites and is higher after HCT with mobilized blood cells as compared with marrow. (A) The most frequently involved organs and sites are the skin, mouth, eyes, gastrointestinal tract, and liver.3 (B) Chronic GVHD can affect all layers of the skin. Photographs of each manifestation in italic may be found in the supplemental Data, available on the Blood Web site. Artwork by Delilah Cohn, MFA, CMI, used with permission.
Systematic and comprehensive assessment of organs and sites possibly affected by chronic GVHD is essential for early diagnosis, early recognition of manifestations associated with high morbidity and disability, and assessment of disease progression and response during treatment (Tables 2-3). Once the clinical diagnosis of chronic GVHD is made, the extent and severity of each affected organ must be ascertained using the NIH chronic GVHD diagnosis and scoring consensus criteria.10 Recommended methods for conducting a chronic GVHD-focused evaluation have been published49 (they can be viewed at http://www.fhcrc.org/en/labs/clinical/projects/gvhd.html).50 Comprehensive evaluations should be done at the time of initial diagnosis, at 3- to 6-month intervals thereafter, and at any time when a major change in therapy is made. Evaluations should continue until at least 12 months after systemic treatment has ended.
Evaluation and frequency of monitoring according symptoms or affected organs
| Evaluation . | Frequency of evaluation/monitoring . | |
|---|---|---|
| Manifestations present . | Manifestations absent . | |
| Review of systems (see Table 3 for chronic GVHD-specific questions) | Every clinic visit | Every clinic visit |
| Physical examination | ||
| Complete skin examination (look, touch, pinch) | Every clinic visit | Every clinic visit |
| Oral examination | Every clinic visit | Every clinic visit |
| Range of motion assessment | Every clinic visit | Every clinic visit |
| Performance score | Every clinic visit | Every clinic visit |
| Nurse assessment | ||
| Weight | Every clinic visit | Every clinic visit |
| Height/adults | Yearly | Yearly |
| Height/children | Every 3-12 mo | Every 3-12 mo |
| Medical photographs | ∼100 d after HCT (baseline), at initial diagnosis of chronic GVHD, every 6 mo if skin or joints are involved and during treatment until at least 1 y after discontinuation of treatment | ∼100 d after HCT (baseline) |
| Other evaluations | ||
| PFTs | ∼100 d after HCT (baseline); see also Table 4 | ∼100 d after HCT and every 3 mo for the first year, then yearly if previous PFTs were abnormal or if continuing systemic treatment; reassess at onset of new symptoms |
| Nutritional assessment | As clinically indicated and yearly if receiving corticosteroids | As clinically indicated |
| Physiotherapy with assessment of range of motion | Every 3 mo if sclerotic features affecting range of motion until resolution | As clinically indicated |
| Dental or oral medicine consultation with comprehensive soft and hard tissue examination, culture, biopsy, or photographs of lesions, as clinically indicated | Every 3-6 mo or more often as indicated | Yearly |
| Ophthalmology consultation with Schirmer test, slit-lamp examination, and intraocular pressure | At initial diagnosis and every 3-6 mo or more often as indicated | ∼100 d after HCT (baseline) and yearly |
| Gynecology examination for vulvar or vaginal involvement | Every 6 mo or more often as indicated | Yearly |
| Dermatology consultation with assessment of extent and type of skin involvement, biopsy, or photographs | As clinically indicated | |
| Neuropsychological testing | As clinically indicated | |
| Bone mineral assessment (DEXA) scan | Yearly during corticosteroid treatment or if prior test was abnormal | ∼100 d after HCT if continuing corticosteroid treatment (baseline) |
| Evaluation . | Frequency of evaluation/monitoring . | |
|---|---|---|
| Manifestations present . | Manifestations absent . | |
| Review of systems (see Table 3 for chronic GVHD-specific questions) | Every clinic visit | Every clinic visit |
| Physical examination | ||
| Complete skin examination (look, touch, pinch) | Every clinic visit | Every clinic visit |
| Oral examination | Every clinic visit | Every clinic visit |
| Range of motion assessment | Every clinic visit | Every clinic visit |
| Performance score | Every clinic visit | Every clinic visit |
| Nurse assessment | ||
| Weight | Every clinic visit | Every clinic visit |
| Height/adults | Yearly | Yearly |
| Height/children | Every 3-12 mo | Every 3-12 mo |
| Medical photographs | ∼100 d after HCT (baseline), at initial diagnosis of chronic GVHD, every 6 mo if skin or joints are involved and during treatment until at least 1 y after discontinuation of treatment | ∼100 d after HCT (baseline) |
| Other evaluations | ||
| PFTs | ∼100 d after HCT (baseline); see also Table 4 | ∼100 d after HCT and every 3 mo for the first year, then yearly if previous PFTs were abnormal or if continuing systemic treatment; reassess at onset of new symptoms |
| Nutritional assessment | As clinically indicated and yearly if receiving corticosteroids | As clinically indicated |
| Physiotherapy with assessment of range of motion | Every 3 mo if sclerotic features affecting range of motion until resolution | As clinically indicated |
| Dental or oral medicine consultation with comprehensive soft and hard tissue examination, culture, biopsy, or photographs of lesions, as clinically indicated | Every 3-6 mo or more often as indicated | Yearly |
| Ophthalmology consultation with Schirmer test, slit-lamp examination, and intraocular pressure | At initial diagnosis and every 3-6 mo or more often as indicated | ∼100 d after HCT (baseline) and yearly |
| Gynecology examination for vulvar or vaginal involvement | Every 6 mo or more often as indicated | Yearly |
| Dermatology consultation with assessment of extent and type of skin involvement, biopsy, or photographs | As clinically indicated | |
| Neuropsychological testing | As clinically indicated | |
| Bone mineral assessment (DEXA) scan | Yearly during corticosteroid treatment or if prior test was abnormal | ∼100 d after HCT if continuing corticosteroid treatment (baseline) |
Modified from Flowers and Vogelsang.69
Chronic GVHD review of systems
| No. . | System/others . | Inquire/description . |
|---|---|---|
| 1 | Skin | Skin feels tight or hard, increased dryness, pruritus, or looks different (ie, new rash, papules, discoloration, shining scar-like, scaly)? |
| 2 | Sweat glands | Inability to sweat or to keep body warm? |
| 3 | Skin appendages | Loss of hair (scalp or body including bows or lashes), or nail changes (ridges or brittle, loss)? |
| 4 | Fasciae/joints | Stiffness or pain in the wrists, fingers, or other joints? |
| 5 | Eyes | Eye dryness, sensitivity to wind or dry environments (air conditioning), pain? |
| 6 | Mouth | Oral dryness, taste alterations, sensitivities (spicy/carbonate drinks, toothpaste), ulcers/sores, pain? |
| 7 | Esophagus | Foods or pills gets stuck upon swallowing? |
| 8 | Lungs | Cough, dyspnea (on exertion or rest) or wheezes? |
| 9 | Genital tract | Vaginal dryness, pain, dyspareunia (female); pain or dysuria due to stenosis of urethra (male)? |
| 10 | Weight loss | Unexplained weight loss or inability to gain weight (pancreatic insufficiency or hypercatabolism)? |
| No. . | System/others . | Inquire/description . |
|---|---|---|
| 1 | Skin | Skin feels tight or hard, increased dryness, pruritus, or looks different (ie, new rash, papules, discoloration, shining scar-like, scaly)? |
| 2 | Sweat glands | Inability to sweat or to keep body warm? |
| 3 | Skin appendages | Loss of hair (scalp or body including bows or lashes), or nail changes (ridges or brittle, loss)? |
| 4 | Fasciae/joints | Stiffness or pain in the wrists, fingers, or other joints? |
| 5 | Eyes | Eye dryness, sensitivity to wind or dry environments (air conditioning), pain? |
| 6 | Mouth | Oral dryness, taste alterations, sensitivities (spicy/carbonate drinks, toothpaste), ulcers/sores, pain? |
| 7 | Esophagus | Foods or pills gets stuck upon swallowing? |
| 8 | Lungs | Cough, dyspnea (on exertion or rest) or wheezes? |
| 9 | Genital tract | Vaginal dryness, pain, dyspareunia (female); pain or dysuria due to stenosis of urethra (male)? |
| 10 | Weight loss | Unexplained weight loss or inability to gain weight (pancreatic insufficiency or hypercatabolism)? |
Close serial monitoring of all organ systems is essential in order to ensure early detection, recognition, and intervention directed toward reversing or preventing progression of chronic GVHD manifestations and treatment-associated toxicities. In particular, periodic pulmonary function tests are essential for early detection of lung involvement manifested as BOS because this complication has an insidious onset, and patients may remain asymptomatic until considerable lung function has been lost. We recommend complete pulmonary function testing in all patients before HCT and at ∼3 months after HCT as a baseline for future comparisons (Table 4).10 Follow-up testing should be done at the onset of chronic GVHD and at 3- to 6-month intervals for the first year or more often if testing shows any significant new airflow obstruction or decline in the percent of predicted FEV1. Patient and physician-directed tools to support chronic GVHD monitoring can be found online (http://www.fhcrc.org/en/treatment/long-term-follow-up/information-for-physicians.html).
General guidelines for monitoring and management of new airflow obstruction
| Guidelines . |
|---|
| A. Significant new airflow obstruction with a % predicted FEV1 ≥70% |
| 1. Initiate inhaled corticosteroid therapy |
| • Fluticasone (Flovent) 440 mcg BID, or |
| • Advair 500/50 mcg BID (if symptoms of airway obstruction are present) |
| • Treatment should continue until either % FEV1 becomes <70% (see below), or until GVHD resolves (ie, resolution of all reversible manifestations of GVHD without exacerbation after at least 6 mo after discontinuation of all systemic immunosuppressive treatment) |
| 2. Other immunosuppressive treatment as indicated to control GVHD in other organs |
| • Treatment should continue until either % FEV1 becomes <70% (see below), or until GVHD resolves (ie, resolution of all reversible manifestation of GVHD without exacerbation after at least 12 mo after discontinuation of all systemic treatments) |
| 3. Monitor PFTs or spirometry monthly for at least 3 mo |
| • If % FEV1 stabilizes, PFTs or spirometry every 3 mo for 1 y, then if stable continue at 6-mo intervals for 1 y and at 6-12 mo intervals thereafter |
| • If % FEV1 continues to decrease, go to B below |
| B. Significant airflow obstruction with a % FEV1 <70% with/without significant air-trapping by high resolution chest CT |
| 1. Consider bronchoscopy to rule out an undetected infectious etiology for airflow obstruction, even if no infiltrate is apparent |
| 2. After infection has been ruled out, evaluate the patient eligibility for clinical trial for treatment of BOS and initiate (or increase) prednisone dose to 1 mg/kg/d |
| • Start standard chronic GVHD taper at 2 wk (Table 5) |
| • Consider continuing inhaled corticosteroids throughout prednisone therapy |
| 3. If % FEV1 decreases further to <70% during treatment, discuss changes of immunosuppressive treatment with transplant physician |
| 4. CMV monitoring in blood per standard practice |
| 5. Monitor PFTs or spirometry monthly for at least 3 mo |
| • If % FEV1 stabilizes, continue PFTs or spirometry every 3 mo for 1 y |
| • If % FEV1 continues to decrease, go to C below |
| C. Corticosteroid-resistant airflow obstruction defined as progressive decline of FEV1 by ≥10% despite treatment with 1 mg/kg/d of prednisone (or similar corticosteroids) |
| 1. May consider increasing the dose of prednisone to 2 mg/kg/d for a maximum of 2 wk, followed by a taper to reach a dose of 1 mg/kg/d by 2-4 wk |
| 2. Another treatment must be considered and discussed with the transplant team |
| 3. Monitor CMV in blood per standard practice |
| 4. Monitor PFTs monthly for at least 3 mo |
| • If % FEV1 stabilizes, monitor PFTs every 3 mo for 1 y |
| D. Additional considerations |
| 1. Consider changing prophylaxis for encapsulated bacterial infection to azithromycin 250 mg on Mondays-Wednesdays-Fridays |
| • Assure patient is receiving adequate prophylaxis for Pneumocystis, varicella virus, and herpes simplex virus infections |
| • Fungal prophylaxis per standard practice |
| 2. Monitor CMV in blood per standard practice |
| 3. May continue inhaled corticosteroids throughout prednisone therapy |
| 4. Discontinuation of inhaled corticosteroid treatment can be considered 12 mo after treatment with prednisone has been discontinued |
| Guidelines . |
|---|
| A. Significant new airflow obstruction with a % predicted FEV1 ≥70% |
| 1. Initiate inhaled corticosteroid therapy |
| • Fluticasone (Flovent) 440 mcg BID, or |
| • Advair 500/50 mcg BID (if symptoms of airway obstruction are present) |
| • Treatment should continue until either % FEV1 becomes <70% (see below), or until GVHD resolves (ie, resolution of all reversible manifestations of GVHD without exacerbation after at least 6 mo after discontinuation of all systemic immunosuppressive treatment) |
| 2. Other immunosuppressive treatment as indicated to control GVHD in other organs |
| • Treatment should continue until either % FEV1 becomes <70% (see below), or until GVHD resolves (ie, resolution of all reversible manifestation of GVHD without exacerbation after at least 12 mo after discontinuation of all systemic treatments) |
| 3. Monitor PFTs or spirometry monthly for at least 3 mo |
| • If % FEV1 stabilizes, PFTs or spirometry every 3 mo for 1 y, then if stable continue at 6-mo intervals for 1 y and at 6-12 mo intervals thereafter |
| • If % FEV1 continues to decrease, go to B below |
| B. Significant airflow obstruction with a % FEV1 <70% with/without significant air-trapping by high resolution chest CT |
| 1. Consider bronchoscopy to rule out an undetected infectious etiology for airflow obstruction, even if no infiltrate is apparent |
| 2. After infection has been ruled out, evaluate the patient eligibility for clinical trial for treatment of BOS and initiate (or increase) prednisone dose to 1 mg/kg/d |
| • Start standard chronic GVHD taper at 2 wk (Table 5) |
| • Consider continuing inhaled corticosteroids throughout prednisone therapy |
| 3. If % FEV1 decreases further to <70% during treatment, discuss changes of immunosuppressive treatment with transplant physician |
| 4. CMV monitoring in blood per standard practice |
| 5. Monitor PFTs or spirometry monthly for at least 3 mo |
| • If % FEV1 stabilizes, continue PFTs or spirometry every 3 mo for 1 y |
| • If % FEV1 continues to decrease, go to C below |
| C. Corticosteroid-resistant airflow obstruction defined as progressive decline of FEV1 by ≥10% despite treatment with 1 mg/kg/d of prednisone (or similar corticosteroids) |
| 1. May consider increasing the dose of prednisone to 2 mg/kg/d for a maximum of 2 wk, followed by a taper to reach a dose of 1 mg/kg/d by 2-4 wk |
| 2. Another treatment must be considered and discussed with the transplant team |
| 3. Monitor CMV in blood per standard practice |
| 4. Monitor PFTs monthly for at least 3 mo |
| • If % FEV1 stabilizes, monitor PFTs every 3 mo for 1 y |
| D. Additional considerations |
| 1. Consider changing prophylaxis for encapsulated bacterial infection to azithromycin 250 mg on Mondays-Wednesdays-Fridays |
| • Assure patient is receiving adequate prophylaxis for Pneumocystis, varicella virus, and herpes simplex virus infections |
| • Fungal prophylaxis per standard practice |
| 2. Monitor CMV in blood per standard practice |
| 3. May continue inhaled corticosteroids throughout prednisone therapy |
| 4. Discontinuation of inhaled corticosteroid treatment can be considered 12 mo after treatment with prednisone has been discontinued |
Before considering treatment, all potential infectious etiologies of airflow obstruction must be investigated and treated if present. Investigations that should be considered (directed by clinical symptoms), include sinus CT scan, nasal washes, sinus aspiration, high-resolution chest CT scan, sputum culture, bronchoalveolar lavage, and lung biopsy.
BID, twice daily; CT, computed tomography scan.
Treatment
Treatment of chronic GVHD is intended to produce a sustained benefit by reducing symptom burden, controlling objective manifestations of disease activity, and preventing damage and disability, without causing disproportionate toxicity or harms related to the treatments themselves. The long-term goal of GVHD treatment is the development of immunologic tolerance, indicated by successful withdrawal of all immunosuppressive treatment without recurrence or clinically significant exacerbation of disease manifestations. The current therapeutic approach functions primarily to prevent immune-mediated damage, while awaiting the development of tolerance. Evidence to suggest that current treatments accelerate the development of immunologic tolerance is lacking. Optimal treatment of chronic GVHD requires a multidisciplinary team approach that includes transplantation specialists, a primary health care provider, organ-specific consultants, nurses, and ancillary services such as social services, vocational specialists, patient and family support groups, and systems.
Systemic therapy for at least 1 year is generally indicated for patients who meet criteria for moderate-to-severe disease according to the NIH consensus criteria: involvement of 3 or more organs, moderate or severe organ involvement in any organ, or any lung involvement.10,51 Systemic treatment is also generally indicated for patients with less severe disease if high-risk features such as thrombocytopenia, hyperbilirubinemia, or onset during corticosteroid treatment are present.2,10,52-54 Symptomatic mild chronic GVHD is often treated with topical therapies alone.13,52 Topical agents may also be used as adjuncts to systemic therapy to improve and accelerate local response. Comprehensive reviews of topical therapies have been published previously.2,13,52 Considerations affecting the choice of treatment include the affected organs or sites, the severity of disease manifestations, the presence of health problems that might be exacerbated by the treatment, possible drug interactions, the intensity of the monitoring needed, and factors that affect access to the treatment such as travel, distance, and cost.
Primary systemic treatment
Management of chronic GVHD has relied on corticosteroids as the mainstay of treatment of >3 decades. Systemic treatment typically begins with prednisone at 0.5 to 1 mg/kg per day, followed by a taper to reach an alternate-day regimen, with or without cyclosporine or tacrolimus. The efficacy of alternate-day vs daily administration of corticosteroids has been reported in pediatric renal transplantation but has not been tested in HCT.55 Prolonged systemic corticosteroid treatment causes significant toxicity, including weight gain, bone loss, myopathy, diabetes, hypertension, mood swings, cataract formation, and increased risk of infection. Many of these toxicities can be mitigated by alternate-day administration of corticosteroids. Alternate-day administration also has an important role in facilitating adrenal recovery long before the end of treatment. In a recent prospective study, the average dose of prednisone was tapered to 0.20 to 0.25 mg/kg per day or 0.4 to 0.5 mg/kg every other day within 3 months after starting treatment.56 Medications used for treatment of chronic GVHD should be withdrawn gradually one at a time after the disease has resolved. As a general principle, withdrawal of systemic treatment should begin with the medication that is most likely to cause long-term toxicity. Withdrawal of prednisone should generally precede withdrawal of a calcineurin inhibitor, unless continued treatment with the calcineurin inhibitor threatens to cause intolerable or irreversible toxicity.
Strategies for tapering the dose of prednisone vary considerably, but as a general principle, efforts should be made to use the minimum dose that is sufficient to control GVHD manifestations (Figure 2). In practice, this means that prednisone doses should be decreased progressively in patients who have had a complete response or a very-good-partial response, and tapering should continue until manifestations begin to recur or show evidence of exacerbation. A prototypic taper schedule (Table 5) is designed to approximate a 20% to 30% dose reduction every 2 weeks, with smaller absolute decrements toward the end of the taper schedule. Toxicity associated with the administration of prednisone may require dose adjustments.
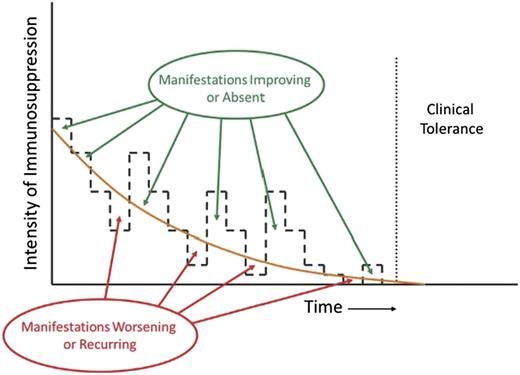
Appropriate management of chronic GVHD requires continuous recalibration of immunosuppressive treatment in order to avoid overtreatment or undertreatment. The intensity of treatment required to control the disease decreases across time. Manifestations of chronic GVHD improve or are absent when the intensity of treatment is above the threshold shown as the orange curve, and they worsen or recur when the intensity of treatment is below the threshold. The slope of the threshold varies among patients and can be determined only by serial attempts to decrease the intensity of treatment. Clinical tolerance is defined by the ability to withdraw all systemic treatment without recurrence of chronic GVHD.
Appropriate management of chronic GVHD requires continuous recalibration of immunosuppressive treatment in order to avoid overtreatment or undertreatment. The intensity of treatment required to control the disease decreases across time. Manifestations of chronic GVHD improve or are absent when the intensity of treatment is above the threshold shown as the orange curve, and they worsen or recur when the intensity of treatment is below the threshold. The slope of the threshold varies among patients and can be determined only by serial attempts to decrease the intensity of treatment. Clinical tolerance is defined by the ability to withdraw all systemic treatment without recurrence of chronic GVHD.
Prednisone taper schedule
| Week . | Dose, mg/kg body weight . |
|---|---|
| 0 | 1.0 |
| 2 | 1.0/0.5* (to begin within 2 wk after objective improvement) |
| 4 | 1.0/0.25* |
| 6 | 1.0 qod (continued until resolution of all clinical manifestations) |
| 8 | 0.70 qod (to begin after resolution of all clinical manifestations) |
| 10 | 0.55 qod |
| 12 | 0.45 qod |
| 14 | 0.35 qod |
| 16 | 0.25 qod |
| 18 | 0.20 qod |
| 20 | 0.15 qod |
| 22 | 0.10 qod |
| Week . | Dose, mg/kg body weight . |
|---|---|
| 0 | 1.0 |
| 2 | 1.0/0.5* (to begin within 2 wk after objective improvement) |
| 4 | 1.0/0.25* |
| 6 | 1.0 qod (continued until resolution of all clinical manifestations) |
| 8 | 0.70 qod (to begin after resolution of all clinical manifestations) |
| 10 | 0.55 qod |
| 12 | 0.45 qod |
| 14 | 0.35 qod |
| 16 | 0.25 qod |
| 18 | 0.20 qod |
| 20 | 0.15 qod |
| 22 | 0.10 qod |
qod, every other day.
Alternate-day administration.
A physician or advanced practitioner should examine the patient before each reduction of the prednisone dose. If exacerbation or recurrence of chronic GVHD is evident at any step of the taper, the dose of prednisone should be increased promptly by 2 levels, with daily administration for 2 to 4 weeks, followed by resumption of alternate-day administration. Treatment should then be continued for at least 3 months before attempting to resume the taper. Cycles of attempted tapering and dose escalation should be repeated as needed until the dose reaches 0.10 mg/kg per day, which equates to adrenal replacement therapy. Administration of prednisone may be discontinued after a minimum of at least 4 weeks of treatment at a dose of 0.10 mg/kg every other day. Some patients have recurrent symptoms with doses at or below 0.10 mg/kg every other day, and in this situation, treatment with very low prednisone doses may be required for a year or more.
Combination therapy with other immunosuppressive agents is often considered in hopes of minimizing toxicity caused by prolonged corticosteroid treatment.54 Randomized trials, however, showed no benefit from adding azathioprine,57 thalidomide,58 mycophenolate mofetil,56 or hydroxychloroquine59 to initial treatment of chronic GVHD. A trial comparing cyclosporine plus prednisone vs prednisone alone showed no statistically significant differences in survival or the duration of treatment.60 The incidence of avascular necrosis was lower in the cyclosporine plus prednisone arm, suggesting that cyclosporine could have had a steroid-sparing effect, but steroid doses across time were not measured in this study. Results are pending from a recent randomized, multicenter phase 2 to 3 clinical trial comparing prednisone and sirolimus with or without a calcineurin inhibitor for initial treatment of chronic GVHD. Premature closure of this trial at the end of phase 2 suggests that the expected benefit of omitting the calcineurin inhibitor was not observed. Wherever possible, clinical trials should be considered as the first option for initial systemic treatment of chronic GVHD.
Secondary systemic treatment
Approximately 50% to 60% of patients with chronic GVHD require secondary treatment within 2 years after initial systemic treatment.61,62 Indications for secondary treatment include worsening manifestations of chronic GVHD in a previously affected organ, development of signs and symptoms of chronic GVHD in a previously unaffected organ, absence of improvement after 1 month of standard primary treatment, inability to decrease prednisone below 1 mg/kg per day within 2 months, or significant treatment-related toxicity. Numerous clinical trials have been carried out to evaluate approaches for secondary treatment of chronic GVHD. Reports from retrospective and prospective studies often indicate high response rates, but results are difficult to interpret because of deficiencies in study design.54
No consensus has been reached regarding the optimal choice of agents for secondary treatment of chronic GVHD, and the published literature provides little useful guidance. Therefore, clinical management requires an empirical approach, as illustrated in “Case summary.” Treatment choices are based on physician experience, ease of use, need for monitoring, risk of toxicity, and potential exacerbation of preexisting comorbidity.63,64 Options for secondary treatment have been recently reviewed and are summarized in Table 6.36
Agents used for secondary treatment of chronic GVHD*
| Treatment . | % Overall response* . | Survival . |
|---|---|---|
| ECP | 65-70 | 70%-78% at 1 y |
| Rituximab | 66-86 | 72% at 1 y |
| Imatinib | 22-79 | 75%-84% at 1.5 y |
| Pentostatin | 53-56 | 34%-60% at 1-3 y |
| Mesenchymal stem cells | 50-74 | 78% at 2 y |
| Mycophenolate mofetil | 26-64 | 67%-96% at 1 y |
| mTOR inhibitor | 76 | 72% at 3 y |
| Interleukin-2 | 52 | Not reported |
| Other therapies summarized in other reviews** | ||
| Calcineurin inhibitor | ||
| High-dose methylprednisolone | ||
| Methotrexate | ||
| Thalidomide | ||
| Hydroxychloroquine | ||
| Clofazimine | ||
| Thoracoabdominal irradiation | ||
| Alefacept | ||
| Infliximab | ||
| Etanercept70 | ||
| Treatment . | % Overall response* . | Survival . |
|---|---|---|
| ECP | 65-70 | 70%-78% at 1 y |
| Rituximab | 66-86 | 72% at 1 y |
| Imatinib | 22-79 | 75%-84% at 1.5 y |
| Pentostatin | 53-56 | 34%-60% at 1-3 y |
| Mesenchymal stem cells | 50-74 | 78% at 2 y |
| Mycophenolate mofetil | 26-64 | 67%-96% at 1 y |
| mTOR inhibitor | 76 | 72% at 3 y |
| Interleukin-2 | 52 | Not reported |
| Other therapies summarized in other reviews** | ||
| Calcineurin inhibitor | ||
| High-dose methylprednisolone | ||
| Methotrexate | ||
| Thalidomide | ||
| Hydroxychloroquine | ||
| Clofazimine | ||
| Thoracoabdominal irradiation | ||
| Alefacept | ||
| Infliximab | ||
| Etanercept70 | ||
Ancillary and supportive care
Ancillary and supportive care therapies are commonly used in addition to systemic treatment of GVHD, although in some cases, their use may circumvent the need for systemic treatment or allow doses of systemic agents to be reduced. A detailed list of site-specific therapies has been reported elsewhere.2,13 Specific dispensary information for topical therapies is available online (http://asbmt.affiniscape.com/associations/11741/files/DispensaryGuidelines.pdf).
Symptoms caused by ocular sicca can be relieved by the frequent application of artificial lubricant tears or by plugging or ligation of the tear ducts. Symptoms can be relieved by using specialized moisture-chamber eyewear available from several vendors. Permanent punctal ligation is usually necessary in more severe cases of ocular sicca. Many patients with severe sicca keratitis have reported significant relief of symptoms with prosthetic replacement of the ocular surface ecosystem (PROSE), which refers to a gas-permeable scleral lens.65 Involvement of an ophthalmologist with expertise in the management of dry eye and corneal and conjunctival disease is strongly recommended for patients with ocular manifestations of chronic GVHD.
Oral cavity erythema, ulceration, and gingivitis are often treated with topical steroid rinses or ointments. Vaginal GVHD may respond to topical steroids and dilator therapy, but management should also address any coexisting estrogen deficiency or coexisting yeast or bacterial infection. Sialogogue therapy (to increase the flow rate of saliva) with agents such as cevimeline or pilocarpine may improve symptoms of oral,66 ocular, and vaginal dryness.
Consistent weight-bearing exercise for 30 minutes daily at least 5 days per week and daily stretching are particularly important for preserving bone health, muscle strength, and mobility. Physical therapy to maintain strength and joint mobility can prevent the development of disability during immunosuppressive treatment of chronic GVHD. Deep tissue massage is a helpful adjunct to preserve or improve range of motion in patients with fasciitis or scleroderma.
Close attention must be paid to complications of glucocorticoid treatment through management of hyperglycemia, hypertension, and bone loss. A balanced, healthy diet low in sodium and free sugars and high in calcium with adequate fluid intake is essential. Calcium (1500 mg per day) and vitamin D (1000 IU per day) intake between food and supplements is recommended to retard the development of osteoporosis during glucocorticoid treatment. Clinical trials have not yet been carried out to determine whether bisphosphonates are effective for prevention of glucocorticoid-induced osteoporosis, but some experts recommend the use of these agents for patients with osteopenia.
Both the disease and its treatment with immunosuppressive agents increase the risk of infection in patients with chronic GVHD.2,51 Antibiotic prophylaxis for Pneumocystis pneumonia and encapsulated bacterial infections should be given until 6 months after discontinuation of all systemic treatment. IV administration of γ globulin may help prevent infection in patients who have serum immunoglobulin G (IgG) concentrations <400 mg/dL or IgG2 or IgG4 subclass deficiencies. Cytomegalovirus (CMV) infection poses risks of CMV disease in patients with a history of viral reactivation and in those with low CD4 counts or cord blood donors. All patients with chronic GVHD who are at risk of CMV infection should have regular blood tests for surveillance of viral reactivation. Preemptive antiviral therapy should be instituted whenever surveillance tests show viral reactivation, before the onset of overt CMV disease. CMV-seronegative recipients with CMV-seronegative donors should receive screened or filtered leukocyte-depleted blood products. In addition, long-term administration of valacyclovir or acyclovir is recommended to prevent reactivation of varicella-zoster virus in patients previously infected with this virus.
Prognosis and outcomes
Duration of treatment
Approximately 50% of patients are cured within 7 years after starting systemic treatment, as indicated by resolution of disease manifestations and permanent withdrawal of systemic treatment. Approximately 10% require continued systemic treatment of an indefinite period beyond 7 years, and the remaining 40% have recurrent malignancy or die within 7 years during treatment of chronic GVHD.30
Growth factor–mobilized apheresis products have replaced marrow as the most frequent source of cells for allogeneic HCT with both related and unrelated donors. The use of mobilized blood cells has been associated with 3 detrimental outcomes with respect to chronic GVHD: a higher incidence, a higher risk of fasciitis and development of fibrotic manifestations affecting the skin and joints, and a longer time to resolution of the disease, development of immunologic tolerance, and withdrawal of systemic treatment.48 The median duration of systemic treatment of chronic GVHD is ∼2 years in patients who had HCT with marrow cells and ∼3.5 years in those who had HCT with mobilized blood cells.
Graft-versus-leukemia associated with chronic GVHD
Chronic GVHD is associated with a reduced risk of recurrent malignancy after hematopoietic cell transplantation, raising the question of whether the intensity of immunosuppression should be attenuated when patients at high risk of recurrent malignancy develop chronic GVHD. This question has not been addressed directly in clinical trials, but several observations are pertinent. First, chronic GVHD increases the risk of nonrelapse mortality, thereby offsetting any benefit gained through the effects on malignant cells in the recipient. The tradeoff between risks of nonrelapse mortality and recurrent malignancy is balanced, such that mortality rates are not affected by the presence or absence of chronic GVHD.67,68 Second, a single-institution study showed that withdrawal of immunosuppression decreased the subsequent risk of recurrent malignancy in patients without prior GVHD but not in those with prior GVHD.67
These results suggest that attenuation of immunosuppression in patients with active manifestations of chronic GVHD might likewise not decrease the risk of recurrent malignancy. Nonetheless, unnecessary immunosuppressive treatment could increase the risk of recurrent malignancy, as suggested by trends in a prospective study evaluating the use of mycophenolate mofetil added to first-line treatment of chronic GVHD.56 Therefore, the intensity of treatment should be calibrated periodically by lowering the dose of immunosuppressive medications to levels that allow disease manifestations to begin emerging before increasing the dose, as described in Figure 2.
Future perspectives
Participation in a clinical trial represents the first option to consider for eligible patients with chronic GVHD. Novel strategies directed toward depleting or modulating B cells, expanding T or B regulatory cells, and targeting the processes implicated in fibrosis are under active investigation and could lead to future advances in treatment of chronic GVHD. Progress toward decreasing the impact of chronic GVHD after HCT will be made not only through improved treatment but also through development of prevention strategies that do not impair the immunological activity of donor cells against malignant cells in the recipient. In the absence of specific interventions to decrease the risk of chronic GVHD, marrow should be preferred over mobilized blood as a source of stem cells for HCT with myeloablative conditioning regimens.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Anne Thompson for assistance with preparing the manuscript and Kevin Bray for assistance with hyperlinks.
This work was supported in part by grant CA18029 from the National Cancer Institute at the National Institutes of Health.
Authorship
Contribution: M.E.D.F. and P.J.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary E. D. Flowers, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-290, PO Box 19024, Seattle, WA 98109-1024; e-mail: mflowers@fredhutch.org.