Key Points
CLEC-2 can be downregulated from circulating platelets by anti–CLEC-2 antibodies through Src-family kinase-dependent internalization.
Platelet-specific Syk deficiency abrogates anti–CLEC-2 antibodies-induced thrombocytopenia, but not CLEC-2 internalization.
Abstract
Platelet aggregation at sites of vascular injury is not only essential for hemostasis, but may also cause acute ischemic disease states such as myocardial infarction or stroke. The hemi-immunoreceptor tyrosine-based activation motif–containing C-type lectinlike receptor 2 (CLEC-2) mediates powerful platelet activation through a Src- and spleen tyrosine kinase (Syk)–dependent tyrosine phosphorylation cascade. Thereby, CLEC-2 not only contributes to thrombus formation and stabilization but also plays a central role in blood-lymphatic vessel development, tumor metastasis, and prevention of inflammatory bleeding, making it a potential pharmacologic target to modulate these processes. We have previously shown that injection of the anti–CLEC-2 antibody, INU1, results in virtually complete immunodepletion of platelet CLEC-2 in mice, which is, however, preceded by a severe transient thrombocytopenia thereby limiting its potential therapeutic use. The mechanisms underlying this targeted CLEC-2 downregulation have remained elusive. Here, we show that INU1-induced CLEC-2 immunodepletion occurs through Src-family kinase–dependent receptor internalization in vitro and in vivo, presumably followed by intracellular degradation. In mice with platelet-specific Syk deficiency, INU1-induced CLEC-2 internalization/degradation was fully preserved whereas the associated thrombocytopenia was largely prevented. These results show for the first time that CLEC-2 can be downregulated from the platelet surface through internalization in vitro and in vivo and that this can be mechanistically uncoupled from the associated antibody-induced thrombocytopenia.
Introduction
Platelet activation at sites of vascular injury is not only crucial to limiting posttraumatic blood loss, but also causes myocardial infarction and stroke.1-3 Mainly 2 major classes of receptors induce platelet activation, characterized by shape change, upregulation of integrin adhesion receptor activity, release of granule content, and enhanced procoagulant activity. Soluble agonists, such as thrombin, adenosine 5′-diphosphate, and thromboxane A2, stimulate receptors that couple to heterotrimeric G proteins and activate downstream effectors.2 The other pathway is triggered by the major activatory platelet collagen receptor, glycoprotein VI (GPVI), which signals via the immunoreceptor tyrosine-based activation motif (ITAM)–bearing Fc receptor (FcR) γ-chain, or by the C-type lectinlike receptor-2 (CLEC-2), where signaling is initiated by tyrosine phosphorylation of a single YxxL sequence, called hemi-ITAM (hemITAM), in its cytoplasmic tail.4
CLEC-2 is a ∼32-kDa type II transmembrane protein, encoded by the Clec1b gene,5 that was originally identified as a transcript in immune cells and later found to be highly expressed in platelets where it serves as the receptor for the powerful platelet-activating snake venom protein rhodocytin (RC).6 Upon ligand engagement of CLEC-2, hemITAM phosphorylation of the receptor is mediated by Src-family kinases (SFKs) and spleen tyrosine kinase (Syk), which is essential for signaling and downstream phosphorylation of effector proteins, including phospholipase Cγ2.6,7
CLEC-2 is a unique platelet receptor, which is critical for developmental processes, most notably for maintaining the separation of blood and lymph vessels8-11 and the formation of lymph nodes.12 Beyond development, it is required for the maintenance of high endothelial venule barrier integrity.13 These functions depend on the interaction of CLEC-2 with its major physiological ligand, podoplanin, a transmembrane glycoprotein widely expressed outside the blood vascular system, most notably on lymphatic endothelial cells, lymph node stromal cells, and some immune cells during inflammation. In addition, platelets can be activated by podoplanin-expressing tumor cells and this has been shown to critically contribute to hematogenous metastasis.14-16
On the other hand, studies in mice have shown that the lack of platelet CLEC-2 affects thrombus stability in vitro and in vivo and protects mice from occlusive arterial thrombus formation while only moderately increasing tail bleeding times,9,17,18 thereby establishing the receptor as a potential target for antithrombotic therapy.17-19 Interestingly, however, CLEC-2 appears to share functional redundancy with GPVI, as mice deficient in both receptors display virtually abolished arterial thrombus formation and a pronounced bleeding defect.18 Furthermore, recent evidence suggests that CLEC-2/GPVI-dependent signaling is of particular significance for the maintenance of vascular integrity under conditions of inflammation.20 Despite its central function in multiple physiological and pathophysiological processes, not much is known about the cellular regulation of CLEC-2 in platelets. This may, however, be of major importance for the development of pharmaceuticals that modulate CLEC-2 function under diseased conditions.
We have previously demonstrated that CLEC-2 can be targeted and specifically depleted from platelets and/or megakaryocytes (MKs) in mice by in vivo administration of the monoclonal antibody, INU1.17 Importantly, however, INU1 injection caused a severe transient thrombocytopenia, with the appearance of newly produced CLEC-2–deficient platelets on day 2 to 3 after injection. It is currently unclear by which mechanism antibody-induced downregulation of the receptor occurs in vivo and whether signaling downstream of the receptor is involved in this process. Similarly, it is unknown whether CLEC-2 is removed from circulating platelets or only downregulated in MKs, thus resulting in the release of CLEC-2–depleted platelets.
Here, we show that INU1-induced CLEC-2 downregulation in vivo occurs through internalization of the receptor in circulating platelets and that this process, as well as the associated thrombocytopenia, can be blocked by inhibition of SFK activity. Remarkably, INU1-induced thrombocytopenia is also strongly attenuated in mice with a platelet-specific Syk deficiency, whereas CLEC-2 downregulation is fully preserved.
Methods
Mice
Male NMRi and C57BL/6JRj mice maintained under specific-pathogen-free conditions were obtained at an age of 6 to 9 weeks (Janvier Labs). Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken). The generation of the Sykfl/fl mouse is described in the supplemental Methods and supplemental Figure 1 (see supplemental Data available on the Blood Web site) and the mice were intercrossed with mice carrying the Cre-recombinase under control of the platelet factor 4 (Pf4) promoter.21
Platelet preparation, western blotting, flow cytometry, immunofluorescence staining, and differentiation of fetal liver cell–derived MKs
CLEC-2 internalization in vivo
Mice were injected with fluorescence-tagged INU1 (INU1-Alexa-F488, referred to as INU1-F488). After 15 minutes, platelets were isolated and allowed to adhere to poly-l-lysine (PLL)–coated cover slips for 30 minutes and further processed as described in the previous section.
Chemicals and data analysis
A list of antibodies and reagents and a statistical data analysis are provided in the supplemental Methods.
Results
INU1-induced thrombocytopenia occurs independently of activatory FcγRs and platelet aggregation
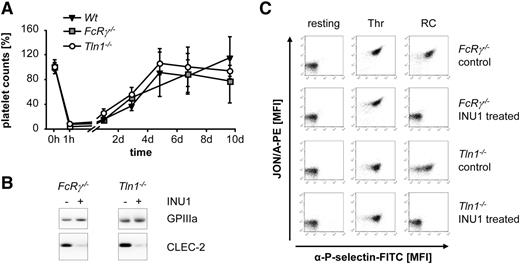
To study the mechanisms underlying targeted CLEC-2 downregulation in vivo, wild-type (Wt) mice were IV injected with 100 µg of the anti-mouse CLEC-2 antibody, INU1 (rat immunoglobulin G1κ [IgG1κ]). Free INU1 was detectable in the plasma by enzyme-linked immunosorbent assay until day 5 postinjection (data not shown). Immunofluorescence staining of whole femora cryosections revealed a robust α-rat IgG-Cy3 signal on MKs (counterstained with anti-GPIb antibodies) that was maintained for 5 days, showing that INU1 efficiently binds to MKs in vivo (supplemental Figure 2). In parallel, circulating platelets were studied ex vivo at different time points after injection (Figure 1). As described previously,17 this treatment caused a severe transient thrombocytopenia with platelet counts dropping below 10% of control within the first hour, recovering back to normal levels on days 3 to 4 postinjection (Figure 1A). Newly generated platelets lacked CLEC-2 for up to day 6, whereas the expression of other major glycoprotein receptors in these platelets was unaltered compared with control (data not shown and May et al17 ). Western blot analysis confirmed the complete loss of platelet CLEC-2 on day 5 after INU1 injection and platelets were refractory toward the CLEC-2 agonist RC, whereas responses to other agonists, such as thrombin, were unaltered compared with control (Figure 1B-C and data not shown) confirming previous results.17 To test whether the INU1-induced thrombocytopenia depends on platelet CLEC-2, we treated mice with a MK/platelet-specific CLEC-2 deficiency (Clec1bfl/fl Pf4-cre,10 further referred to as Clec-2−/−) with 100 µg of the antibody. As expected, this treatment did not cause thrombocytopenia (Figure 1A).
INU1-induced thrombocytopenia depends on platelet CLEC-2 expression. (A) Wt and Clec2−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (B) Western blot analysis of CLEC-2 levels, before and on day 5 post-INU1 injection in platelet lysates of Wt and Clec2−/− mice. GPIIIa served as a loading control. (C) Flow cytometric analysis of αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure on platelets on day 5 post-INU1 injection. Washed blood was incubated with the indicated agonist for 15 minutes and analyzed on a FACSCalibur (RC, 0.12 µg/mL; thrombin (Thr), 0.1 U/mL). Results are representative of 3 individual experiments. FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; PE, phycoerythrin; SD, standard deviation.
INU1-induced thrombocytopenia depends on platelet CLEC-2 expression. (A) Wt and Clec2−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (B) Western blot analysis of CLEC-2 levels, before and on day 5 post-INU1 injection in platelet lysates of Wt and Clec2−/− mice. GPIIIa served as a loading control. (C) Flow cytometric analysis of αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure on platelets on day 5 post-INU1 injection. Washed blood was incubated with the indicated agonist for 15 minutes and analyzed on a FACSCalibur (RC, 0.12 µg/mL; thrombin (Thr), 0.1 U/mL). Results are representative of 3 individual experiments. FITC, fluorescein isothiocyanate; MFI, mean fluorescence intensity; PE, phycoerythrin; SD, standard deviation.
One possible explanation for the rapid and severe thrombocytopenia in Wt mice upon INU1 injection might be Fcγ receptor (FcγR)–dependent clearance of the antibody-opsonized platelets. To address this directly, we used FcR γ-chain–deficient mice (Fcer1g−/−,22 further referred to as FcRγ−/−), which lack the activatory FcγRs (FcRγI, III, and IV) and thus their macrophages are unable to phagocytose antibody-opsonized particles.22,26 Remarkably, IV injection of 100 µg of INU1 in FcRγ−/− mice caused a profound thrombocytopenia and a complete loss of the CLEC-2 protein in newly generated platelets comparable to Wt mice (Figure 2; day 5 postinjection).
INU1-induced thrombocytopenia is independent of platelet integrin activation and FcγRs. (A) Wt, FcRγ−/−, and Tln1−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (B) Western blot analysis of CLEC-2 levels, before and on day 5 post-INU1 injection in platelet lysates of FcRγ−/− and Tln1−/− mice. GPIIIa served as a loading control. (C) Flow cytometric analysis of αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure on platelets on day 5 post-INU1 injection (RC, 0.12 µg/mL; Thr, 0.1 U/mL). Results are representative of 3 individual experiments.
INU1-induced thrombocytopenia is independent of platelet integrin activation and FcγRs. (A) Wt, FcRγ−/−, and Tln1−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (B) Western blot analysis of CLEC-2 levels, before and on day 5 post-INU1 injection in platelet lysates of FcRγ−/− and Tln1−/− mice. GPIIIa served as a loading control. (C) Flow cytometric analysis of αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure on platelets on day 5 post-INU1 injection (RC, 0.12 µg/mL; Thr, 0.1 U/mL). Results are representative of 3 individual experiments.
We have previously shown that binding of INU1 to CLEC-2 potently induces aggregation of mouse platelets in vitro.17 Therefore, we speculated that INU1 may induce the formation of platelet aggregates which are then filtered out by the capillary bed in the lungs and/or cleared by the reticuloendothelial system, thereby causing the severe thrombocytopenia. To test this directly, we analyzed the effect of INU1 treatment in conditional talin1-deficient mice (Tln1fl/fl PF4-cre,23 further referred to as Tln1−/−), which are unable to activate their platelet integrins and thus fail to form platelet aggregates. Unexpectedly, INU1 treatment of Tln1−/− mice resulted in severe thrombocytopenia and CLEC-2 deficiency in newly generated platelets comparable to Wt mice (Figure 2A-B; day 5 postinjection). Similar results were obtained when integrin αIIbβ3 was functionally blocked with Fab fragments of the anti-αIIbβ3 antibody, JON/A27 (100 μg 1 hour before the experiment; supplemental Figure 3A-C). Together, these results indicated that INU1-induced thrombocytopenia depends on binding of the antibody to platelet CLEC-2, but can occur independently of FcRγ-mediated phagocytosis or integrin-mediated platelet aggregation.
INU1-induced thrombocytopenia depends on CLEC-2 signaling
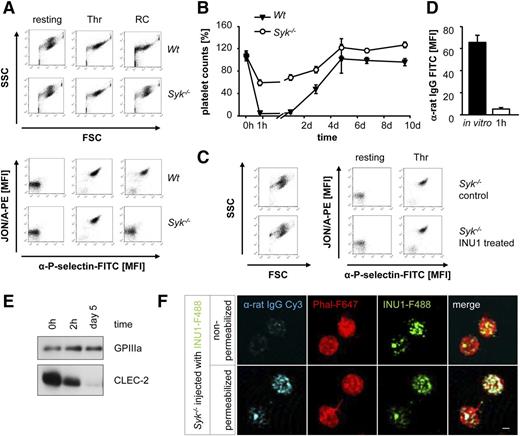
To test whether signaling downstream CLEC-2 is required for INU1-induced CLEC-2 downregulation and thrombocytopenia, we analyzed mice with a platelet-specific Syk deficiency (Sykfl/fl PF4-cre, further referred to as Syk−/−). Platelets from these mice display abolished activation upon stimulation with ITAM-specific agonists whereas activation with thrombin and other G-protein-coupled receptors agonists is normal (Figure 3A, Séverin et al7 and Königsberger et al28 ). Surprisingly, Syk deficiency in platelets markedly attenuated INU1-triggered thrombocytopenia (1 hour after injection: 56% ± 5% of control in Syk−/− mice vs 1% ± 0% in Wt; Figure 3B) and the platelets were in a resting state as revealed by flow cytometric measurement of forward scatter (FSC)/side scatter (SSC) characteristics, integrin αIIbβ3 activation, and degranulation-dependent P-selectin exposure (Figure 3C). Additionally, these platelets showed an unaltered ultrastructure in electron microscopy and a normal activation response to agonists, such as thrombin (data not shown and Figure 3C). CLEC-2 surface levels in circulating platelets were, however, strongly reduced 1 hour after antibody injection indicating that the receptor had been efficiently downregulated from the surface independently of Syk activity and cellular activation (Figure 3D). Western blot analysis of platelet lysates 2 hours after the injection revealed the presence of reduced but still robustly detectable CLEC-2 protein levels in these platelets (66% ± 7% of initial protein levels), strongly suggesting that the antibody-opsonized receptor had been internalized (Figure 3E). Similar to Wt mice,17 platelets of Syk−/− mice completely lacked CLEC-2 on day 5 after INU1 injection, indicating that the internalized receptor underwent intracellular degradation (Figure 3E). Together, these results demonstrate for the first time that targeting of CLEC-2 by INU1 can efficiently trigger downregulation of the receptor in circulating platelets. Furthermore, this process is independent of Syk and cellular activation and can be mechanistically uncoupled from the undesired antibody-induced transient severe thrombocytopenia.
Syk is required for INU1-induced thrombocytopenia. (A) Flow cytometric analysis of naive Wt and Syk−/− platelets incubated with various agonists. Displayed are the FSC and SSC characteristics as well as integrin αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure (RC, 0.12 µg/mL; Thr, 0.1 U/mL). (B) Wt and Syk−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (C) Flow cytometric analysis of control and INU1-treated Syk−/− platelets 1 hour after injection. Displayed are the FSC and SSC as well as the αIIbβ3 activation and degranulation-dependent P-selectin exposure. (D) Syk−/− mice were bled 1 hour after INU1 injection and washed blood was incubated with an α-rat Ig-FITC antibody for 15 minutes and subsequently analyzed on a FACSCalibur. As in vitro control, untreated mice were bled, blood was incubated with 20 µg/mL INU1 for 15 minutes, washed, and incubated with an α-rat Ig-FITC antibody for 15 minutes. (E) Western blot analysis of CLEC-2 levels at the indicated time points post-INU1 injection in platelets lysates of Syk−/− mice. GPIIIa served as a loading control. (F) Syk−/− mice were injected with 100 µg of INU1-F488 antibody, 15 minutes after injection platelets were isolated, allowed to adhere to PLL-coated cover slips, fixed and stained with α-rat IgG-Cy3 under permeabilizing or nonpermeabilizing conditions, and stained subsequently with PhalF647 diluted in permeabilizing buffer. Samples were visualized using a Leica TCS SP5 confocal microscope equipped with a 100×/1.4 oil objective. Scale bar represents 1 µm. Results are representative of 3 individual experiments.
Syk is required for INU1-induced thrombocytopenia. (A) Flow cytometric analysis of naive Wt and Syk−/− platelets incubated with various agonists. Displayed are the FSC and SSC characteristics as well as integrin αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure (RC, 0.12 µg/mL; Thr, 0.1 U/mL). (B) Wt and Syk−/− mice were IV injected with 100 µg of INU1 and platelet counts were determined on a FACSCalibur at the indicated time points. Results are mean ± SD in percentage of the initial platelet counts (n = 5 mice per group). (C) Flow cytometric analysis of control and INU1-treated Syk−/− platelets 1 hour after injection. Displayed are the FSC and SSC as well as the αIIbβ3 activation and degranulation-dependent P-selectin exposure. (D) Syk−/− mice were bled 1 hour after INU1 injection and washed blood was incubated with an α-rat Ig-FITC antibody for 15 minutes and subsequently analyzed on a FACSCalibur. As in vitro control, untreated mice were bled, blood was incubated with 20 µg/mL INU1 for 15 minutes, washed, and incubated with an α-rat Ig-FITC antibody for 15 minutes. (E) Western blot analysis of CLEC-2 levels at the indicated time points post-INU1 injection in platelets lysates of Syk−/− mice. GPIIIa served as a loading control. (F) Syk−/− mice were injected with 100 µg of INU1-F488 antibody, 15 minutes after injection platelets were isolated, allowed to adhere to PLL-coated cover slips, fixed and stained with α-rat IgG-Cy3 under permeabilizing or nonpermeabilizing conditions, and stained subsequently with PhalF647 diluted in permeabilizing buffer. Samples were visualized using a Leica TCS SP5 confocal microscope equipped with a 100×/1.4 oil objective. Scale bar represents 1 µm. Results are representative of 3 individual experiments.
SFK activity is essential for INU1-induced receptor internalization
To address the hypothesis that INU1-induced CLEC-2 downregulation occurs through internalization in more detail, Syk−/− mice were injected with 100 µg of Alexa F488–conjugated INU1 (INU1-F488) and after 15 minutes, platelets were isolated and fixed on PLL-coated cover slips. To determine the localization of INU1, the cells were then stained with α-rat IgG-Cy3 antibodies with or without membrane permeabilization. After removal of unbound antibody, all samples were counterstained with phalloidin-Atto647N (Phal-F647) under permeabilizing conditions to visualize the filamentous actin cytoskeleton. Under these experimental conditions, we consistently found a punctate distribution of INU1-F488 close to the cell membrane. This bound INU1-F488 antibody could, however, only be counterstained with α-rat IgG-Cy3 antibodies under permeabilizing, but not under nonpermeabilizing, conditions demonstrating internalization of the INU1/CLEC-2 complex (Figure 3F).
These findings prompted us to test whether INU1 could also induce internalization of CLEC-2 in vitro and whether lNU1 binding to CLEC-2 alone is sufficient to induce this process. Therefore, platelets of Wt and Syk−/− mice were incubated with INU1-F488 for 15 minutes and then fixed on PLL-coated slides for 30 minutes followed by staining with α-rat IgG-Cy3 antibodies with or without membrane permeabilization. Nonpermeabilized Wt or Syk−/− platelets yielded only a very weak staining with α-rat IgG-Cy3 antibodies, whereas permeabilization of the cells resulted in strong intracellular staining with a pattern resembling and colocalizing with that of INU1-F488, confirming that the CLEC-2/INU1 complex had efficiently been internalized in both Wt and Syk−/− platelets (Figure 4A). In Wt but not in Syk−/− platelets, INU1 induced a marked shape change, reflecting cellular activation. Accordingly, the INU1-F488 signal was centralized in Wt but not in Syk−/− platelets (Figure 4A), the latter displaying a punctate staining of CLEC-2 similar to that observed in platelets from INU1-treated Syk−/− mice. To test whether the altered staining pattern for internalized CLEC-2/INU1 complexes in Syk−/− platelets was indeed due to defective CLEC-2 signaling, we repeated the experiment in the presence of thrombin which indeed induced a strong centralization of the INU1-F488 signal in Syk−/− platelets comparable to Wt platelets (Figure 4B). These results suggested that INU1 binding to platelet CLEC-2 potently induces receptor internalization under both in vivo and in vitro conditions independently of Syk and platelet activation.
CLEC-2 downregulation occurs through Syk-independent internalization. (A) Wt and Syk−/− platelets were in vitro incubated with 20 µg/mL INU1-F488 for 15 minutes. Thereafter, platelets were stained and imaged as described in Figure 3F. Scale bars represent 1 µm. (B) Wt and Syk−/− platelets were in vitro incubated with 20 µg/mL INU1-F488 for 15 minutes followed by 2-minute incubation with Thr (0.1 U/mL). Subsequently, platelets were stained and imaged as described in Figure 3F. Results are representative of 3 individual experiments.
CLEC-2 downregulation occurs through Syk-independent internalization. (A) Wt and Syk−/− platelets were in vitro incubated with 20 µg/mL INU1-F488 for 15 minutes. Thereafter, platelets were stained and imaged as described in Figure 3F. Scale bars represent 1 µm. (B) Wt and Syk−/− platelets were in vitro incubated with 20 µg/mL INU1-F488 for 15 minutes followed by 2-minute incubation with Thr (0.1 U/mL). Subsequently, platelets were stained and imaged as described in Figure 3F. Results are representative of 3 individual experiments.
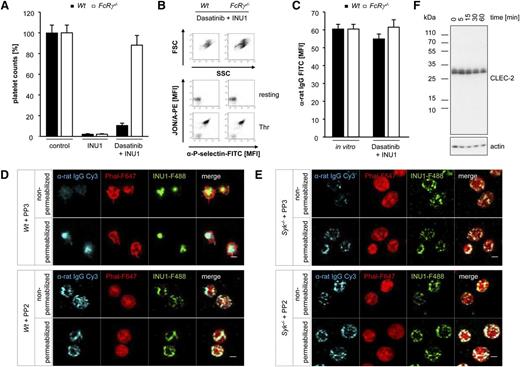
A previous study has shown that the antibody-induced tyrosine phosphorylation of both CLEC-2 and the downstream kinase Syk is completely blocked by PP2, a potent SFK inhibitor.7 Thus, we hypothesized that phosphorylation of the hemITAM of CLEC-2 might be sufficient to trigger antibody-induced internalization of the receptor. To test this directly, Wt mice were injected with the SFK inhibitor Dasatinib (5 mg/kg) or vehicle.29 In line with previous data,30 we detected an ITAM-specific activation defect in platelets of Dasatinib-treated mice, whereas other signaling pathways were unaffected, confirming the selectivity of the inhibitor (supplemental Figure 4). Surprisingly, injection of 100 µg of INU1 in Dasatinib-treated Wt mice resulted in thrombocytopenia after 2 hours, albeit to a lesser extent than in vehicle-pretreated mice (Figure 5A). The remaining platelets were in a resting state as revealed by flow cytometric measurement of FSC/SSC characteristics, integrin αIIbβ3 activation, and P-selectin exposure and showed a normal activation response to agonists such as thrombin (Figure 5B). Importantly, INU1 was detected at maximal levels on the surface of these circulating platelets (Figure 5C), indicating that activation of SFK upon antibody-induced CLEC-2 dimerization is a prerequisite for the internalization of the receptor. Because under these conditions circulating platelets were opsonized with INU1, we speculated that they might be cleared by Fc-dependent mechanisms. Therefore, we treated FcRγ−/− mice with Dasatinib (5 mg/kg) or vehicle 1 hour before the injection of INU1. In line with our previous results (Figure 2A), vehicle-treated FcRγ−/− mice became thrombocytopenic upon INU1 injection. In sharp contrast, platelet counts of Dasatinib-treated FcRγ−/− mice remained at 88% ± 9% of control (Figure 5A), indicating that the antibody-opsonized platelets were indeed cleared by FcγR-dependent mechanisms. The circulating platelets displayed robust CLEC-2 expression on their surface, which was comparable to that of in vitro INU1-stained control platelets (Figure 5C). Additionally, these platelets were in a resting state and showed a normal activation response to agonists such as thrombin (Figure 5B). Collectively, these results provide strong in vivo evidence that CLEC-2 immunodepletion is an SFK-dependent internalization process.
CLEC-2 internalization depends on SFK activity. (A) Wt and FcRγ−/− mice were pretreated with Dasatinib (5 mg/kg) for 1 h followed by IV injection of 100 µg of INU1. Platelet counts were determined 2 hours after injection on a FACSCalibur. Results are mean ± SD in percentage of starting values (n = 5 mice per group). (B) Flow cytometric analysis of INU1-injected Dasatinib-treated Wt and FcRγ−/− platelets 2 hours after injection. Displayed are the FSC and SSC as well as the αIIbβ3 activation and degranulation-dependent P-selectin exposure (Thr, 0.1 U/mL). (C) Two hours after INU1 injection, washed blood of Dasatinib-treated Wt and FcRγ−/− mice was incubated for 15 minutes with an α-rat Ig-FITC antibody and subsequently analyzed on a FACSCalibur. As in vitro control untreated mice were bled, blood was incubated with 20 µg/mL INU1 for 15 minutes, washed and incubated with an α-rat Ig-FITC antibody for 15 minutes. (D) Wt and (E) Syk−/− platelets were in vitro incubated for 15 minutes with 25 µM PP3 (negative control) or PP2 followed by an incubation with 20 µg/mL INU1-F488 for 15 minutes. Thereafter, platelets were stained and imaged as described in Figure 3F. (F) Wt platelets were in vitro incubated for 5 minutes with eptifibatide (40 µg/mL) and 20 µg/mL INU1 antibody. Subsequently, platelets were lysed at the indicated time points. Western blot analysis was performed with an antibody recognizing the intracellular N-terminal region of CLEC-2. β-actin served as a loading control. Results are representative of 3 individual experiments.
CLEC-2 internalization depends on SFK activity. (A) Wt and FcRγ−/− mice were pretreated with Dasatinib (5 mg/kg) for 1 h followed by IV injection of 100 µg of INU1. Platelet counts were determined 2 hours after injection on a FACSCalibur. Results are mean ± SD in percentage of starting values (n = 5 mice per group). (B) Flow cytometric analysis of INU1-injected Dasatinib-treated Wt and FcRγ−/− platelets 2 hours after injection. Displayed are the FSC and SSC as well as the αIIbβ3 activation and degranulation-dependent P-selectin exposure (Thr, 0.1 U/mL). (C) Two hours after INU1 injection, washed blood of Dasatinib-treated Wt and FcRγ−/− mice was incubated for 15 minutes with an α-rat Ig-FITC antibody and subsequently analyzed on a FACSCalibur. As in vitro control untreated mice were bled, blood was incubated with 20 µg/mL INU1 for 15 minutes, washed and incubated with an α-rat Ig-FITC antibody for 15 minutes. (D) Wt and (E) Syk−/− platelets were in vitro incubated for 15 minutes with 25 µM PP3 (negative control) or PP2 followed by an incubation with 20 µg/mL INU1-F488 for 15 minutes. Thereafter, platelets were stained and imaged as described in Figure 3F. (F) Wt platelets were in vitro incubated for 5 minutes with eptifibatide (40 µg/mL) and 20 µg/mL INU1 antibody. Subsequently, platelets were lysed at the indicated time points. Western blot analysis was performed with an antibody recognizing the intracellular N-terminal region of CLEC-2. β-actin served as a loading control. Results are representative of 3 individual experiments.
To further confirm these in vivo findings under in vitro conditions, we preincubated Wt and Syk−/− platelets with the SFK inhibitor PP2 (25 µM) or PP3 (25 µM), its inactive control, followed by incubation with 20 µg/mL INU1-F488 (15 minutes). PP3 did not affect INU1-induced responses of Wt and Syk−/− platelets, resulting in CLEC-2 receptor internalization in both genotypes, and centralization of the antibody/receptor complex in Wt, but not Syk−/−, platelets (Figure 5D-E). In sharp contrast, antibody-induced downregulation of CLEC-2 was abolished in PP2-pretreated Wt and Syk−/− platelets, as revealed by the robust detection of INU1-F488 with the α-rat IgG-Cy3 antibody in the absence of permeabilization (Figure 5D-E). To further corroborate these findings, we performed flow cytometric time-course experiments in Wt and Syk−/− platelets. Platelets were preincubated with eptifibatide (40 µg/mL), to prevent aggregate formation, and PP2 (25 µM) or PP3 followed by incubation with 20 µg/mL INU1 for up to 3 hours. Wt platelets preincubated with PP3 had already internalized 30% of surface CLEC-2-INU1 complexes after 15 minutes and only a weak signal was detectable on the surface after 60 minutes, demonstrating efficient internalization of INU1-opsonized CLEC-2 (supplemental Figure 5). In Syk−/− platelets, similar observations were made, albeit with a delayed time course. In sharp contrast, PP2-treated Wt as well as Syk−/− platelets did not show a decrease in INU1 IgG surface signal over time (supplemental Figure 5). This clearly demonstrates that CLEC-2 internalization occurs independently of Syk, but requires SFK activity.
Finally, to investigate whether INU1-induced receptor shedding of CLEC-2 contributes to the loss of the receptor from the surface, western blot analysis of Wt platelets, incubated in vitro for different time periods with INU1 (20 µg/mL) in the presence of eptifibatide (40 µg/mL), was performed (Figure 5F). Using an antibody recognizing the N-terminal intracellular portion of CLEC-2, we did not observe any fragments or changes in the molecular weight of the CLEC-2 band, but a gradual decrease of the CLEC-2 signal at later time points (>30 minutes). In light of our previous experiments, it appears conceivable that intracellular degradation of INU1-bound CLEC-2, but not CLEC-2 downregulation through ectodomain shedding, is responsible for the loss of the CLEC-2 signal, which is in agreement with a previous study.31
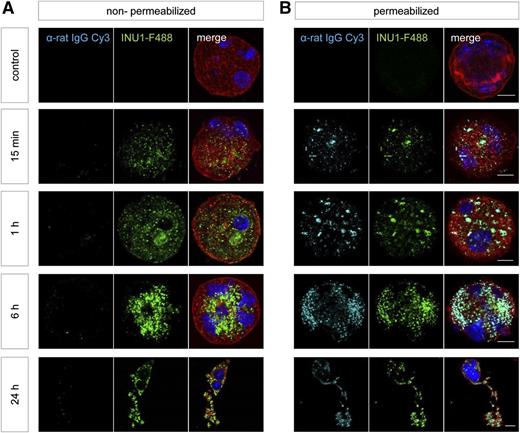
One question that remained was how CLEC-2–deficient platelets are generated in Wt animals where platelets undergo activation after INU1 binding. To address this in vitro, differentiated fetal liver cell–derived MKs were incubated with INU1-F488 for different time periods, fixed and stained with α-rat-IgG-Cy3 under membrane permeabilizing or nonpermeabilizing conditions, as well as with phalloidin-F647 and 4,6 diamidino-2-phenylindole (DAPI) diluted in permeabilizing buffer. A robust INU1-F488 signal was detectable at all time points, demonstrating the presence of the INU1/CLEC-2 complex (Figure 6). However, like in platelets, this could only be counterstained by α-rat IgG-Cy3 antibodies under permeabilizing conditions, arguing for the internalization of the INU1/CLEC-2 complex also in MKs (Figure 6). In contrast to platelets, no significant loss of total CLEC-2 was observed in MKs over time (Figure 6, supplemental Figure 2). This may be explained by the fact that MKs, in contrast to platelets, possess a nucleus and a highly active transcriptional machinery which may resynthesize CLEC-2 upon loss. Of note, on both in vitro and in situ stained MKs, we observed a signal for the INU1/CLEC-2 complex on proplatelets indicating that degradation of the internalized CLEC-2 might occur in platelets very early in the course of their biogenesis.
INU1-induced CLEC-2 internalization in fetal liver–derived MKs. The livers of 13.5- to 14.5-day-old mouse embryos were isolated from time-mated mice and a single-cell suspension was cultured for 72 hours; mature MKs were enriched on day 3 of culturing using a bovine serum albumin density gradient. These MKs were treated with INU1-F488 for the indicated time points. Subsequently, they were spun onto glass slides, fixed, and stained with α-rat IgG-Cy3 under (B) permeabilizing or (A) nonpermeabilizing conditions, as well as with phalloidin-F647 (red) diluted in permeabilizing buffer. Nuclei were stained using DAPI. Samples were visualized with a Leica TCS SP5 confocal microscope. Scale bar represents 10 µm.
INU1-induced CLEC-2 internalization in fetal liver–derived MKs. The livers of 13.5- to 14.5-day-old mouse embryos were isolated from time-mated mice and a single-cell suspension was cultured for 72 hours; mature MKs were enriched on day 3 of culturing using a bovine serum albumin density gradient. These MKs were treated with INU1-F488 for the indicated time points. Subsequently, they were spun onto glass slides, fixed, and stained with α-rat IgG-Cy3 under (B) permeabilizing or (A) nonpermeabilizing conditions, as well as with phalloidin-F647 (red) diluted in permeabilizing buffer. Nuclei were stained using DAPI. Samples were visualized with a Leica TCS SP5 confocal microscope. Scale bar represents 10 µm.
Discussion
In this study, we show that the targeted downregulation of CLEC-2 occurs through SFK-dependent, but Syk-independent, internalization in circulating platelets. In contrast, anti–CLEC-2 antibody-induced thrombocytopenia depends on SFK- and Syk-mediated platelet activation, thereby allowing the mechanistic uncoupling of these 2 processes.
CLEC-2 has increasingly been recognized as a central activating platelet receptor in thrombus formation and stabilization as well as tumor metastasis and maintenance of vascular integrity during inflammation. Our data provide the first evidence that anti–CLEC-2 antibody treatment results in CLEC-2 immunodepletion via internalization, presumably followed by intracellular degradation. To our knowledge, CLEC-2 is so far the second receptor to be downregulated from the surface of circulating platelets through antibody targeting, with this process previously described for GPVI in mouse and human platelets.32,33 However, in the case of GPVI, the major pathway immunodepletion is ectodomain shedding, whereas internalization becomes the prevailing mechanism only under conditions of impaired GPVI signaling.34 Our data show for the first time that the major route of CLEC-2 downregulation in platelets and MKs is internalization whereas there is no evidence for ectodomain shedding of CLEC-2 under any of the tested experimental conditions in vitro and in vivo. This finding is in line with a previous in vitro study, which also found no evidence for ectodomain shedding of CLEC-2 following autoactivation or in response to activation of ITAM receptors in human platelets.31 Interestingly, however, in that study, western blot and flow cytometric analyses indicated that CLEC-2 surface expression was not regulated at all.31 Different experimental conditions and reagents may explain why CLEC-2 internalization was not detected in this previous study. For example, in our in vitro studies, we used the αIIbβ3 blocker eptifibatide to inhibit platelet aggregate formation and enable the analysis of activated single platelets. Under these conditions, INU1 binding very efficiently induces virtually complete internalization of CLEC-2 in platelets in vitro. Importantly, however, and in agreement with previous findings, we could not observe any downregulation of surface CLEC-2 after stimulation with other agonists (data not shown).31 Furthermore, the previous study used a different anti–CLEC-2 antibody (AYP1), which detects CLEC-2 on human platelets. It is likely that AYP1 binds a different epitope in the extracellular domain of CLEC-2 than INU1. Thus, epitope-specific differences could be 1 reason for the discrepancies between the 2 studies. This would, however, stand in stark contrast to the targeted downregulation of GPVI, which occurs epitope independently.35 The contradictory findings may as well be explained by species-specific differences, however, this is considered very unlikely. Unfortunately, INU1 antibody does not cross-react with human CLEC-2 thus excluding studies on human platelets. In conclusion, further studies and development of new anti–CLEC-2 antibodies will be required to assess whether CLEC-2 is similarly downregulated in human platelets.
Our results demonstrate that INU1-induced CLEC-2 internalization is strictly dependent on SFK activity in vitro and in vivo, strongly suggesting that phosphorylation of the CLEC-2 hemITAM is a critical step required for this process to occur. Indeed, Dasatinib, a widely used anti-cancer drug for patients with, for example, Imatinib-resistant chronic myelogenous leukemia or prostate cancer36-39 that efficiently inhibits SFK activity prevented INU1-induced CLEC-2 downregulation in vivo (Figure 5). It was previously shown that upon stimulation of CLEC-2 by multimeric ligands such as rhodocytin, and presumably podoplanin, hemITAM phosphorylation by Syk is sufficient to trigger downstream signaling and platelet activation. In contrast, for dimeric activation with ligands such as antibodies, SFKs are essential,7 indicating that signal strength might affect CLEC-2 phosphorylation and thereby CLEC-2 regulation. Although it is currently unclear under which conditions CLEC-2 internalization occurs in normal physiology, it is likely that this mechanism may regulate platelet reactivity. Given the multimeric nature of podoplanin it appears unlikely that CLEC-2 is internalized upon binding to this ligand. Podoplanin is, however, not present in the blood stream and the relevance of CLEC-2 for thrombus formation and stabilization17 argues for the presence of different, hitherto unidentified, CLEC-2 ligands, which could trigger internalization of the receptor. Further studies are required to identify novel CLEC-2 ligands and assess their effect on CLEC-2 surface expression. Of note, a decrease in CLEC-2 surface expression upon stimulation with rhodocytin was reported recently, which was, however, ascribed to steric hindrance between RC and the anti–CLEC-2 antibody.31 In the context of thrombus formation CLEC-2 downregulation might also represent a negative feedback regulation of platelet reactivity. This could be of particular relevance because it was recently shown that CLEC-2–mediated platelet activation is insensitive toward classical inhibitors such as nitric oxide or prostacyclin (PGI2).40 Further studies are required to understand whether this might play a role in limiting stability of a growing thrombus, or during lymph-vessel development, thereby preventing overshooting aggregation responses.
Our data clearly show that whereas INU1-induced CLEC-2 downregulation is strictly dependent on hemITAM signaling through the receptor, it still efficiently occurs in the absence of Syk in vitro and in vivo (Figures 3F, 4A). This result was unexpected as we found, in line with previous reports,6,7,28 that Syk−/− platelets are entirely refractory to stimulation with CLEC-2 agonists, including RC and INU1 (Figure 3A). Thus, we propose that a hemITAM-triggered signaling pathway downstream of CLEC-2 exists in platelets that triggers internalization of the receptor independently of classic cellular activation. This is of particular relevance, as the severe transient thrombocytopenia seen in INU1-treated Wt mice was not observed in Syk−/− mice (Figure 3B), and hence seems to depend on the classical activation pathway. Together, our data demonstrate for the first time that the targeted downregulation of CLEC-2 occurs in circulating platelets in vivo and can be mechanistically uncoupled from the undesired associated thrombocytopenia.
Interestingly, a similar downregulation mechanism has been described for the T-cell receptor that is as well dependent on SFK, in this case Lck, but independent of Syk.41 Phosphorylation of the T-cell receptor by Lck results in recruitment of adaptor proteins and ubiquitin ligases leading to internalization and degradation.41 It was recently shown that the anti–CLEC-2 antibody-induced phosphorylation of the CLEC-2 hemITAM depends on the SFK Lyn7 and our results support the concept of SFK being upstream of Syk in hemITAM signaling.
Our study also demonstrated that INU1 can induce thrombocytopenia in 2 different ways: one that is platelet activation-dependent and FcγR–independent and a second one that depends on FcγRs. The first mechanism applies to the situation in Wt mice, where platelets are rapidly activated and internalize the CLEC2/INU1 complexes. These activated platelets are then rapidly cleared from the circulation independently of FcγRs (Figures 1-2). FcγR-dependent platelet clearance, however, becomes relevant in Dasatinib-treated mice, where INU1 does not cause platelet activation and remains on the surface of the cells (due to blocked internalization). In Syk−/− mice, however, INU1 does not cause platelet activation and is rapidly internalized and thus inaccessible for FcγRs. As a result, INU1-induced thrombocytopenia is largely prevented in these animals (Figure 3). The partial drop in platelet counts in Syk-deficient mice most likely represents a short time frame of FcγR-dependent platelet clearance until full internalization of the CLEC-2 has occurred. In line with this, blockade of FcγRs further reduced INU1-induced thrombocytopenia in Syk-deficient mice (supplemental Figure 6).
Taken together, our results provide the first evidence of an active mechanism that regulates CLEC-2 surface abundance in mouse platelets that strongly depends on phosphorylation events mediated via SFK. Moreover, Syk appears to be the central molecular checkpoint that is dispensable for CLEC-2 immunodepletion but required for INU1-induced thrombocytopenia. This offers the possibility of mechanistically uncoupling targeted CLEC-2 downregulation from the undesired anti–CLEC-2–induced platelet activation and consumption. Furthermore, our data show that MKs can also downregulate CLEC-2 from their surface through internalization, which may explain the appearance of CLEC-2–deficient platelets in the circulation of Wt mice 2 to 3 days after anti–CLEC-2 antibody treatment. These results may have implications for the development of therapeutic agents to interfere with CLEC-2 activity in thrombotic, inflammatory, or malignant diseases.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stefanie Hartmann for excellent technical assistance, and the microscopy platform of the Bioimaging Center (Rudolf Virchow Center) for providing technical infrastructure.
This work was supported by the British Heart Foundation (CH/03/003), the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688 [B.N.] and Sonderforschungsbereich 914/TP A02 [B.W.]), and the Rudolf Virchow Center. S.S. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg. For the generation of Sykfl/fl ES cells, J.S. was supported by EMBO Short-term Fellowship ASTF 291.00-2006.
Authorship
Contribution: V.L., D.S., S.S., and T.V. performed experiments, analyzed data, and wrote the manuscript; B.W., F.K., S.P.W., J.S., and W.W. contributed vital new reagents and mice; and B.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.S. is Department Respiratory Diseases Research, Boehringer-Ingelheim Pharma GmbH & Co KG, Biberach, Germany.
Correspondence: Bernhard Nieswandt, University Hospital Würzburg and Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Josef-Schneider-Strasse 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal