Key Points
Most AMLs lack ASS1, which allows synthesis of arginine, and so depend on exogenous sources.
Depletion of arginine via ADI-PEG 20 reduces the burden of primary AML in vivo and in vitro.
Abstract
The strategy of enzymatic degradation of amino acids to deprive malignant cells of important nutrients is an established component of induction therapy of acute lymphoblastic leukemia. Here we show that acute myeloid leukemia (AML) cells from most patients with AML are deficient in a critical enzyme required for arginine synthesis, argininosuccinate synthetase-1 (ASS1). Thus, these ASS1-deficient AML cells are dependent on importing extracellular arginine. We therefore investigated the effect of plasma arginine deprivation using pegylated arginine deiminase (ADI-PEG 20) against primary AMLs in a xenograft model and in vitro. ADI-PEG 20 alone induced responses in 19 of 38 AMLs in vitro and 3 of 6 AMLs in vivo, leading to caspase activation in sensitive AMLs. ADI-PEG 20–resistant AMLs showed higher relative expression of ASS1 than sensitive AMLs. This suggests that the resistant AMLs survive by producing arginine through this metabolic pathway and ASS1 expression could be used as a biomarker for response. Sensitive AMLs showed more avid uptake of arginine from the extracellular environment consistent with their auxotrophy for arginine. The combination of ADI-PEG 20 and cytarabine chemotherapy was more effective than either treatment alone resulting in responses in 6 of 6 AMLs tested in vivo. Our data show that arginine deprivation is a reasonable strategy in AML that paves the way for clinical trials.
Introduction
Malignant cells show increased uptake and metabolism of glucose and amino acids.1,2 These metabolic changes are believed to supply the cancer cells with the biomass to proliferate. Starving malignant cells of metabolic resources is one strategy that has been successfully used in the treatment of acute lymphoblastic leukemia where asparaginase is an important part of induction chemotherapy.
The amino acid arginine is used in a number of metabolic pathways including the synthesis of nitric oxide, polyamines, and amino acids such as glutamate and proline.3,4 Cells may either import arginine or synthesize it from citrulline through the enzymes argininosuccinate synthetase-1 (ASS1) and argininosuccinate lyase (ASL) (Figure 1A).4-6 ASS1 is not expressed by many malignant cells, and this appears to confer a proliferative advantage to the cells.7-9 Overexpression and silencing of ASS1 in cancer cell lines reduce and increase proliferation, respectively.8,9 The survival of patients with cancer that does not express ASS1 is worse than for patients whose tumors express ASS1.7-9
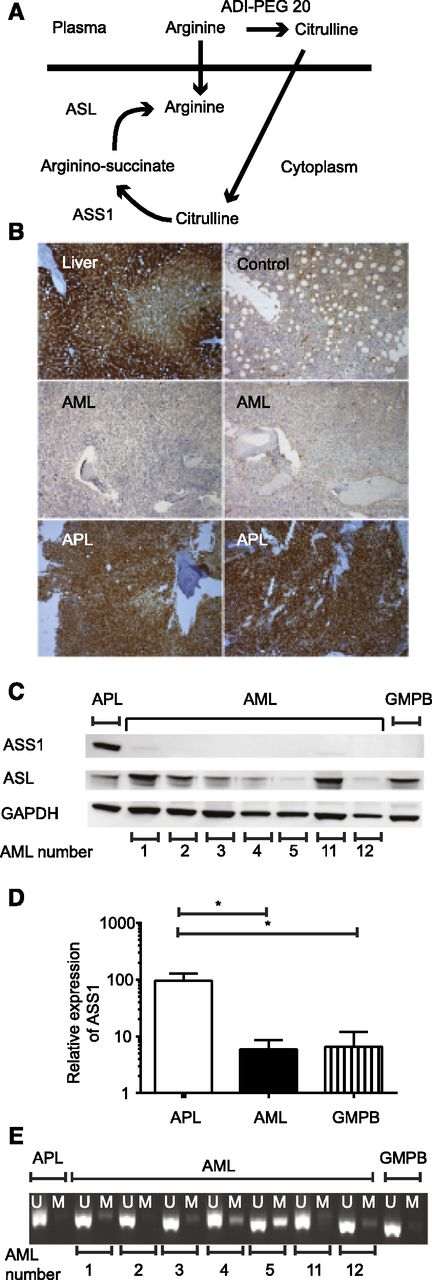
ASS1 is not expressed in most AML. (A) The 2 enzymes required for endogenous arginine synthesis are shown together with the products of arginine following ADI-PEG 20 therapy. (B) ASS1 expression was assessed in bone marrow using immunohistochemistry: positive control, liver (top left); control bone marrow (top right). The rest of the panels are pictures of trephines with the diagnosis indicated on each panel. The 2 AML samples are negative (middle right, sample T19; middle left, sample T22), whereas the 2 APL samples express ASS1 (bottom right, sample T3; bottom left, sample T2). Slides were analyzed using a Leica DM2500 microscope with ×100 magnification, and images were acquired by a Leica DFC320 camera and processed using Leica IM50 software. (C) The expression of ASS1 and ASL in primary AML lysates was tested using western blotting. Results for 7 AML, 1 APL, and 1 granulocyte–colony-stimulating factor mobilized peripheral blood (GMPB) samples are shown. (D) The relative expression of ASS1 was quantified by real-time PCR in 4 APL, 38 AML, and 11 GMPB samples. Relative expression of ASS1 was greater in APL than AML or GMPB. *P < .001. (E) Methylation of the ASS1 gene was assessed in 7 primary AML, 1 APL, and 1 GMPB samples. Six of 7 AML samples show some methylation, whereas the APL does not. U, unmethylated DNA; M methylated DNA.
ASS1 is not expressed in most AML. (A) The 2 enzymes required for endogenous arginine synthesis are shown together with the products of arginine following ADI-PEG 20 therapy. (B) ASS1 expression was assessed in bone marrow using immunohistochemistry: positive control, liver (top left); control bone marrow (top right). The rest of the panels are pictures of trephines with the diagnosis indicated on each panel. The 2 AML samples are negative (middle right, sample T19; middle left, sample T22), whereas the 2 APL samples express ASS1 (bottom right, sample T3; bottom left, sample T2). Slides were analyzed using a Leica DM2500 microscope with ×100 magnification, and images were acquired by a Leica DFC320 camera and processed using Leica IM50 software. (C) The expression of ASS1 and ASL in primary AML lysates was tested using western blotting. Results for 7 AML, 1 APL, and 1 granulocyte–colony-stimulating factor mobilized peripheral blood (GMPB) samples are shown. (D) The relative expression of ASS1 was quantified by real-time PCR in 4 APL, 38 AML, and 11 GMPB samples. Relative expression of ASS1 was greater in APL than AML or GMPB. *P < .001. (E) Methylation of the ASS1 gene was assessed in 7 primary AML, 1 APL, and 1 GMPB samples. Six of 7 AML samples show some methylation, whereas the APL does not. U, unmethylated DNA; M methylated DNA.
Tumor cells that lack ASS1 are therefore dependent on exogenous arginine, which in normal circumstances is available from the extracellular environment.4-6 However, ASS1-negative tumor cells are potentially vulnerable to systemic arginine depletion, having no means to synthesize their own. A pegylated form of arginine deiminase (ADI-PEG 20), a mycoplasma-derived enzyme that degrades arginine to citrulline, is undergoing clinical trials in ASS1-negative solid tumors.10-12 The drug efficiently depletes arginine from plasma in humans10,13 and is well tolerated with injection site discomfort being the commonest side effect and no grade 3 or 4 events in 39 patients with melanoma.10 This favorable side effect profile is consistent with the expression of ASS1 in normal cells as these can generate arginine in contrast to malignant ASS1-deficient cells. The principal potential problem with ADI-PEG 20 is immunogenicity against this formulation containing a mycoplasma-derived protein with loss of arginine depletion related to antibodies against ADI-PEG 20 (ADI is found in microorganisms not humans).11
Acute myeloid leukemia (AML) remains a challenging illness particularly in the older patients and/or where adverse genetic features are present. The backbone of AML induction therapy, cytarabine with an anthracycline antibiotic, has remained unchanged for the past 50 years. Here, we show that most primary AMLs lack ASS1 expression and are therefore potentially susceptible to arginine deprivation therapy. We demonstrate that the leukemic burden is reduced by ADI-PEG 20 therapy alone in 3 of 6 primary AMLs tested in a xenograft model and in all 6 AMLs when combined with cytarabine. ADI-PEG 20 induced apoptosis in sensitive AMLs. Resistant AMLs expressed relatively higher levels of ASS1 messenger RNA (mRNA) than sensitive AMLs suggesting that the mechanism of resistance is through production of arginine. By contrast, sensitive AMLs took up arginine from the extracellular environment more avidly than resistant AMLs. Regeneration of normal hematopoietic cells was seen in mice following successful clearance of AML by ADI-PEG 20 indicating that there is relative selectivity for AML, and this appears to be because of differential expression of ASS1. Lastly, we show that normal human hematopoietic stem-progenitor cells express higher levels of ASS1 than sensitive AMLs and resist killing by ADI-PEG 20, providing the rationale for a selective effect of arginine deprivation on AML.
Patients and methods
Primary cells
Blood and bone marrow samples were collected from patients with AML after written informed consent at St. Bartholomew’s Hospital. The protocol was approved by the East London and City Research Ethics Committee. All studies comply with the rules of the Review Board and by the revised Helsinki protocol. The samples were collected at untreated presentation or relapse. Details of the patient samples are listed in Table 1 and supplemental Table 1 (available on the Blood Web site).14
AML characteristics and responses to ADI-PEG 20 in vitro and in vivo
| AML number . | Cytogenetics . | MRC cytogenetic risk group14 . | NPM . | FLT3 . | AML responds to . | |||
|---|---|---|---|---|---|---|---|---|
| ADI-PEG 20 in vitro . | ADI-PEG 20 in vivo . | Ara-C in vivo . | Ara-C + ADI-PEG 20 in vivo . | |||||
| 1 | Failed | NA | Mutant | ITD | No | No | No | Yes |
| 2 | Normal | Intermediate | Mutant | ITD | Yes | Yes | Yes | Yes |
| 3 | t(6;11) MLL rearranged | Poor | WT | NT | Yes | Yes | Yes | Yes |
| 4 | Normal | Intermediate | WT | ITD | No | No | Yes | Yes |
| 5 | Normal | Intermediate | WT | ITD | Yes | Yes | Yes | Yes |
| 6 | del(7q), +8, add(11q) | Poor | WT | WT | No | No | No | Yes |
| 7 | inv(16) | Good | NT | NT | Yes | |||
| 8 | inv(16) | Good | NT | NT | Yes | |||
| 9 | t(8;21) | Good | NT | NT | No | |||
| 10 | t(8;21) | Good | NT | NT | Yes | |||
| 11 | +13 | Intermediate | Mutant | ITD | Yes | |||
| 12 | +13 | Intermediate | Mutant | WT | Yes | |||
| 13 | +13, t(3;14) | Intermediate | Mutant | WT | No | |||
| 14 | add(21p) | Intermediate | Mutant | WT | Yes | |||
| 15 | Normal | Intermediate | WT | WT | No | |||
| 16 | Normal | Intermediate | Mutant | ITD | No | |||
| 17 | Normal | Intermediate | Mutant | WT | Yes | |||
| 18 | Normal | Intermediate | Mutant | WT | No | |||
| 19 | Normal | Intermediate | Mutant | ITD | Yes | |||
| 20 | Normal | Intermediate | Mutant | ITD | Yes | |||
| 21 | Normal | Intermediate | Mutant | WT | Yes | |||
| 22 | Normal | Intermediate | Mutant | WT | Yes | |||
| 23 | t(11;19) MLL rearranged | Intermediate | WT | WT | No | |||
| 24 | t(15;16) | Intermediate | WT | ITD | Yes | |||
| 25 | t(6;9) | Intermediate | WT | ITD | No | |||
| 26 | t(6;9) | Intermediate | WT | ITD | No | |||
| 27 | t(9;11) MLL rearranged | Intermediate | WT | WT | Yes | |||
| 28 | Failed | NA | Mutant | WT | No | |||
| 29 | Complex | Poor | NT | NT | No | |||
| 30 | Complex | Poor | NT | NT | No | |||
| 31 | Complex | Poor | NT | NT | No | |||
| 32 | Complex | Poor | NT | NT | No | |||
| 33 | del(7q) | Poor | NT | NT | No | |||
| 34 | del(7q) | Poor | NT | NT | No | |||
| 35 | t(11;17) MLL rearranged | Poor | WT | WT | No | |||
| 36 | t(3;3), −7 | Poor | NT | NT | Yes | |||
| 37 | t(6;11) MLL rearranged | Poor | NT | NT | Yes | |||
| 38 | t(6;11) MLL rearranged | Poor | NT | NT | Yes | |||
| AML number . | Cytogenetics . | MRC cytogenetic risk group14 . | NPM . | FLT3 . | AML responds to . | |||
|---|---|---|---|---|---|---|---|---|
| ADI-PEG 20 in vitro . | ADI-PEG 20 in vivo . | Ara-C in vivo . | Ara-C + ADI-PEG 20 in vivo . | |||||
| 1 | Failed | NA | Mutant | ITD | No | No | No | Yes |
| 2 | Normal | Intermediate | Mutant | ITD | Yes | Yes | Yes | Yes |
| 3 | t(6;11) MLL rearranged | Poor | WT | NT | Yes | Yes | Yes | Yes |
| 4 | Normal | Intermediate | WT | ITD | No | No | Yes | Yes |
| 5 | Normal | Intermediate | WT | ITD | Yes | Yes | Yes | Yes |
| 6 | del(7q), +8, add(11q) | Poor | WT | WT | No | No | No | Yes |
| 7 | inv(16) | Good | NT | NT | Yes | |||
| 8 | inv(16) | Good | NT | NT | Yes | |||
| 9 | t(8;21) | Good | NT | NT | No | |||
| 10 | t(8;21) | Good | NT | NT | Yes | |||
| 11 | +13 | Intermediate | Mutant | ITD | Yes | |||
| 12 | +13 | Intermediate | Mutant | WT | Yes | |||
| 13 | +13, t(3;14) | Intermediate | Mutant | WT | No | |||
| 14 | add(21p) | Intermediate | Mutant | WT | Yes | |||
| 15 | Normal | Intermediate | WT | WT | No | |||
| 16 | Normal | Intermediate | Mutant | ITD | No | |||
| 17 | Normal | Intermediate | Mutant | WT | Yes | |||
| 18 | Normal | Intermediate | Mutant | WT | No | |||
| 19 | Normal | Intermediate | Mutant | ITD | Yes | |||
| 20 | Normal | Intermediate | Mutant | ITD | Yes | |||
| 21 | Normal | Intermediate | Mutant | WT | Yes | |||
| 22 | Normal | Intermediate | Mutant | WT | Yes | |||
| 23 | t(11;19) MLL rearranged | Intermediate | WT | WT | No | |||
| 24 | t(15;16) | Intermediate | WT | ITD | Yes | |||
| 25 | t(6;9) | Intermediate | WT | ITD | No | |||
| 26 | t(6;9) | Intermediate | WT | ITD | No | |||
| 27 | t(9;11) MLL rearranged | Intermediate | WT | WT | Yes | |||
| 28 | Failed | NA | Mutant | WT | No | |||
| 29 | Complex | Poor | NT | NT | No | |||
| 30 | Complex | Poor | NT | NT | No | |||
| 31 | Complex | Poor | NT | NT | No | |||
| 32 | Complex | Poor | NT | NT | No | |||
| 33 | del(7q) | Poor | NT | NT | No | |||
| 34 | del(7q) | Poor | NT | NT | No | |||
| 35 | t(11;17) MLL rearranged | Poor | WT | WT | No | |||
| 36 | t(3;3), −7 | Poor | NT | NT | Yes | |||
| 37 | t(6;11) MLL rearranged | Poor | NT | NT | Yes | |||
| 38 | t(6;11) MLL rearranged | Poor | NT | NT | Yes | |||
Ara-C, cytosine arabinoside; FLT3, Fms-related tyrosine kinase 3; MLL, mixed lineage leukemia; MRC, Medical Research Council; NA, not applicable; NPM, nucleophosmin; NT, not tested; WT, wild type.
ASS1 staining of trephines
Bone marrow trephines were stained with antibody to ASS1 after fixation and decalcification. In cases where >20% of cells expressed ASS1, the sample was deemed to be positive.
Animal experiments
Nonobese diabetic/severe combined immunodeficiency disease/interleukin 2 receptor γ chain null (NOD/SCID/IL2rγ−/−) mice were a kind gift of Leonard Shultz and were used as detailed previously.15,16 All animal experiments were performed in accordance with Home Office and Cancer Research UK guidelines. The protocol was reviewed by the animal ethics committee of the Cancer Research UK London Research Institute.
The 6 AML samples chosen for in vivo experiments were screened for ability to generate a graft in immunodeficient mice before use. Ten to 13 weeks after transplantation, drugs were administered to the mice as follows. ADI-PEG 20 (provided as a gift by Polaris Pharmaceuticals) was administered by intraperitoneal injection (5 IU) once per week for 4 weeks. This dose was derived from previous studies.17 Cytarabine was administered by subcutaneous injection for 10 days at dose of 0.2 mg daily, the approximate dose that humans receive with the low-dose 20 mg twice-daily schedule.18 Phosphate-buffered saline vehicle was given to control mice.
Mice were euthanized at day 23 to 28 after starting therapy. Bone marrow cells were removed by crushing bones as previously described.19 Engraftment was assessed by immunophenotyping as described previously.15 In 2 experiments, an extra cohort of mice was set up, and these mice received 2 cycles of treatment (ie, a total of 8 weeks of ADI-PEG 20 given weekly). These mice were euthanized 56 to 63 days after the start of therapy.
Quantification of arginine and other amino acids
Ultra-high-performance liquid chromatography mass spectrometry/mass spectrometry was used to quantify arginine in plasma as described.20
Methylation of ASS1 gene
To examine the methylation status of the ASS1 gene, methylation-specific polymerase chain reaction (PCR) was carried out as described.21
In vitro effect of ADI-PEG 20 treatment on AML primary cells
The stromal coculture was performed as described.22 AML cells were cultured at a density of 1.2 million cells per mL in the presence and absence of ADI-PEG 20 (0.75 μg/mL). The dose of ADI-PEG 20 was derived from leukemia cell line studies and prior publications.21,23 Three days after treatment, cells were harvested and counted using a Vi-CELL Cell Viability Analyzer (Beckman).
Statistics
Generalized linear models based on the negative binomial distribution were used to assess statistical significance of the difference between engraftment percentages following xenografting. The Student t test was used elsewhere to assess differences unless stated. Error bars are standard error of mean.
Further methodologic details are found in supplemental Methods.
Results
ASS1 is highly expressed in APL but not most AML samples
We used immunohistochemistry to identify ASS1 expression in bone marrow trephines from 30 patients with AML and 2 with acute promyelocytic leukemia (APL). Two of 30 (6.7%) AML trephines showed staining for ASS1 in >20% of leukemia cells, whereas both APL samples expressed ASS1 (Figure 1B; supplemental Table 1). Thus, 93% (28 of 30) were ASS1 deficient. None of the 10 control bone marrow trephines expressed ASS1 in >20% of cells (from patients with lymphoma without bone marrow involvement).
ASS1 expression was also assessed in cell lysates from 19 different primary AML samples using western blotting. ASS1 was strongly expressed by only 2 of 19 AMLs, whereas APL strongly expressed ASS1 (representative data are shown in Figure 1C). A different anti-ASS1 antibody was used to confirm the results of the western blotting on 11 AML samples (supplemental Figure 1). The combined data from the immunohistochemistry and the western blotting therefore showed that only 8% (4 of 49) of AMLs strongly expressed ASS1. Donor GMPB samples were used as a control for AML; 4 of 4 GMPB samples did not express ASS1 strongly.
The relative expression of ASS1 to glyceraldehyde-3-phosphate dehydrogenase was quantified by real-time PCR in primary AML cells and GMPB. APL expressed higher levels of ASS1 than AML, consistent with the western blotting and immunohistochemistry (Figure 1D). GMPB expressed similar amounts of ASS1 to AML. Therefore, both AML and normal hematopoietic cells are potentially vulnerable to arginine deprivation.
By contrast, ASL was expressed in 7 of 7 AML samples (Figure 1C) indicating that these AMLs could convert argininosuccinate into arginine if they had access to this substrate (Figure 1A). GMPB also expressed ASL. Similar data were obtained using mRNA expression analysis (supplemental Figure 2).
We tested whether the expression of ASS1 is low in AML because of methylation of the ASS1 promoter as has been described in lymphoma cell lines.21 Twenty-one of 28 AML samples had evidence of methylation of the ASS1 promoter, whereas neither APL nor GMPB did (representative data are shown in Figure 1E). These data suggest that epigenetic suppression of ASS1 may be common in AML.
Administration of ADI-PEG 20 depletes plasma arginine in vivo and leads to reductions in AML burden
We tested whether arginine deprivation would affect the growth of primary AML cells in a xenograft model. We transplanted 6 primary AML samples (samples 1-6) into 123 NSG mice. Ten to 13 weeks posttransplantation, once the AML grafts were established, we treated mice with 4 doses of ADI-PEG 20 (given weekly), cytarabine, or vehicle. Ninety-five mice were euthanized 23 to 28 days after starting therapy to evaluate impact of therapies on the AML cells. Twenty-eight mice received another 4 doses of ADI-PEG 20 or further cytarabine or vehicle and were euthanized 56 to 63 days after starting therapy.
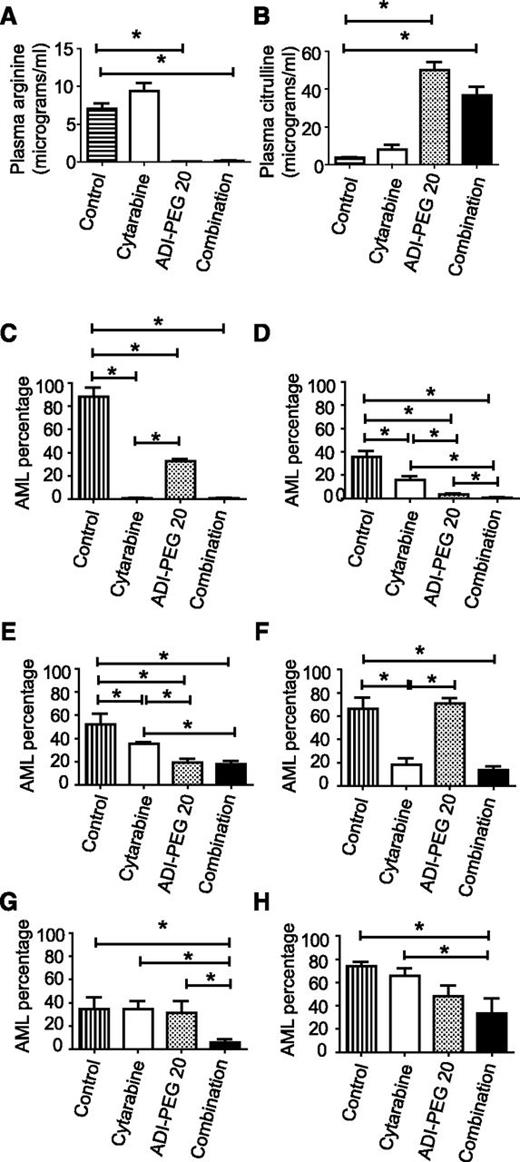
We confirmed that arginine was depleted from the plasma by ADI-PEG 20 treatment using liquid chromatography tandem mass spectrometry when the mice were euthanized (Figure 2A). The product of arginine deimination, citrulline was elevated in the plasma (Figure 2B).
Arginine is depleted in mice receiving ADI-PEG 20, and this leads to reduced AML burden. (A) Plasma arginine was reduced in mice receiving ADI-PEG 20 either alone or in combination with cytarabine (data from 95 mice are shown). (B) Plasma citrulline levels were increased in mice receiving ADI-PEG 20 (data from 95 mice are shown). (C-H) The effects of ADI-PEG 20, cytarabine, and combination therapy on bone marrow AML percentage is shown. (C-E) ADI-PEG 20 alone reduced the percentage of AML in 3 experiments (AML samples 2, 3, and 5). (C-F) Cytarabine alone reduced the percentage of AML in 4 experiments (AML samples 2, 3, 4, and 5). (G-H) Neither drug alone was effective for AML samples 1 and 6, but the combination was effective in both. *P < .05.
Arginine is depleted in mice receiving ADI-PEG 20, and this leads to reduced AML burden. (A) Plasma arginine was reduced in mice receiving ADI-PEG 20 either alone or in combination with cytarabine (data from 95 mice are shown). (B) Plasma citrulline levels were increased in mice receiving ADI-PEG 20 (data from 95 mice are shown). (C-H) The effects of ADI-PEG 20, cytarabine, and combination therapy on bone marrow AML percentage is shown. (C-E) ADI-PEG 20 alone reduced the percentage of AML in 3 experiments (AML samples 2, 3, and 5). (C-F) Cytarabine alone reduced the percentage of AML in 4 experiments (AML samples 2, 3, 4, and 5). (G-H) Neither drug alone was effective for AML samples 1 and 6, but the combination was effective in both. *P < .05.
ADI-PEG 20 significantly reduced the percentage of AML cells in the bone marrow in 3 of 6 experiments compared with vehicle-treated mice (samples 2, 3, and 5) (Figure 2C-E and Table 1). Cytarabine reduced the percentage of AML in 4 of 6 experiments (samples 2-5) (Figure 2C-F). Four AMLs showed differential sensitivity to the agents; AMLs 2 and 4 (Figure 2C,F) showed a significantly greater sensitivity to cytarabine than ADI-PEG 20, and AMLs 3 and 5 (Figure 2D-E) showed a significantly greater sensitivity to ADI-PEG 20 than cytarabine. AMLs 1 and 6 were resistant to each agent given individually, but the combination of ADI-PEG 20 and cytarabine had a significant effect on the AML percentage (Figure 2G-H). Furthermore, the combination was significantly more effective than chemotherapy alone in 4 experiments (AML samples 1, 3, 5, and 6) (Figure 2D-E,G-H).
We tested whether 8 weeks of therapy would improve the response or whether there would be evidence of resistance development in 2 AML samples. For sample 1, the data at 4 and 8 weeks were similar; there was no significant effect of ADI-PEG 20 or cytarabine alone at 8 weeks whereas the combination induced a significant reduction in AML percentage (supplemental Figure 3A and Figure 2G). For sample 2, the effect of ADI-PEG 20 on AML did not reach statistical significance at 8 weeks, whereas the combination was effective (supplemental Figure 3B and Figure 2C). These experiments do not reveal a major difference between 4 and 8 weeks of therapy.
Normal mouse hematopoiesis expresses ASS1 and resists killing by ADI-PEG 20 in vivo
To determine whether ADI-PEG 20 killed normal mouse hematopoietic tissue, we examined bone marrow sections from mice treated with ADI-PEG 20. Sheets of blasts were seen in control mice but little normal hematopoiesis (Figure 3A). By contrast, in mice in which AML responded to ADI-PEG 20 therapy, normal hematopoeisis was observed indicating that normal hematopoietic cells are relatively resistant to ADI-PEG 20 (Figure 3B). ASS1 expression was lower in vehicle-treated mice (Figure 3C) than ADI-treated mice (Figure 3D). To ensure the ASS1 expressing cells were not AML cells, we stained mouse bone marrow sections with antibodies to ASS1 (the antibody to ASS1 reacts with both human and mouse ASS1) and human CD45. No detectable ASS1 was seen in AML cells (Figure 3E-F), whereas mouse bone marrow cells expressed ASS1 (Figure 3F). The differential expression of ASS1 presumably explains the relative sensitivity of AML; normal mouse hematopoietic cells express ASS1 and can therefore synthesize arginine from argininosuccinate. The expression of ASS1 was also seen in the bone marrow of untransplanted mice that had not received ADI-PEG 20; therefore, the expression of ASS1 is not in reaction to ADI-PEG 20 (supplemental Figure 4). ASS1 expression was higher in mouse bone marrow than human bone marrow (Figure 1B).
ASS1-expressing normal hematopoietic cells are seen following suc-cessful AML clearance by ADI-PEG 20. Bone marrow sections from mice transplanted with AML sample 3 following treatment with vehicle or ADI-PEG 20 were stained with hematoxylin and eosin to assess residual hematopoiesis. The majority of cells in mice receiving vehicle were leukemic (A), whereas normal hematopoietic cells, including megakaryocytes (arrowed), are seen in mice treated with ADI-PEG 20 (B). ASS1 expression was lower in mice receiving vehicle (C) than mice receiving ADI-PEG 20 (D). Slides were analyzed using a Leica DM2500 microscope (magnification ×400 [A-B], ×200 [C-D]), and images were acquired by a Leica DFC320 camera and processed using Leica IM50 software. ASS1 was not expressed by AML cells (E) but was expressed by nonhuman marrow cells in mice treated with ADI-PEG 20 (F). Images were captured on an LSM510 Meta confocal laser microscope using a 40 × 1.3 oil immersion objective.
ASS1-expressing normal hematopoietic cells are seen following suc-cessful AML clearance by ADI-PEG 20. Bone marrow sections from mice transplanted with AML sample 3 following treatment with vehicle or ADI-PEG 20 were stained with hematoxylin and eosin to assess residual hematopoiesis. The majority of cells in mice receiving vehicle were leukemic (A), whereas normal hematopoietic cells, including megakaryocytes (arrowed), are seen in mice treated with ADI-PEG 20 (B). ASS1 expression was lower in mice receiving vehicle (C) than mice receiving ADI-PEG 20 (D). Slides were analyzed using a Leica DM2500 microscope (magnification ×400 [A-B], ×200 [C-D]), and images were acquired by a Leica DFC320 camera and processed using Leica IM50 software. ASS1 was not expressed by AML cells (E) but was expressed by nonhuman marrow cells in mice treated with ADI-PEG 20 (F). Images were captured on an LSM510 Meta confocal laser microscope using a 40 × 1.3 oil immersion objective.
ADI-PEG 20 induces apoptosis in half of primary AML cells in vitro
We wanted to develop an in vitro assay to test ADI against AML, so we tested ADI-PEG 20 against the 6 AML cells we had used in the xenograft work in an MS-5 stromal coculture.22 ADI-PEG 20 depleted arginine in supernatant efficiently (supplemental Table 2). Three of 6 AML samples (2, 3, and 5) showed induction of apoptosis in response to ADI-PEG 20 as evidenced by significantly increased expression of active caspase 3/7 and annexin V and reduced numbers of viable cells (supplemental Figure 5 and Table 1). The 3 AMLs that were sensitive to single agent ADI-PEG 20 in vitro had been sensitive to ADI-PEG 20 in vivo and the 3 AMLs that were resistant in vitro were resistant in vivo, suggesting that the in vitro system reflects the in vivo assay.
We expanded the in vitro testing to include a total of 38 primary AML samples (4 good risk, 20 intermediate risk, 12 poor risk, and 2 with failed karyotype). Intracellular depletion of arginine was achieved by ADI-PEG 20, whereas while citrulline was increased (supplemental Table 2). There was significantly more caspase activation (n = 38; paired Student t test, P < .0002), more annexin V expression (n = 23; paired Student t test, P = .001) and reduced AML cell numbers (n = 38; paired Student t test, P < .0001) in AML samples exposed to ADI-PEG 20 than AML samples cultured without ADI-PEG 20. The AML samples did not behave homogeneously. Nineteen AML samples showed significant induction of apoptosis as evidenced by caspase activation (and increased expression of annexin V) following ADI-PEG 20 and 19 did not (Figure 4 and Table 1). Of the 19 samples that showed caspase activation, all but 2 showed a significant reduction in the number of live cells after 72 hours culture with ADI-PEG 20. By contrast, none of the 19 samples without caspase activation showed a reduction in live cell numbers. Therefore, ADI-PEG 20 induces apoptosis in approximately half of AML samples in vitro. There was no significant difference in response rates between the different cytogenetic risk groups (P > .3, binomial test).
Apoptosis induction by ADI-PEG 20 in resistant and sensitive AML in vitro. ADI-PEG 20 induced apoptosis in half the AML samples in vitro. The expression of active caspase 3/7 following ADI-PEG 20 administration is shown in the ADI-sensitive (n = 19) and ADI-resistant AMLs (n = 19) as well as GMPB (n = 9) and normal hematopoietic stem cells (HSCs; n = 4) (A). Similar data were observed for annexin-V expression (B) for ADI-PEG 20–sensitive (n = 13) and ADI-PEG 20–resistant AML (n = 10) and GMPB (n = 7). (C) Cell death as defined by 4,6-diamidino-2-phenylindole staining was increased in sensitive AMLs (n = 13) but not resistant AML (n = 10), GMPB (n = 7), or normal HSCs (n = 4). Numbers of viable AML cells were reduced in ADI-PEG 20–sensitive AMLs (n = 19) but not ADI-PEG 20–resistant AML (n = 19) nor GMPB (n = 9) (D). *P < .05
Apoptosis induction by ADI-PEG 20 in resistant and sensitive AML in vitro. ADI-PEG 20 induced apoptosis in half the AML samples in vitro. The expression of active caspase 3/7 following ADI-PEG 20 administration is shown in the ADI-sensitive (n = 19) and ADI-resistant AMLs (n = 19) as well as GMPB (n = 9) and normal hematopoietic stem cells (HSCs; n = 4) (A). Similar data were observed for annexin-V expression (B) for ADI-PEG 20–sensitive (n = 13) and ADI-PEG 20–resistant AML (n = 10) and GMPB (n = 7). (C) Cell death as defined by 4,6-diamidino-2-phenylindole staining was increased in sensitive AMLs (n = 13) but not resistant AML (n = 10), GMPB (n = 7), or normal HSCs (n = 4). Numbers of viable AML cells were reduced in ADI-PEG 20–sensitive AMLs (n = 19) but not ADI-PEG 20–resistant AML (n = 19) nor GMPB (n = 9) (D). *P < .05
We tested whether there was synergy between cytarabine and ADI-PEG 20 in vitro using the method of Chou and Talalay.24 Synergy was seen in 3 of 3 ADI-PEG 20–resistant AMLs (samples 1, 4, and 6) tested with combination indices (CIs) of <0.1, <0.1, and 0.09, respectively (where a CI of <1 indicates synergism). The in vivo data were comparable for samples 1 and 6 (Figure 2G-H). Synergy was seen in 1 of 3 ADI-PEG 20–sensitive AMLs (3, 20, and 24) with CIs of 1.2, 0.63, and 1.4, respectively.
We tested whether normal human hematopoietic cells were sensitive to ADI-PEG 20 using the same coculture system. GMPB from healthy donors was used as a source of normal hematopoietic cells. ADI induced neither caspase (n = 9; paired Student t test, P = .8) nor annexin V expression (n = 7; paired Student t test, P = .9) nor affected cell numbers (n = 9; paired Student t test, P = .8), indicating relative resistance of normal human hematopoiesis (Figure 4). ADI-PEG 20 did not induce apoptosis in normal human bone marrow HSCs (CD34+ CD38− cells) (Figure 4).
ADI-PEG 20–resistant AML cells show increased relative expression of ASS1
In order to test whether those AML samples that resist ADI-PEG 20 might have greater expression of ASS1 and hence be able to generate arginine endogenously, we used real-time PCR to quantify ASS1 expression in AML cells. The AMLs that were resistant to ADI-PEG 20 (n = 18) showed significantly higher relative expression of ASS1 than nonresponders (n = 19; P = .03) (Figure 5A). There was higher expression of ASL in resistant AML also (P = .048) (Figure 5B). These data suggest that resistance is mediated by increased expression of enzymes responsible for endogenous arginine production in some AMLs.
Expression of ASS1 and ASL is higher in ADI-PEG 20–resistant AML and normal HSCs. The relative expression of ASS1 mRNA (A) and ASL mRNA (B) was significantly higher in ADI-PEG 20–resistant AML (n = 18) than in ADI-PEG 20–sensitive AML (n = 19). ASS1 expression was higher in AML (n = 13) and GMPB (n = 4) after in vitro culture following ADI-PEG 20 exposure compared with control (C). Normal human bone marrow HSCs expressed greater amounts of ASS1 than lymphocytes or other marrow cells (CD34− CD3− CD19−) cells (n = 6) (D). (E) The uptake of labeled 13C6 arginine after 10 minutes was greater in sensitive AML (n = 6) than resistant AML (n = 6). The uptake of labeled arginine was significantly reduced by cationic amino acid transporter-1 (CAT-1) inhibitor in sensitive AML cells only. (F) Plasma arginine concentration was measured in patients at diagnosis of AML (n = 15). The arginine concentration was significantly lower than in healthy controls (n = 5). *P < .05.
Expression of ASS1 and ASL is higher in ADI-PEG 20–resistant AML and normal HSCs. The relative expression of ASS1 mRNA (A) and ASL mRNA (B) was significantly higher in ADI-PEG 20–resistant AML (n = 18) than in ADI-PEG 20–sensitive AML (n = 19). ASS1 expression was higher in AML (n = 13) and GMPB (n = 4) after in vitro culture following ADI-PEG 20 exposure compared with control (C). Normal human bone marrow HSCs expressed greater amounts of ASS1 than lymphocytes or other marrow cells (CD34− CD3− CD19−) cells (n = 6) (D). (E) The uptake of labeled 13C6 arginine after 10 minutes was greater in sensitive AML (n = 6) than resistant AML (n = 6). The uptake of labeled arginine was significantly reduced by cationic amino acid transporter-1 (CAT-1) inhibitor in sensitive AML cells only. (F) Plasma arginine concentration was measured in patients at diagnosis of AML (n = 15). The arginine concentration was significantly lower than in healthy controls (n = 5). *P < .05.
The expression of ASS1 was not significantly different in GMPB compared with ADI-PEG 20–resistant AML (Figures 1D and 5A), and this may explain why GMPB is resistant to ADI-PEG 20. Although the mean ASS1 expression was higher in GMPB than sensitive AML, this did not reach statistical significance (P = .15), and other mechanisms may be at play.
We compared the expression of ASS1 in AML cells with a leukemic stem cell phenotype (CD34+ CD38−) with the bulk population of AML cells in 10 CD34-positive AMLs. There was no significant difference in ASS1 expression between the phenotypically defined leukemia stem cells (LSCs) and the bulk of AML cells (relative expression of ASS1 in bulk AML population = 16.4 ± 13.9 vs 7.2 ± 4 in LSCs; P = .5).
We looked at expression of ASS1 after culture (72 hours) of AML samples (n = 13) with or without ADI-PEG 20 to test whether the malignant cells might adapt to arginine deprivation. ASS1 levels were significantly increased in those samples cultured with ADI-PEG 20 (Figure 5C), and similar changes were seen in normal GMPB cells, indicating that hematopoietic cells can upregulate the enzyme that synthesizes arginine in response to exposure to ADI-PEG 20.
Normal human bone marrow HSCs are enriched for ASS1 expression
Although <20% of human bone marrow cells expressed ASS1, using immunohistochemistry it was clear that some cells did express ASS1. To determine which normal hematopoietic cells express ASS1 in human bone marrow, we sorted HSCs (CD34+ CD38−), progenitors (CD34+ CD38+), lymphocytes (CD3+ and CD19+), and other cells (CD34− CD3− CD19−) and assessed ASS1 mRNA expression. Cells with an HSC phenotype expressed ASS1 at a higher level than the other fractions (Figure 5D). Furthermore, both normal bone marrow HSC and progenitor populations expressed higher levels of ASS1 than ADI-PEG 20–sensitive AML (P < .02 for each) (Figure 5A,D). This difference between the normal stem-progenitor cells and the sensitive AMLs should allow ADI-PEG 20 to be relatively selective.
ADI-PEG 20–sensitive AML have increased uptake of extracellular arginine via CAT-1
AML samples auxotrophic for arginine would be expected to take up more arginine from extracellular sources than nonauxotrophic AML samples. To test this we measured the uptake of labeled 13C6 arginine into AML cells.25 Significantly more labeled arginine was found inside ADI-PEG 20–sensitive AMLs cells than inside resistant AML cells (Figure 5E). To test whether the increased uptake of arginine is because of the CAT-1, we inhibited CAT-1 using N-ethylmaleimide.25 The CAT-1 inhibitor reduced the uptake of labeled arginine into sensitive but not resistant AML (Figure 5E), consistent with increased uptake of arginine via CAT-1 in ADI-PEG 20–sensitive AMLs.
AML-derived arginase depletes plasma of arginine but not as effectively as ADI-PEG 20
Recent data suggest AML secretes arginase in order to impair T-cell function,26 but depletion of arginine might be expected to kill some AML cells based on the data presented previously. In the work of Mussai and colleagues, arginase activity was increased, but blood arginine levels were not measured directly.26 To test whether AML actually depletes plasma arginine and to what degree, we quantified arginine in plasma from patients with AML at presentation as well as healthy controls.
Plasma arginine levels were significantly reduced in AML consistent with work showing arginase secretion by AML (Figure 5F). However, the degree of arginine depletion induced by arginase secreted by AML was much less than is seen following ADI-PEG 20 administration. Plasma arginine concentration in AML patients is 18.2 ± 2.7 μg/mL, whereas levels are <0.3 μg/mL following ADI-PEG 20 administration in humans.10 This suggests that AML can tolerate a degree of arginine depletion because of its own arginase but not severe depletion as induced by ADI-PEG 20.
Discussion
We demonstrate here that many AMLs are deficient in ASS1, one of the key enzymes in endogenous arginine synthesis. Because of this, the AML cells are vulnerable to arginine deprivation. ADI-PEG 20 alone induced a response in half the AML samples tested in vitro and in vivo. Responses to ADI-PEG 20 were seen in good-, intermediate-, and poor-risk cytogenetic AML. There was no significant difference between the response rate and the cytogenetic risk group. The relative ASS1 expression was, however, higher in AML samples that were resistant to ADI-PEG 20. This suggests that the mechanism of resistance may be through increased endogenous synthesis of arginine. The other enzyme in the arginine synthesis pathway, ASL, was also more highly expressed in ADI-PEG 20–resistant AMLs. ADI-PEG 20–sensitive AMLs showed increased uptake of extracellular arginine mediated via CAT-1 supporting the hypothesis that these AMLs are auxotrophic for arginine.
The expression of ASS1 in normal and leukemic cells was higher after in vitro culture with ADI-PEG 20 (Figure 5C). This suggests the cells are adapting to arginine deprivation by enhancing their capacity to synthesize endogenous arginine, although we cannot exclude the possibility that the ASS1-expressing cells have been selected for. ASS1 upregulation in response to arginine deprivation has been reported previously in melanoma cell lines.27 ASS1 upregulation might lead to resistance following prolonged arginine deprivation; however, this adaption may impair tumor growth because overexpression of ASS1 slows tumor growth in vitro.8 Thus, although ASS1 upregulation may provide a means for tumors to survive arginine deprivation, a by-product of this adaption may be slower growth of the surviving tumor cells. Therefore, even if arginine deprivation does not kill tumor cells, it may slow their growth. Consistent with this, sarcoma cell lines showed reduced cycling in response to ADI-PEG 20 therapy.7
ASS1 was not detectable in most ADI-PEG 20–resistant AML cells using immunohistochemistry or western blotting (Figure 1) despite having relatively high mRNA expression. There was a better correlation between the expression of ASS1 mRNA and response to ADI-PEG 20 than there was between ASS1 expression tested by western blotting and response to ADI-PEG 20 (supplemental Figure 6). There were 16 AML samples in which we have data on both ASS1 expression by western blotting and response to ADI-PEG 20 data in vitro. Two of 16 samples expressed ASS1 by western blotting and were resistant, but 6 of 16 samples were negative for ASS1 by western blotting but resistant to ADI-PEG 20. By contrast, 15 of 18 resistant AML samples had relative ASS1 mRNA expression of >1, whereas only 1 of 19 sensitive AMLs did. These data suggest the antibody based technique is less sensitive than real-time PCR as a marker of functional arginine synthesizing capacity. Relative ASS1 mRNA expression appears to be a promising potential biomarker for clinical trials of arginine deprivation in AML. Studies in other malignancies have shown a similar correlation between ASS1 expression and sensitivity to arginine deprivation.8,9
The leukemic stem cell compartment did not show higher expression of ASS1 than the bulk of AML cells. By contrast, there was heterogeneity of ASS1 expression in normal human bone marrow cells. The normal HSCs expressed higher levels than more differentiated CD34− cells. This may explain why patients with solid tumors who receive ADI-PEG 20 can develop moderate neutropenia but tend not to develop more extensive hematopoietic toxicity.10,28 Critically, the normal HSCs and progenitors populations both expressed higher ASS1 levels than ADI-PEG 20–sensitive AMLs and this should allow a selective therapeutic effect.
Our data suggest that ADI-PEG 20 induces apoptosis via caspase activation in AML in vitro. Both annexin-V expression and caspase 3/7 activity were increased in sensitive AMLs and this correlated with viable cell numbers. Apoptosis induction following arginine deprivation has been observed in breast cancer and lymphoma cell lines.9,21 In breast and prostate cancer cell lines, ADI-PEG 20 led to cell death by causing mitochondrial dysfunction, a process that was dependent on autophagy.9,29 Further studies are required to delineate the full mechanism of apoptosis induction in AML.
We compared the effect of ADI-PEG 20 on AML in vivo with that of cytarabine chemotherapy. Two of 6 AMLs responded better to ADI-PEG 20, and 2 of 6 AMLs responded better to cytarabine. This suggests that cytarabine and ADI-PEG 20 may be working by different pathways. In support of this all 6 AMLs responded to the combination of ADI-PEG 20 and cytarabine, even when 2 of the 6 AMLs did not respond to either agent alone. Of note, these 6 AMLs had adverse genetic or cytogenetic features. Furthermore, synergism between cytarabine and ADI-PEG 20 was demonstrated in vitro. These data support a clinical trial of combination ADI-PEG 20 and cytarabine in AML.
We have shown that arginine depletion can negatively affect the growth of AML, yet paradoxically AML produces arginase.26 ADI-PEG 20 produces greater systemic depletion of arginine than AML-derived arginase as evidenced by our data based on measurements of plasma arginine concentration in newly diagnosed AML patients. This suggests that there is a threshold level of arginine, below which the AML cells suffer a growth disadvantage.
Alternative enzymic methods for arginine depletion exist; a recent publication used arginase I cobalt to deplete arginine in the treatment of leukemia cell lines.30 This arginase may be less immunogenic than ADI-PEG 20 as it is a human protein. However, arginase I cobalt has a narrow therapeutic window, causing bone marrow necrosis and death in mice at higher doses.31 The critical question will be whether arginase I cobalt is able to reduce arginine in human plasma to below the threshold level to induce AML cell killing while avoiding significant toxicity.
In conclusion, we have provided evidence that ADI-PEG 20 has activity against many primary AML cells in vitro and in vivo and that assessment of relative ASS1 mRNA levels may be useful to predict who responds. These data provide a strong rationale for testing ADI-PEG 20 in clinical trials in combination with standard chemotherapy.
Presented in abstract form at the 55th annual meeting of the American Society for Hematology, New Orleans, LA, December 2013.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chathunissa Gnanaranjan and Phuong Luong for additional technical support and Prof Art Frankel and Dr Mike Potter for helpful discussions.
This work was supported by a grant from Leukaemia Lymphoma Research (grant 12059) (D.C.T.) and by Cancer Research UK (D.B.) and the National Institute for Health Research RM/ICR Biomedical Research Centre. This study was supported in part by research funding from Polaris Pharmaceuticals Inc. (P.W.S.).
Authorship
Contribution: F.M.-M., E.G., J.S.B., J.G.G., P.W.S., D.B., and D.C.T. designed the research, analyzed the data, and wrote the manuscript; J.C. wrote the manuscript; F.M.-M., E.G., L.A.-M., K.A.H., A.C., F.A.-A., K.L., M.G., and F.S. performed the experiments.
Conflict-of-interest disclosure: D.C.T. received funding from Polaris Pharmaceuticals to attend the American Society of Hematology annual meeting. P.W.S. is a recipient of research funding support from Polaris Pharmaceuticals Inc. J.S.B. is an employee of Polaris Group and holds stock options in Polaris Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Correspondence: David C. Taussig, Haemato-Oncology, Royal Marsden Hospital, Downs Rd, Sutton, Surrey, SM2 5PT, United Kingdom; e-mail: d.taussig@qmul.ac.uk.
References
Author notes
F.M.-M., E.G.. and L.A.-M. contributed equally to this study.
D.B., P.W.S., and D.C.T. contributed equally to this study.

![Figure 3. ASS1-expressing normal hematopoietic cells are seen following suc-cessful AML clearance by ADI-PEG 20. Bone marrow sections from mice transplanted with AML sample 3 following treatment with vehicle or ADI-PEG 20 were stained with hematoxylin and eosin to assess residual hematopoiesis. The majority of cells in mice receiving vehicle were leukemic (A), whereas normal hematopoietic cells, including megakaryocytes (arrowed), are seen in mice treated with ADI-PEG 20 (B). ASS1 expression was lower in mice receiving vehicle (C) than mice receiving ADI-PEG 20 (D). Slides were analyzed using a Leica DM2500 microscope (magnification ×400 [A-B], ×200 [C-D]), and images were acquired by a Leica DFC320 camera and processed using Leica IM50 software. ASS1 was not expressed by AML cells (E) but was expressed by nonhuman marrow cells in mice treated with ADI-PEG 20 (F). Images were captured on an LSM510 Meta confocal laser microscope using a 40 × 1.3 oil immersion objective.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/26/10.1182_blood-2014-10-608133/4/m_4060f3.jpeg?Expires=1765939187&Signature=OwFU7bTKKz8ehC~3UPKC3ZsP~dZDl4MB1g3tjX3PJNPdHsg2nbGp-LVCwf-1yZS9pTiLeHvf5GzxIADA25Lw2oegSp0RVRywNNvIEjPiOX69FArQFch-T8C51LT4d~Ob-fG67mKbMD4P7z2JZfWJ-RdqWH-t0JTpPYa0wGK4Vs0MXoUhPvXOq9Nfds7SmLPJsskzFa8jX0ROmZyClEHGWE6~rQzPWaYKILHpXgn0HmHvxszH6U8tzA6SB~j9DxZW77sgVcaAvrQ-uIpCwPv7PZuQPz44bXGNLfOx2yfwt5DBQRQI29Sw0iPrh9~XDJu58og6b5ricUGU65buouuXaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

