Key Points
MYXV binds human T lymphocytes but does not enter and infect T cells until after activation.
MYXV-infected T lymphocytes proliferate less and secrete less inflammatory cytokines but deliver oncolytic virus to augment GVM.
Abstract
Allogeneic hematopoietic cell transplant (allo-HCT) can be curative for certain hematologic malignancies, but the risk of graft-versus-host disease (GVHD) is a major limitation for wider application. Ideally, strategies to improve allo-HCT would involve suppression of T lymphocytes that drive GVHD while sparing those that mediate graft-versus-malignancy (GVM). Recently, using a xenograft model, we serendipitously discovered that myxoma virus (MYXV) prevented GVHD while permitting GVM. In this study, we show that MYXV binds to resting, primary human T lymphocytes but will only proceed into active virus infection after the T cells receive activation signals. MYXV-infected T lymphocytes exhibited impaired proliferation after activation with reduced expression of interferon-γ, interleukin-2 (IL-2), and soluble IL-2Rα, but did not affect expression of IL-4 and IL-10. MYXV suppressed T-cell proliferation in 2 patterns (full vs partial) depending on the donor. In terms of GVM, we show that MYXV-infected activated human T lymphocytes effectively deliver live oncolytic virus to human multiple myeloma cells, thus augmenting GVM by transfer of active oncolytic virus to residual cancer cells. Given this dual capacity of reducing GVHD plus increasing the antineoplastic effectiveness of GVM, ex vivo virotherapy with MYXV may be a promising clinical adjunct to allo-HCT regimens.
Introduction
Allogeneic hematopoietic cell transplant (allo-HCT) can be curative for patients with certain hematologic malignancies. However, graft-versus-host disease (GVHD) remains a major challenge after allo-HCT.1-3 An increasing number of experimental GVHD prophylaxis efforts have exploited T-cell depletion strategies.4-7 Unfortunately, these approaches delay the time to donor engraftment, increase risk for disease relapse, and increase risk for opportunistic infections.
Recently, we discovered that ex vivo virotherapy with the oncolytic poxvirus, myxoma virus (MYXV), selectively targets malignant human hematopoietic cells like acute myeloid leukemia and multiple myeloma, while sparing normal human hematopoietic stem and progenitor cells.8-10 MYXV is a viral oncolytic agent that is nonpathogenic to humans and mice but has natural tropism for a variety of human cancers.11-13 In the course of developing MYXV as an ex vivo purging agent for transplant, we serendipitously discovered that NSG mice receiving human HCT xenografts treated ex vivo with MYXV developed no GVHD, lived longer, and yet still exhibited robust human hematopoietic engraftment in the recipient bone marrow.14 We hypothesized that MYXV impaired the GVHD capacity of alloreactive donor T lymphocytes. To test this prediction and dissect mechanisms by which MYXV suppresses GVHD, we examined human T-lymphocyte responses after MYXV exposure.
Methods
Virus binding and infection conditions
MYXV virion binding to cells was carried out by incubating resting human T cells with vMyx-Venus/M093L at a multiplicity of infection (MOI) of 10 for 1 hour on ice.15 MYXV infections were performed by incubating human resting or activated T cells with vMyx–green fluorescent protein (GFP)16 or vMyx-GFP/tomato red fluorescent protein (TrFP)17 (at MOI = 10) for 1 hour at room temperature. For both binding and infection, mock-treated cells were incubated in complete media containing no virus under the same incubation conditions. Furthermore, heat- and UV-inactivated vMyx-GFP were used as controls to assess whether virus replication competency is needed for the inhibition of T-cell proliferation (for details, see supplemental Methods, available on the Blood Web site).
Proliferation analysis and 1-way MLR assays
Isolated human CD3+ T cells were first labeled using the CellTrace violet (CTV) cell proliferation kit (Invitrogen), as per the manufacturer’s recommendations (see supplemental Methods for details). Next, T cells were either mock-treated, or infected with vMyx-GFP (MOI = 10), and plated in a 96-well round-bottom plate. Then, cells were either stimulated (ie, by adding anti-CD3/CD28–coated microbeads) or left unstimulated. Cells were cultured in a humidified chamber at 37°C and 5% CO2, during 72 or 96 hours. Proliferation of T cells was evaluated using flow cytometry (see supplemental Methods for details). One-way mixed lymphocyte reaction (MLR) assays were performed using mononuclear cells (MNCs) derived from peripheral blood mononuclear cells (PBMCs) or cord blood (CB) from healthy donors (see supplemental Methods for details).18,19
Graft-versus-malignancy assays
Mock-treated or MYXV-treated T lymphocytes (either unstimulated or anti-CD3/CD28 activated) were cultured for 48 hours at 37°C, 5% CO2. At this point, the human multiple myeloma cell line U266, was mixed with the T cells at a ratio of 1:1, and this mixture was cultured for an additional 48 hours at 37°C, 5% CO2. Multiple myeloma (MM) cell infection was analyzed by analyzing GFP+ fluorescence in CD138+ cells using direct microscopy and flow cytometry (see supplemental Methods for details).
Results
MYXV binds to human T lymphocytes but stimulation of T lymphocytes is required for productive infection
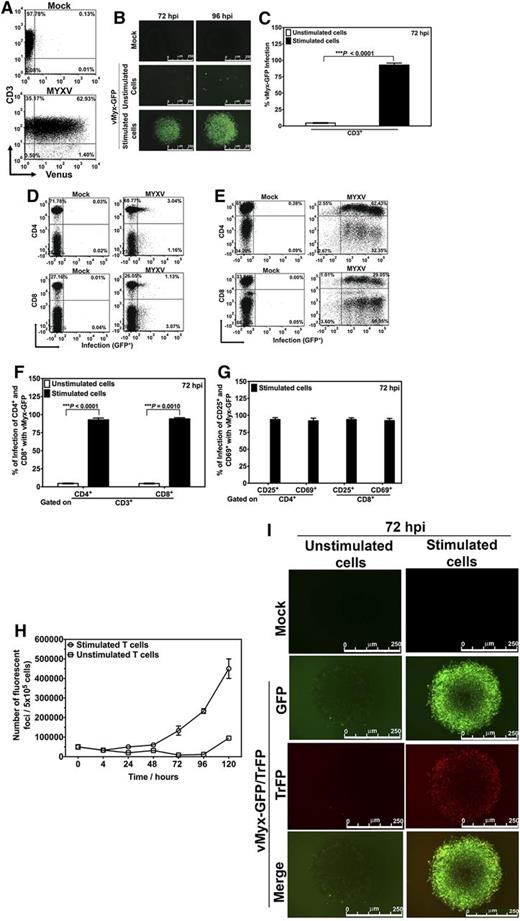
Our first question was whether MYXV can bind or infect resting human T lymphocytes. Primary human CD3+ T cells, isolated from healthy donor peripheral blood, were incubated with fluorescently labeled MYXV (vMyx-Venus/M093L15 ) for 1 hour. After 1-hour adsorption, the T cells were washed of free virus and then analyzed by flow cytometry for evidence of MXYV binding. The T lymphocytes showed Venus-tagged MYXV binding (Figure 1A), ranging from 13.00% to 62.93% that varied by donor (supplemental Table 1). Because the lower limit of sensitivity of this binding assay with Venus-tagged MYXV is ∼500 virus particles per cell, these binding percentages are likely underestimations of the actual percentage of T lymphocytes with bound MYXV.
MYXV binds to unstimulated human T lymphocytes but activation of human T lymphocytes is required for MYXV replication. (A) To investigate whether MYXV binds to unstimulated human T lymphocytes, T cells were isolated using an EasySep negative selection HLA T cell enrichment kit (up to >95% purity). Approximately 1 × 106 of these negatively isolated T lymphocytes were incubated with recombinant vMyx-Venus/M093L at an MOI of 10 for 1 hour on ice to allow virus binding but not entry. After this, unbound virus was washed twice with cold 1× PBS + 5% FBS. T cells were then stained with anti-CD3 antibody, and the levels of Venus+ labeling in the CD3+ population were determined by flow cytometry (bottom panel). A representative experiment from 1 donor is shown. To investigate virus infection, ∼3-4 × 106 isolated human T lymphocytes were incubated with recombinant vMyx-GFP at an MOI of 10 for 1 hour at room temperature to allow virus adsorption. After this, mock-treated and infected T cells were stimulated with anti-CD3/CD28 beads at a cell:bead ratio of 1:1. This was followed by incubation at 37°C for 72 hours or 96 hours. The unstimulated (ie, without adding beads) mock- and MYXV-treated T lymphocytes were subjected to the same culturing conditions. (B) Seventy-two and 96 hours after culturing, expression of virus-expressed GFP was monitored using fluorescence microscopy. (C-F) To quantify the levels of infection of different T populations, 72 hours after vMyx-GFP exposure, cells were stained with antibodies against CD3, CD4, and CD8, and the levels of GFP+ in each population were quantified by using flow cytometry. Likewise, in panel G, levels of infection of T lymphocyte activation proteins CD25 and CD69 were also quantified using flow cytometry. Data reported are representative of al least 6 independent experiments. Significance (ie, P < .05) was determined using the Student t test. To investigate whether MYXV launches productive virus replication in stimulated human T lymphocytes, we performed 1-step growth curves. (H) T cells were infected with vMyx-GFP at an MOI of 10. Infected/unstimulated T cells, and infected/stimulated T cells, were harvested, cells were lysed using repeated freeze-thaw, and the amount of infectious virus in each sample was quantified using foci formation on BSC40 cells. (I) Stimulated or unstimulated T cells were infected with recombinant vMyx-GFP/TrFP at an MOI of 10. Expression of GFP (expressed at both early and late times postinfection) and TrFP (expressed only at late stages of virus infection) was determined 72 hours after infection using fluorescence microscopy. FBS, fetal bovine serum; PBS, phosphate-buffered saline.
MYXV binds to unstimulated human T lymphocytes but activation of human T lymphocytes is required for MYXV replication. (A) To investigate whether MYXV binds to unstimulated human T lymphocytes, T cells were isolated using an EasySep negative selection HLA T cell enrichment kit (up to >95% purity). Approximately 1 × 106 of these negatively isolated T lymphocytes were incubated with recombinant vMyx-Venus/M093L at an MOI of 10 for 1 hour on ice to allow virus binding but not entry. After this, unbound virus was washed twice with cold 1× PBS + 5% FBS. T cells were then stained with anti-CD3 antibody, and the levels of Venus+ labeling in the CD3+ population were determined by flow cytometry (bottom panel). A representative experiment from 1 donor is shown. To investigate virus infection, ∼3-4 × 106 isolated human T lymphocytes were incubated with recombinant vMyx-GFP at an MOI of 10 for 1 hour at room temperature to allow virus adsorption. After this, mock-treated and infected T cells were stimulated with anti-CD3/CD28 beads at a cell:bead ratio of 1:1. This was followed by incubation at 37°C for 72 hours or 96 hours. The unstimulated (ie, without adding beads) mock- and MYXV-treated T lymphocytes were subjected to the same culturing conditions. (B) Seventy-two and 96 hours after culturing, expression of virus-expressed GFP was monitored using fluorescence microscopy. (C-F) To quantify the levels of infection of different T populations, 72 hours after vMyx-GFP exposure, cells were stained with antibodies against CD3, CD4, and CD8, and the levels of GFP+ in each population were quantified by using flow cytometry. Likewise, in panel G, levels of infection of T lymphocyte activation proteins CD25 and CD69 were also quantified using flow cytometry. Data reported are representative of al least 6 independent experiments. Significance (ie, P < .05) was determined using the Student t test. To investigate whether MYXV launches productive virus replication in stimulated human T lymphocytes, we performed 1-step growth curves. (H) T cells were infected with vMyx-GFP at an MOI of 10. Infected/unstimulated T cells, and infected/stimulated T cells, were harvested, cells were lysed using repeated freeze-thaw, and the amount of infectious virus in each sample was quantified using foci formation on BSC40 cells. (I) Stimulated or unstimulated T cells were infected with recombinant vMyx-GFP/TrFP at an MOI of 10. Expression of GFP (expressed at both early and late times postinfection) and TrFP (expressed only at late stages of virus infection) was determined 72 hours after infection using fluorescence microscopy. FBS, fetal bovine serum; PBS, phosphate-buffered saline.
We next questioned whether MYXV actively infects these human T lymphocytes using a vMyx-GFP that expresses GFP encoded in the viral genome and driven by a synthetic early/late viral promoter, so that the very earliest stages of virus replication can be monitored by the expression of GFP. When human T lymphocytes were in an unstimulated state, MYXV initiated its infection cycle in only a very small fraction of the T cells by 72 or 96 hours after incubation (Figure 1B middle panels). In contrast, upon stimulation of T cells with anti-CD3/CD28 beads, the GFP-tagged MYXV infected the activated T cells at much higher levels (GFP+) (Figure 1B bottom panels). Together, these data show that MYXV binds resting human T cells, but enters and initiates infection only after T-cell activation. In contrast, in the absence of stimulation, there is a very early block in MYXV replication prior to early viral gene expression. Importantly, nearly 100% of the activated T cells became infected with GFP-tagged MYXV (Figure 1C), confirming that some input virus was initially bound to essentially all of the available T cells in the culture, regardless of whether the donor exhibited high (50%-60%) or low (10%-20%) binding levels of input Venus-tagged virus.
These results were confirmed with flow cytometric analysis. In all cases, after 72 hours, MYXV successfully infected over 90% of stimulated T cells (Figure 1E) as compared with unstimulated T cells (Figure 1D). Infection of CD4+ T cells and CD8+ T cells by MYXV were similar (Figure 1F). Interestingly, 2-dimensional (2D) plots of infected and stimulated T cells showed different subpopulations and levels of infection of T cells, as assessed by GFP intensities (right panels of Figure 1E). Within activated lymphocyte subsets, 94% of CD25+ cells were infected with MYXV and 92% of CD69+ cells were infected (Figure 1G; supplemental Table 2). Thus, we observe no particular bias among the various subclasses of CD3+ T lymphocytes, in terms of their ability to become infected by MYXV following cell activation with anti-CD3/CD28.
MYXV replication generates low levels of progeny virus in stimulated human T lymphocytes
To determine whether the viral replication cycle was completed with the concomitant production of new infectious progeny virus, we performed single step viral growth analysis (Figure 1H). To do this, unstimulated or stimulated T cells were incubated with MYXV, washed, sampled at serial time points, pelleted, and harvested. The infectious virus was released by sequential freeze-thaw, and the titer of live virus in each sample was determined as previously described.20 Notably, we observed that even though MYXV effectively initiated infection in stimulated T lymphocytes, only low amounts of new viral progeny were produced by the activated T cells. As expected, in unstimulated T lymphocytes essentially no new viral progeny were detected. These data show that MYXV is unable to replicate in resting T cells but shows a limited capacity to productively replicate in activated cells and generate progeny virus.
To confirm these results, we performed fluorescence microscopy analysis (Figure 1I) following incubation with vMyx-GFP/TrFP, a recombinant MYXV expressing both GFP driven by a synthetic early/late viral promoter and TrFP driven by poxvirus late viral promoter. Thus, the successful progression of the virus replication from early to late times can be monitored by the progression of infected cells from green (GFP+) to green plus red (GFP+ + TrFP+). We found that MYXV replication efficiently progresses in stimulated T lymphocytes from early stages (ie, GFP+) to the late viral stages (ie, TrFP+). Our conclusion is that all stages of viral replication occur in stimulated T cells, but the extent of final progeny virus assembly is somewhat less efficient than in fully permissive mammalian cells (such as rabbit cells or many classes of human cancer cells).
Inhibition of activation-induced T-lymphocyte proliferation by MYXV infection
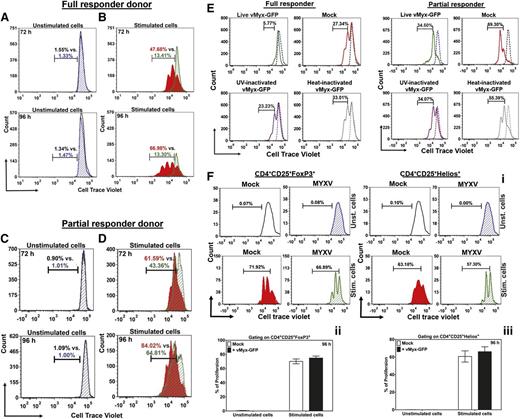
We next analyzed the impact of this infection on lymphocyte proliferation in response to the T-cell activation signals. As expected, unstimulated donor human T lymphocytes, whether mock-treated or MYXV-treated, showed no proliferation after 72 hours or 96 hours in culture (Figure 2A,C). Stimulated T cells that were mock-treated showed the expected increase in cell proliferation at 72 and 96 hours (Figure 2B red histograms). But, when stimulated T cells were MYXV-treated, 2 different proliferation patterns were found depending on the donor: some donors were classified as “full responders” because MYXV completely inhibited the activation-induced proliferation of their T lymphocytes (Figure 2B green histograms). Interestingly, for these full responders, MYXV’s inhibitory effect on activation-induced proliferation was unchanged over time. Some normal donors, however, were classified as “partial responders” because MYXV decreased but did not fully suppress the proliferation of stimulated T cells (Figure 2D). These data (Figure 2; supplemental Figure 1A-D; and supplemental Table 3) support the concept that although MYXV mitigates a proliferative response for all donors tested (N = 8), the extent of antiproliferative effects (ie, full vs partial) is donor dependent.
MYXV impairs activation-induced proliferation of human effector T lymphocytes. To determine whether MYXV can impair the postactivation functions of T lymphocytes, the levels of cell proliferation of stimulated T lymphocytes were assessed using flow cytometry. T cells were preloaded with the tracking dye CTV at 37°C for 20 minutes and then either mock-treated or incubated with ± anti-CD3/CD28 microbeads as described in “Methods.” T lymphocytes were then incubated in a humidified chamber at 37°C, and 5% CO2 for 72 hours or 96 hours to allow for the proliferation of stimulated T cells. At the indicated time points, cells were stained for CD3, CD4, CD8, CD25, and CD69. FCS-Express version 4 was used to analyze the characteristic subpopulations of dividing lymphocytes, and to determine the percentage of proliferation, the proliferation index (PI) and the division index (DI) (see supplemental Methods for a detailed description). (A) Histograms showing populations of mock-treated T lymphocytes (black outline) and MYXV-treated T lymphocytes (blue diagonal) in unstimulated conditions at 72 and 96 hours. The histograms reveal no CTV shift to the left, indicating low numbers in proliferation, and complete overlap when treated with MYXV, indicating no effect of MYXV on lymphocyte proliferation of unstimulated T cells. (B) In stimulated conditions, the mock-treated T lymphocytes (red) proliferate as evidence by leftward CTV stain shifting of the population. However, the MYXV-treated T cells (green diagonal) remains unchanged, indicating full suppression of T cell proliferation. This case is representative of a full responder donor. (C) Control treatments, in unstimulated conditions, showing lack of T-cell proliferation in a partial responder donor. (D) Under stimulated conditions with a partial responder donor, the mock-treated T lymphocytes (red) proliferate as evidence by leftward shifting of the CTV-stained population. However, the MYXV-treated T cells (green diagonal) exhibit an intermediate CTV shifted pattern, indicating partial suppression proliferation by MYXV. (E) To determine whether live virus is needed to suppress the proliferation of T cells, T lymphocytes were incubated with live vMyx-GFP, heat-inactivated vMyx-GFP, or UV-inactivated vMyx-GFP at an equivalent MOI = 10 and ± anti-CD3/CD28 beads. After 72 hours, the proliferation of CTV-tagged T cells was evaluated using flow cytometry. Data indicate that proliferation of T cells is suppressed only in the presence of live MYXV. In contrast, inactivated MYXV did not affect the activation-induced proliferation of T cells. (F) To investigate whether MYXV can affect Tregs, proliferation of nTregs was evaluated using flow cytometry. (i) Histograms of 1 representative donor, showing the proliferation patterns of CTV-tagged CD4+CD25+FoxP3+ (left panels) and CD4+CD25+Helios+ (right panels). (ii-iii) Summaries of the percentage of proliferation of CTV-tagged CD4+CD25+FoxP3+ and CD4+CD25+Helios+, respectively, of different donors (N = 4).
MYXV impairs activation-induced proliferation of human effector T lymphocytes. To determine whether MYXV can impair the postactivation functions of T lymphocytes, the levels of cell proliferation of stimulated T lymphocytes were assessed using flow cytometry. T cells were preloaded with the tracking dye CTV at 37°C for 20 minutes and then either mock-treated or incubated with ± anti-CD3/CD28 microbeads as described in “Methods.” T lymphocytes were then incubated in a humidified chamber at 37°C, and 5% CO2 for 72 hours or 96 hours to allow for the proliferation of stimulated T cells. At the indicated time points, cells were stained for CD3, CD4, CD8, CD25, and CD69. FCS-Express version 4 was used to analyze the characteristic subpopulations of dividing lymphocytes, and to determine the percentage of proliferation, the proliferation index (PI) and the division index (DI) (see supplemental Methods for a detailed description). (A) Histograms showing populations of mock-treated T lymphocytes (black outline) and MYXV-treated T lymphocytes (blue diagonal) in unstimulated conditions at 72 and 96 hours. The histograms reveal no CTV shift to the left, indicating low numbers in proliferation, and complete overlap when treated with MYXV, indicating no effect of MYXV on lymphocyte proliferation of unstimulated T cells. (B) In stimulated conditions, the mock-treated T lymphocytes (red) proliferate as evidence by leftward CTV stain shifting of the population. However, the MYXV-treated T cells (green diagonal) remains unchanged, indicating full suppression of T cell proliferation. This case is representative of a full responder donor. (C) Control treatments, in unstimulated conditions, showing lack of T-cell proliferation in a partial responder donor. (D) Under stimulated conditions with a partial responder donor, the mock-treated T lymphocytes (red) proliferate as evidence by leftward shifting of the CTV-stained population. However, the MYXV-treated T cells (green diagonal) exhibit an intermediate CTV shifted pattern, indicating partial suppression proliferation by MYXV. (E) To determine whether live virus is needed to suppress the proliferation of T cells, T lymphocytes were incubated with live vMyx-GFP, heat-inactivated vMyx-GFP, or UV-inactivated vMyx-GFP at an equivalent MOI = 10 and ± anti-CD3/CD28 beads. After 72 hours, the proliferation of CTV-tagged T cells was evaluated using flow cytometry. Data indicate that proliferation of T cells is suppressed only in the presence of live MYXV. In contrast, inactivated MYXV did not affect the activation-induced proliferation of T cells. (F) To investigate whether MYXV can affect Tregs, proliferation of nTregs was evaluated using flow cytometry. (i) Histograms of 1 representative donor, showing the proliferation patterns of CTV-tagged CD4+CD25+FoxP3+ (left panels) and CD4+CD25+Helios+ (right panels). (ii-iii) Summaries of the percentage of proliferation of CTV-tagged CD4+CD25+FoxP3+ and CD4+CD25+Helios+, respectively, of different donors (N = 4).
Additionally, we found that live virus is required for the suppression of proliferation of stimulated T cells (Figure 2E). In contrast, when cells were treated with the inactivated viruses, the T cells proliferate similarly to mock-treated stimulated cells (Figure 2E).
Proliferation of naturally occurring regulatory T cells (nTregs) of at least 4 healthy donors was also evaluated and revealed that MYXV does not suppress nTregs as shown in the histograms (data of 1 representative donor) (Figure 2F left and right panels) and the bar graphs (CD4+CD25+FoxP3+: 74.84% ± 2.79%; CD4+CD25+Helios+: 66.13% ± 5.56%, MYXV-treated cells) as compared with mock controls (CD4+CD25+FoxP3+: 70.14% ± 3.41%; CD4+CD25+Helios+: 60.69% ± 6.38%) (Figure 2Fii-iii).
To investigate whether MYXV affects the differentiation of T lymphocytes, T cells of 4 different donors were mock-treated, or MYXV-treated ± anti-CD3/CD28 stimulation. Quantification of intracellular transcription factors and proliferation of Tregs of 4 different donors, each one in duplicate, was determined using flow cytometry. Compared with mock treatment, MYXV treatment reduced differentiation into Th1 (Tbet-expressing) cells (mock: 63.57% ± 6.59%, vs MYXV: 40.21% ± 6.50%, not significant [NS]), Th2 (GATA3-expressing) cells (mock: 62.66% ± 8.54%, vs MYXV: 57.04% ± 0.16%, NS), and Th17 (RORγt-expressing) cells (mock: 88.70% ± 5.97%, vs MYXV: 73.52% ± 4.16%, P = .05) (supplemental Figure 2). Together, the results indicate a preferential attenuation of Th1 polarization and Th17, with relatively low attenuation of Th2, and Treg differentiation.
MYXV infection of activated human T cells affects viability
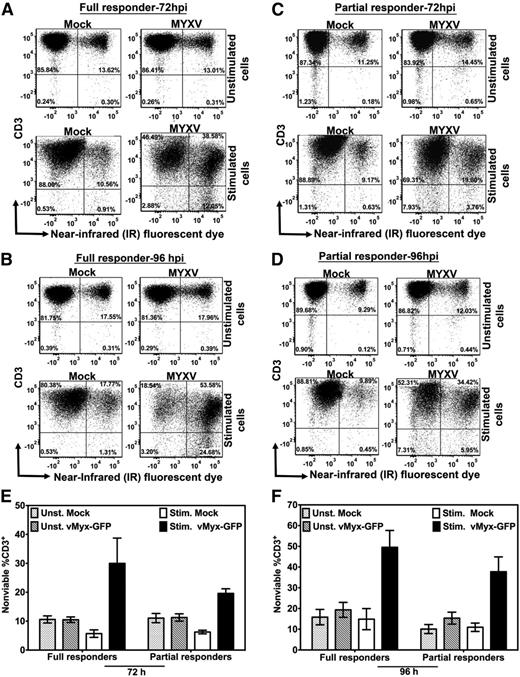
MYXV infection plus stimulation with anti-CD3/CD28 beads resulted in increased percentage of nonviable T lymphocytes for all donors tested (Figure 3). Specifically, this additive effect was observed in T-cell samples from either full responders (Figure 3A-B) or from partial responders (Figure 3C-D), suggesting that such augmented decline in T-cell viability is donor independent (Figure 3E-F). Even though a slightly higher frequency of cell death was observed as a trend for full responders vs partial responders infected with MYXV and stimulated with anti-CD3/CD28 beads, it was not statistically significant (ie, at 72 hours after culturing: 30.00% ± 8.73% full responders vs 19.59% ± 1.60% partial responders, P = .32; and at 96 hours after culturing: 49.54% ± 8.01% full responders vs 37.73% ± 7.14% partial responders, P = .12). On the other hand, unstimulated T cells mock-treated or MYXV-treated, exhibited low and very similar frequencies of cell death (Figure 3E-F).
MYXV infection and stimulation of human T cells reduces their viability. Cell death of T cells was evaluated 72 hours and 96 hours after mock-treatment or MYXV treatment, and ± anti-CD3/CD28 stimulation, using flow cytometry. To assess cell viability, T cells were labeled with the live/dead near-infrared (IR) fluorescent dye, an amine-reactive dye that binds covalently to intracellular and extracellular amines, generating a bright signal that allows the distinction between live/dead cells in a single channel. In addition, the staining pattern of this dye is preserved following cell fixation. (A-B) A representative full responder donor. (C-D) A representative partial responder donor. The percentage of cells was evaluated under unstimulated (top panels) and stimulated conditions (bottom panels). The data revealed that MYXV infection plus stimulation of T cells increased the percentage of cell death of the CD3+ population in culture. (E-F) Summaries of the profile of cell death among donors (N = 4 for each type of donor).
MYXV infection and stimulation of human T cells reduces their viability. Cell death of T cells was evaluated 72 hours and 96 hours after mock-treatment or MYXV treatment, and ± anti-CD3/CD28 stimulation, using flow cytometry. To assess cell viability, T cells were labeled with the live/dead near-infrared (IR) fluorescent dye, an amine-reactive dye that binds covalently to intracellular and extracellular amines, generating a bright signal that allows the distinction between live/dead cells in a single channel. In addition, the staining pattern of this dye is preserved following cell fixation. (A-B) A representative full responder donor. (C-D) A representative partial responder donor. The percentage of cells was evaluated under unstimulated (top panels) and stimulated conditions (bottom panels). The data revealed that MYXV infection plus stimulation of T cells increased the percentage of cell death of the CD3+ population in culture. (E-F) Summaries of the profile of cell death among donors (N = 4 for each type of donor).
MYXV infection of stimulated human T lymphocytes downregulates a subset of activation-inducible cytokines
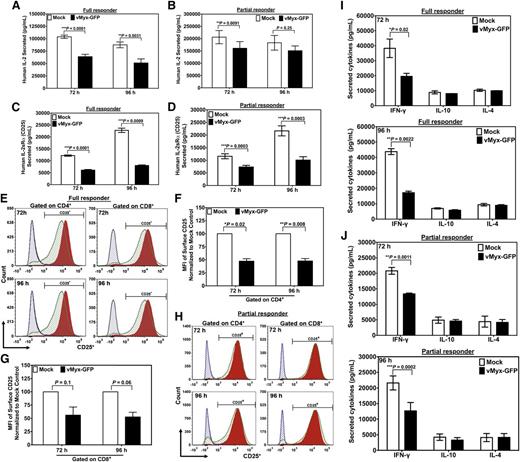
Excessive levels of interleukin-2 (IL-2) have been implicated in the pathophysiology of acute GVHD.21 As expected, supernatants of unstimulated T lymphocytes treated with MYXV, or mock-treated controls, showed no detectable levels of secreted IL-2 (not shown). However, supernatants from stimulated T lymphocytes, either mock-treated or infected with MYXV, now contained measurable levels of IL-2 (Figure 4A-B). For donors assessed as full responders, in terms of activation-induced proliferation, MYXV treatment of stimulated T lymphocytes significantly reduced IL-2 expression compared with mock treatment of stimulated T cells by 72 and 96 hours. For partial responders, MYXV also decreased the IL-2 expression 72 and 96 hours after infection and stimulation (Figure 4B). Thus, both full and partial responders exhibited significantly reduced levels of secreted activation-induced IL-2.
MYXV downregulates the expression IL-2, IL2Rα, and IFN-γ in activated human T cells. To determine whether MYXV infection affects the expression of IL-2 and the IL-2α chain receptor (IL-2Rα, aka CD25), about 1 × 106 of mock-treated (ie, without adding virus) or MYXV-treated human T cells and stimulated with anti-CD3/CD28–coated microbeads were culturing for 72 hours, or 96 hours at 37°C, 5% CO2. Supernatants were collected and analyzed using human IL-2 or human IL-2Rα ELISA. (A-B) MYXV decreases the secretion of IL-2 compared with mock-treated and stimulated T cells. (C-D) Soluble IL-2Rα was significantly downregulated upon infection of activated T cells with MYXV at the indicated time points. (E) Histograms generated from the flow cytometric analysis suggest that MYXV also inhibits the expression of the surface IL-2Rα (CD25) in stimulated and full responder samples (green histograms) as compared with mock-treated and stimulated samples (red histograms). Black histograms correspond to mock-treated T cells and unstimulated T cells, whereas blue histograms correspond to MYXV-treated and unstimulated T cells. (F-G) From the histograms of stimulated samples shown in panel E, the mean fluorescent intensity (MFI) was calculated and is reported as the percentage relative to mock. (F) The MFI of CD25 gated on CD4+. (G) The MFI of CD25 gated on CD8+. Results represent the mean ± standard error of the mean (SEM) of at least 4 different donors. (H) MYXV did not affect the levels of expression of CD25 in the surface of activated lymphocytes of partial responders. (I-J) MYXV affects the expression of IFN-γ, IL-4, and IL-10 in activated human T cells. After culturing activated T cells for 72 hours or 96 hours, with or without virus infection, 1 × 106 of cells were pelleted and the supernatants of both (I) full responders and (J) partial responders used to evaluate the levels of secreted cytokines such as IL-4, IL-10, and IFN-γ utilizing a Luminex platform. MYXV inhibited the secretion of IFN-γ in all donors tested (I-J) at 72 hours, or 96 hours following stimulation as compared with mock-treated samples; whereas the secretion of the cytokines IL-4 and IL-10 was not affected by MYXV-treated vs mock-treated T cells. Results shown correspond to the mean ± SEM of at least 3 different full responder donors, and 3 different partial responder donors.
MYXV downregulates the expression IL-2, IL2Rα, and IFN-γ in activated human T cells. To determine whether MYXV infection affects the expression of IL-2 and the IL-2α chain receptor (IL-2Rα, aka CD25), about 1 × 106 of mock-treated (ie, without adding virus) or MYXV-treated human T cells and stimulated with anti-CD3/CD28–coated microbeads were culturing for 72 hours, or 96 hours at 37°C, 5% CO2. Supernatants were collected and analyzed using human IL-2 or human IL-2Rα ELISA. (A-B) MYXV decreases the secretion of IL-2 compared with mock-treated and stimulated T cells. (C-D) Soluble IL-2Rα was significantly downregulated upon infection of activated T cells with MYXV at the indicated time points. (E) Histograms generated from the flow cytometric analysis suggest that MYXV also inhibits the expression of the surface IL-2Rα (CD25) in stimulated and full responder samples (green histograms) as compared with mock-treated and stimulated samples (red histograms). Black histograms correspond to mock-treated T cells and unstimulated T cells, whereas blue histograms correspond to MYXV-treated and unstimulated T cells. (F-G) From the histograms of stimulated samples shown in panel E, the mean fluorescent intensity (MFI) was calculated and is reported as the percentage relative to mock. (F) The MFI of CD25 gated on CD4+. (G) The MFI of CD25 gated on CD8+. Results represent the mean ± standard error of the mean (SEM) of at least 4 different donors. (H) MYXV did not affect the levels of expression of CD25 in the surface of activated lymphocytes of partial responders. (I-J) MYXV affects the expression of IFN-γ, IL-4, and IL-10 in activated human T cells. After culturing activated T cells for 72 hours or 96 hours, with or without virus infection, 1 × 106 of cells were pelleted and the supernatants of both (I) full responders and (J) partial responders used to evaluate the levels of secreted cytokines such as IL-4, IL-10, and IFN-γ utilizing a Luminex platform. MYXV inhibited the secretion of IFN-γ in all donors tested (I-J) at 72 hours, or 96 hours following stimulation as compared with mock-treated samples; whereas the secretion of the cytokines IL-4 and IL-10 was not affected by MYXV-treated vs mock-treated T cells. Results shown correspond to the mean ± SEM of at least 3 different full responder donors, and 3 different partial responder donors.
In addition, supernatants of unstimulated T lymphocytes treated with MYXV, or mock-treated, showed no detectable levels of IL-2Rα (not shown). Supernatants of stimulated but uninfected T lymphocytes showed high levels of activation-induced IL-2Rα. However, MYXV-treated stimulated T lymphocytes secreted significantly less IL-2Rα than mock-treated stimulated controls. Notably, this inhibitory pattern was observed in both full responders (Figure 4C) and in partial responders (Figure 4D) at 72 and 96 hours after infection and stimulation.
When we analyzed the samples of full responder donors, we found that MYXV decreased the expression of IL-2Rα receptor (CD25) (Figure 4E) by ∼50% on CD4+ lymphocytes and by ∼40% on CD8+ lymphocytes (Figure 4F-G). Interestingly, MYXV did not affect the activation-induced surface levels of CD25 of partial responders (Figure 4H; supplemental Table 4).
Besides IL-2, other lymphocyte-derived cytokines involved in GVHD include IL-4, IL-10, and interferon-γ (IFN-γ).22-24 Of these cytokines, only activation-induced soluble IFN-γ was significantly decreased after MYXV infection as compared with mock-treated and stimulated samples (Figure 4). Notably, we observed a similar inhibitory pattern for T cells derived from both types of donors at 72 hours and at 96 hours (Figure 4I-J). Intracellular staining of IFN-γ confirmed these findings (supplemental Table 5).
Infection with MYXV decreases the proliferation and cytokine production of allostimulated T cells
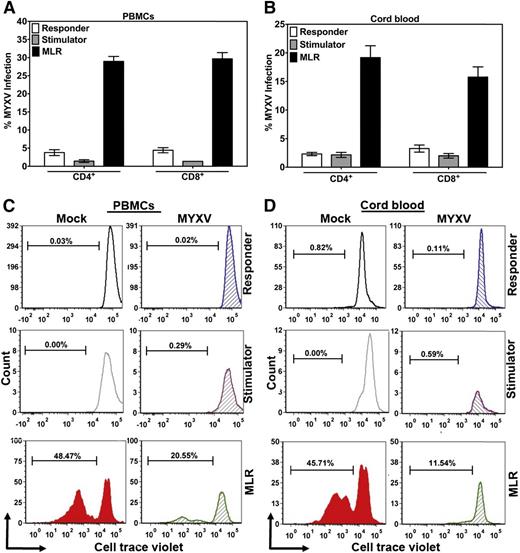
We next used MLRs to recapitulate allostimulation conditions in GVHD. Responder cells were labeled with CTV dye to track proliferation. Mock- or MYXV-treated responder cells were mixed with irradiated unmatched cells (stimulator), and the levels of infection and proliferation were quantified using flow cytometry after 72 hours or 6 days after culturing MLRs. Upon allostimulation of MNCs from PBMCs, we observed that 30.00% ± 1.30% of CD4+ T cells and 30.00% ± 1.72% of CD8+ T cells in the MLR were infected with MYXV (Figure 5A). In contrast, only 3.76% ± 0.79% of responder CD4+ T cells alone and 4.41% ± 1.71% of responder CD8+ T cells alone were infected (Figure 5A). As expected, only 1.44% ± 0.38% of stimulator CD4+ T cells alone and 1.37 ± 0.0 of stimulator CD8+ T cells alone were infected (Figure 5A). When the MNCs from cord blood were allostimulated by MLR, 19.19% ± 1.77% of CD4+ T cells and 16.00% ± 2.10% of CD8+ T cells were infected with MYXV (Figure 5B). As expected, 2.15% ± 0.43% of responder CD4+ T cells alone and 3.25% ± 0.62% of responder CD8+ T cells alone were infected (Figure 5B). Likewise, 2.15% ± 0.43% of CD4+ stimulator T cells alone and 1.99% ± 0.39% of stimulator CD8+ T cells alone were infected (Figure 5B).
Effect of MYXV on infection, proliferation, IL-2 and IL-2Rα secretion of T cells allostimulated via MLR. To investigate whether MYXV affects the functionality of T cells in the context of allostimulation, in vitro MLR assays were carried out. PBMCs and CB from healthy donors were used to isolate MNCs. Stimulator cells were irradiated using 3000 cGy from a Cs157 and 5 × 105 of cells were plated in triplicate into 96-well plates. Responder cells were mock-treated or MYXV-treated and then 1 × 105 cells were seeded in triplicate into empty wells or in wells containing irradiated stimulator cells. After culturing for 72 hours or 96 hours, levels of infection (A-B) and proliferation (C-D) were evaluated using flow cytometry.
Effect of MYXV on infection, proliferation, IL-2 and IL-2Rα secretion of T cells allostimulated via MLR. To investigate whether MYXV affects the functionality of T cells in the context of allostimulation, in vitro MLR assays were carried out. PBMCs and CB from healthy donors were used to isolate MNCs. Stimulator cells were irradiated using 3000 cGy from a Cs157 and 5 × 105 of cells were plated in triplicate into 96-well plates. Responder cells were mock-treated or MYXV-treated and then 1 × 105 cells were seeded in triplicate into empty wells or in wells containing irradiated stimulator cells. After culturing for 72 hours or 96 hours, levels of infection (A-B) and proliferation (C-D) were evaluated using flow cytometry.
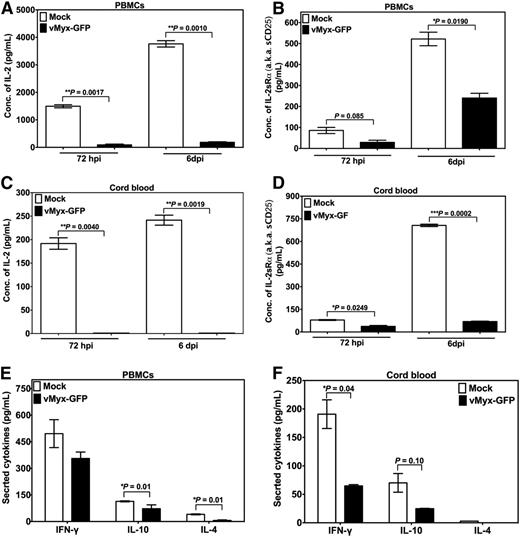
Furthermore, infection with MYXV and allostimulation of cells from PBMCs or CB via MLR resulted in significant inhibition of proliferation of responder T lymphocytes (Figure 5C and D, respectively) as compared with mock-treated cells. As expected, responder or irradiated stimulator T cells alone without MLR did not proliferate. Next, we performed enzyme-linked immunosorbent assay (ELISA) or multiplex assays to determine the levels of secreted cytokines in MLRs. Low levels of soluble IL-2, IL-2Rα, IL-4, IL-10, and IFN-γ were observed after infection and allostimulation of either PBMCs (Figure 6A,B,E) or CB cells (Figure 6C,D,F). These results were consistent with those obtained upon stimulation of T cells with anti-CD3/CD28 beads (Figures 2A-D and 4). In terms of infection, proliferation pattern, and cytokine production, no major differences were found for PBMCs or CB cells, suggesting that both PBMCs and CB are almost equally susceptible to MYXV.
Effect of MYXV on the expression of IFN-γ, IL-4, and IL-10 by T cells allostimulated via MLR. To determine whether MYXV infection affects the expression of IL-2 and the IL-2 α chain receptor (IL-2Rα, aka CD25) in the setting of allostimulation via MLR, PBMCs and CB cells, from healthy donors were used to isolate MNCs. A total of 1 × 105 mock-treated (ie, no virus) responder cells or MYXV-treated responder cells were mixed with 5 × 105 irradiated stimulator cells. Cocultures were incubated for 72 hours or 6 days at 37°C, 5% CO2. At the indicated time points, supernatants were collected to carry out ELISA and multiplex assays. (A-B) ELISAs of IL-2 and IL-2Rα of PBMCs. (C-D) ELISAs of IL-2 and IL-2Rα of CB. (E-F) Multiplex assays were used to quantify the levels of secretion of IFN-γ, IL-4, and IL10 from PBMCs and CB cells, respectively.
Effect of MYXV on the expression of IFN-γ, IL-4, and IL-10 by T cells allostimulated via MLR. To determine whether MYXV infection affects the expression of IL-2 and the IL-2 α chain receptor (IL-2Rα, aka CD25) in the setting of allostimulation via MLR, PBMCs and CB cells, from healthy donors were used to isolate MNCs. A total of 1 × 105 mock-treated (ie, no virus) responder cells or MYXV-treated responder cells were mixed with 5 × 105 irradiated stimulator cells. Cocultures were incubated for 72 hours or 6 days at 37°C, 5% CO2. At the indicated time points, supernatants were collected to carry out ELISA and multiplex assays. (A-B) ELISAs of IL-2 and IL-2Rα of PBMCs. (C-D) ELISAs of IL-2 and IL-2Rα of CB. (E-F) Multiplex assays were used to quantify the levels of secretion of IFN-γ, IL-4, and IL10 from PBMCs and CB cells, respectively.
Activated T lymphocytes efficiently transfer MYXV and kill susceptible cancer cells
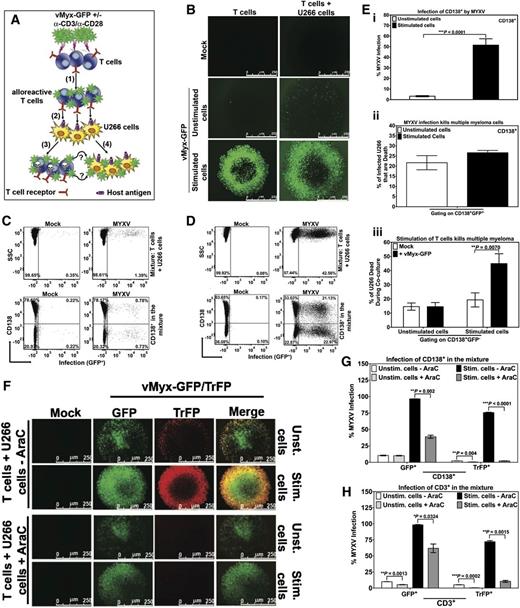
The purpose of allo-HCT is twofold: (1) to replace the hematopoietic system of the recipient using hematopoietic stem/progenitor cells from a closely matched donor and (2) to attack and eliminate residual cancer cells in the recipient by means of alloreactive donor T cells. Any viable adjunct therapy to allo-HCT to prevent GVHD should not come at the price of either reducing engraftment of the normal stem cells or reducing the efficiency of graft-versus-malignancy (GVM). Previously, when we used a xenograft model of human MM in immunodeficient mice, we found that ex vivo treatment of the donor human bone marrow with MYXV, while leaving normal stem cell engraftment unaffected, not only prevented GVHD but also preserved GVM against preseeded myeloma.14 However, the mechanisms of GVM after MYXV treatment of the donor transplant were unknown in our prior study. Therefore, we designed in vitro modeling experiments to examine the mechanistic basis for the observed GVM preservation (Figure 7A). Briefly, human T lymphocytes were isolated by negative selection from healthy donor PBMCs. The T cells were then mock-treated or treated with vMyx-GFP for 1 hour at room temperature to allow for virus binding. Unbound virus was removed by washing and the cells were cultured in complete media, either with or without anti-CD3/CD28 stimulation for 48 hours at 37°C. Next, the T cells were mixed with human MM cells (U266 cells) that have previously shown susceptibility to MYXV oncolysis.10,14,15 This mixture of T cells and MM cells was incubated at 37°C for an additional 48 hours. As controls, T cells alone or MM cells alone were subjected to the same MYXV treatment, ± anti-CD3/CD28 activation and culture conditions. The levels of MYXV infection in the MM cells (monitored as CD138+) were evaluated at 96 hours after MYXV treatment of unstimulated or stimulated T cells (corresponding to 48 hours after mixing T cells with MM cells). MYXV infection levels of either the donor T cells or MM cells were assessed using fluorescence microscopy and flow cytometry. As expected, MYXV did not productively infect unstimulated T cells (Figure 7B left middle panel). Although a slight increase in the number of MYXV-infected T cells was observed when unstimulated/infected T cells were mixed with MM cells (Figure 7B right middle panel), the percentage of infection in all cells (ie, T cells and MM cells) was only 1.39% (Figure 7C top right panel). The percentage of infection of MM cells in this mixture was only 0.78% (Figure 7C bottom right panel). This low level of MYXV infection was expected because only a very small fraction of unstimulated T cells was infected with MYXV.
Input MYXV and virus progeny from activated human T cells are both efficiently transferred to human multiple myeloma cells. To investigate whether MYXV infection of unstimulated vs activated T cells can secondarily target and infect virus-susceptible human U266 MM cells, an in vitro virus transfer assay was performed and is described in diagram (A). Experimental schematic depicting human T lymphocytes incubated with MYXV in the presence or absence of activating anti-CD3/CD28 microbeads, (1) MYXV binding to T cells (alloreactive T cells), free MYXV washed from culture, (2) admixture of human MM (U266 cells). As a result a dual action of MYXV is proposed: (3) MYXV mediates infection/suppression of alloreactive T cells when they interact with host U266 antigens, and (4) the infection of malignant cells by passing of virus from activated T cells to myeloma cells (GFP+). (B) Fluorescence micrographs showing minimal increase in MYXV infection (GFP+) in unstimulated conditions when T cells were mixed with MM cells (middle panels). However, after stimulation there was a significant increase in MYXV infection of all cells (bottom panels). (C) Flow cytometry was used to quantify infection in cell subsets. CD138+ myeloma cells showed only minimal MYXV infection in unstimulated conditions (bottom right plot). (D) In stimulated conditions, there was a significant increase in the percentage of CD138+ myeloma cells with MYXV infection (bottom right panel). Compared with unstimulated conditions, activated T lymphocytes caused more than a 25-fold increase in myeloma infection with MYXV (from 0.78% to 21.13%). (E) (i) Bar graph showing percentage of the CD138+ myeloma cell population with MYXV infection in the unstimulated (white) vs stimulated (black) conditions. (ii) Bar graph showing the percentage of MM dead (CD138+) induced by MYXV infection (GFP+) (ie, gating on CD138+GFP+) under unstimulated (white) vs stimulated (black) conditions (ie, 21% vs 27%, respectively). (iii) Gating on CD138+GFP−, bar graph showing percentage of MM dead (CD138+). Mock-treated (white), or MYXV-treated (black) T cells and under stimulation with anti-CD3/CD28 resulted on 19.30% and 44.96% of MM dead, respectively. On the other hand, mock-treated (white), MYXV-treated (black) T cells without anti-CD3/CD28, resulted in <15% of MM dead. T cells from 3 different donors were tested and showed reproducibly consistent virus-transfer and killing results. (F) To determine whether progeny and/or input virus is transferred from activated T cells to MM cells, late gene expression, and therefore the generation of progeny MYXV, was blocked by using AraC. Briefly, after exposure of T cells with vMyx-GFP/TrFP, 10 μg/mL AraC (+AraC) or vehicle only (-AraC) were added to the T cells with or without α-CD3/α-CD28 stimulation. After 48 hours of culturing T cells ± AraC, U266 cells were added to the culture and incubated at 37°C 5% CO2 for an additional 48 hours (see supplemental Methods for more details). Infection was evaluated using florescence microscopy. Flow cytometry was used to quantify the levels of infection of (G) MM cells (CD138+) and (H) T cells (CD3+). Data indicate that after infection of activated T cells, MYXV replicates, but both input virus (unaffected by AraC) and virus progeny (inhibited by AraC) are both then transferred to myeloma cells to initiate infection of these cells. At least 3 independent experiments were performed.
Input MYXV and virus progeny from activated human T cells are both efficiently transferred to human multiple myeloma cells. To investigate whether MYXV infection of unstimulated vs activated T cells can secondarily target and infect virus-susceptible human U266 MM cells, an in vitro virus transfer assay was performed and is described in diagram (A). Experimental schematic depicting human T lymphocytes incubated with MYXV in the presence or absence of activating anti-CD3/CD28 microbeads, (1) MYXV binding to T cells (alloreactive T cells), free MYXV washed from culture, (2) admixture of human MM (U266 cells). As a result a dual action of MYXV is proposed: (3) MYXV mediates infection/suppression of alloreactive T cells when they interact with host U266 antigens, and (4) the infection of malignant cells by passing of virus from activated T cells to myeloma cells (GFP+). (B) Fluorescence micrographs showing minimal increase in MYXV infection (GFP+) in unstimulated conditions when T cells were mixed with MM cells (middle panels). However, after stimulation there was a significant increase in MYXV infection of all cells (bottom panels). (C) Flow cytometry was used to quantify infection in cell subsets. CD138+ myeloma cells showed only minimal MYXV infection in unstimulated conditions (bottom right plot). (D) In stimulated conditions, there was a significant increase in the percentage of CD138+ myeloma cells with MYXV infection (bottom right panel). Compared with unstimulated conditions, activated T lymphocytes caused more than a 25-fold increase in myeloma infection with MYXV (from 0.78% to 21.13%). (E) (i) Bar graph showing percentage of the CD138+ myeloma cell population with MYXV infection in the unstimulated (white) vs stimulated (black) conditions. (ii) Bar graph showing the percentage of MM dead (CD138+) induced by MYXV infection (GFP+) (ie, gating on CD138+GFP+) under unstimulated (white) vs stimulated (black) conditions (ie, 21% vs 27%, respectively). (iii) Gating on CD138+GFP−, bar graph showing percentage of MM dead (CD138+). Mock-treated (white), or MYXV-treated (black) T cells and under stimulation with anti-CD3/CD28 resulted on 19.30% and 44.96% of MM dead, respectively. On the other hand, mock-treated (white), MYXV-treated (black) T cells without anti-CD3/CD28, resulted in <15% of MM dead. T cells from 3 different donors were tested and showed reproducibly consistent virus-transfer and killing results. (F) To determine whether progeny and/or input virus is transferred from activated T cells to MM cells, late gene expression, and therefore the generation of progeny MYXV, was blocked by using AraC. Briefly, after exposure of T cells with vMyx-GFP/TrFP, 10 μg/mL AraC (+AraC) or vehicle only (-AraC) were added to the T cells with or without α-CD3/α-CD28 stimulation. After 48 hours of culturing T cells ± AraC, U266 cells were added to the culture and incubated at 37°C 5% CO2 for an additional 48 hours (see supplemental Methods for more details). Infection was evaluated using florescence microscopy. Flow cytometry was used to quantify the levels of infection of (G) MM cells (CD138+) and (H) T cells (CD3+). Data indicate that after infection of activated T cells, MYXV replicates, but both input virus (unaffected by AraC) and virus progeny (inhibited by AraC) are both then transferred to myeloma cells to initiate infection of these cells. At least 3 independent experiments were performed.
In contrast, when MYXV-treated stimulated T lymphocytes were mixed with MM cells, there was a significant increase in the percentage of infected MM cells (Figure 7B bottom right panel). Under conditions of T-cell stimulation, the level of MYXV infection in MM cells increased 100-fold (from 0.78% to 21.13%) (Figure 7D bottom right panel). T lymphocytes from 3 different donors were tested in a similar fashion and showed reproducibly consistent infection levels of the target MM cells (Figure 7Ei). Furthermore, we show evidence that CD138+ cell death is induced by MYXV infection (ie, CD138+GFP+) under both unstimulated and stimulated conditions. For instance, up to 21.74% ± 3.50% and 27.00% ± 1.15% of CD138+ MM cells die, under unstimulation and stimulation conditions, respectively (Figure 7Eii). These results confirm the oncolytic effects of MYXV infection on human MM cells. Interestingly, we also show enhanced and significant killing of even noninfected MM cells (ie, CD138+GFP−) after MYXV infection of T cells and stimulation with anti-CD3/CD28 (44.96% ± 6.94%) as compared with mock-treated and stimulated T cells (ie, 19.30% ± 4.99%; **P = 0070) (Figure 7Eiii). On the other hand, when comparing MM cell killing of uninfected CD138+ MM cells (ie, GFP−) under the unstimulated conditions, no significant differences were found between MYXV-treated resting T cells (ie, 14.52% ± 2.93%) and mock-treated resting T cells (ie, 14.58% ± 2.57%; P = .3984, NS) (Figure 7Eiii). This latter result suggests that MYXV-infected/stimulated T cells that do not donate virus to MM are nevertheless now better cytotoxic killers of MM cells than mock-treated/stimulated T cells. We speculate that T cells exposed to MYXV are now also better armed to kill cancer cells by cytotoxic T lymphocyte killing. Together, our results indicate that MYXV enhances the beneficial effects of GVM.
To determine whether input and/or progeny virus are transferred from infected/stimulated T cells to MM cells, cytosine arabinoside (AraC), a known inhibitor of viral DNA replication and late gene expression of MYXV, was used. When T cells were exposed to vMyx-GFP/TrFP followed by the addition of AraC and stimulation with anti-CD3/CD28 beads, the late gene expression of MYXV (TrFP+) was inhibited (Figure 7F), which resulted in very low levels of viral late gene expression in both MM cells (Figure 7G) and T cells (Figure 7H). Lower levels of early and late gene expression (GFP+) were observed in activated T cells treated with AraC, indicating that GFP expression was aborted by the AraC, as expected (Figure 7H). Importantly, GFP expression in the MM cells, which are infected by MYXV donated from the stimulated T cells, was reduced but not eliminated by the AraC treatment. This means that both progeny virus (inhibited by AraC) and input virus (unaffected by AraC) can infect the target MM cells. Together, these results support the notion that both input and progeny virus, derived from stimulated T cells are handed-off or delivered into the MM cells, which results in their productive infection.
Discussion
A major clinical challenge after allo-HCT is the prevention or control of GVHD. Because GVHD is driven by resident CD3+ T lymphocytes in the donor allograft, one of the most effective treatments for GVHD is the prophylactic depletion or inhibition of alloreactive T cells.24,25 However, intensifying T-cell purging by conventional methods increases the risk for life-threatening infections due to delayed immune recovery, graft failure, and disease relapse.26,27 At a minimum, optimizing outcomes after allo-HCT simultaneously requires control of GVHD, sparing of normal hematopoietic stem/progenitor cell engraftment, and permission for GVM.
We previously demonstrated efficient human hematopoietic engraftment with no GVHD after xenotransplant of MYXV-treated primary human hematopoietic stem/progenitor cells.14 The safety of using MYXV with human hematopoietic stem/progenitor cells has been correlated to the virus’ inability to bind or infect normal human CD34+ hematopoietic cells.9,10 Of the hundreds of immunocompromised mice that we have transplanted with MYXV-treated cells, none have shown pox ulcerations or any semblance of viral infection whatsoever. Moreover, MYXV has been widely distributed in the Australian environment as a means to control feral populations of European rabbits, and no human infections have ever been reported.28
The data in this report are the first to reveal mechanisms by which ex vivo virotherapy with MYXV controls GVHD yet does not compromise GVM.14 Herein, we present direct evidence that MYXV binds unstimulated human CD3+ T lymphocytes but T-cell activation is required to initiate productive virus infection, which can then be delivered to susceptible cancer cells.
In addition to efficiently infecting stimulated human T lymphocytes from all normal donors tested, MYXV also impaired T-cell functionality by (1) reducing T-cell proliferation and (2) downregulating T-cell signaling pathways of known importance in GVHD.21 Specifically, we found that MYXV infection consistently decreased activation-induced T-lymphocyte secretion of IL-2, IL-2Rα, and IFN-γ, but not the secretion of IL-4 or IL-10. Inhibition of secretion of IFN-γ is consistent with lower levels of Tbet (Th1) expression, and not variation in the expression of GATA3 (Th2).
The reason(s) why MYXV completely suppresses the proliferation of stimulated T cells in some donors (full responders) and only partially inhibits the proliferation of stimulated T cells in other donors (partial responders) is still unknown and requires more investigation. We found that the levels of initial virus binding could not be directly correlated with the type of donor (ie, full responder vs partial responder). Because the limit of detection of binding of Venus-tagged MYXV to cells by fluorescence-activated cell sorter is in the order of several hundred virus particles per cell, we can only note that the majority of T cells from all donors tested became infected following T-cell stimulation, suggesting that sufficient virus binds all of the cells to at least initiate infection following cell activation.
Although depletion or inhibition of T-cell activation helps to control GVHD, a detrimental result of this strategy is that the beneficial effects of GVM may also be compromised. A previous report by our group demonstrated that ex vivo virotherapy with MYXV can control GVHD in NSG mice xenografted with human PBMCs and yet still retain the beneficial effects of GVM against preseeded human MM in the bone marrow of the recipients.14 Results in our present study support the notion that both input and progeny MYXV derived from infected/stimulated T cells can be efficiently transferred to susceptible target human cancer cells and mediate oncolytic effects against these cancer cells via the antigenic stimulation of the donor T cells. Our results are somewhat distinct from those of Cole et al, in which viral vectors hitchhike on nonactivated T cells, and virus delivery to cancer cells does not involve virus replication.29 In contrast, we report here that activation of T cells is required to efficiently deliver both input and progeny oncolytic MYXV to target human myeloma cells.
Overall, our results provide new insights into the specific mechanisms used by MYXV to control GVHD after allo-HCT. We now show that the ex vivo virotherapy regimen has the potential to arm resident resting T cells residing in the donor allograft with adsorbed MYXV, which is then triggered into the replication cycle only after the T cells encounter antigenic stimulation. At this point, if the stimulating host cells are from normal tissues, the triggered virus replication retards or blocks subsequent T-cell proliferation. But if the stimulating host cells are cancerous, the oncolytic virus is efficiently transferred from T lymphocytes to target and kill cancer cells. In essence, GVM by the donor transplant now becomes augmented by virus-versus-malignancy (VVM). Because productive viral infection of a tumor can also induce an in situ vaccine effect and initiate systemic antitumor immunity, an added benefit of using the ex vivo MYXV approach is that infection of malignant cells in vivo via T-cell–mediated delivery might also create an in situ vaccination effect against tumor antigens. Therefore, for all these reasons, ex vivo MYXV pretreatment of donor allografts may be a promising clinical adjunct to allo-HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank The Diabetes Institute at the University of Florida, Department of Pathology, Immunology and Laboratory Medicine for providing them with reagents, tools, and instrumentation, and very helpful discussions. They also thank Dr Shannon Wallet from the department of Oral Biology at the University of Florida for providing the Luminex instrument facility.
This work was supported by a Florida Bankhead-Coley Cancer Research Program grant 1BT02 and a National Institutes of Health National Cancer Institute grant R01 CA138541-01 (G.M.). This work was also supported by the Gatorade Trust, which was administered by the University of Florida Department of Medicine.
The Leukemia & Lymphoma Society supported C.R.C. with a Scholar in Clinical Research award (2400-13). N.Y.V. was supported by a Professorship in Blood & Marrow Transplant.
Authorship
Contribution: C.R.C., G.M., and N.Y.V. conceived the study concept, designed the experiments, analyzed the results, and wrote the manuscript; N.Y.V., C.H.W., A.M.M., E.W., and W.C. conducted the experiments and edited the manuscript; and J.R.W. edited the manuscript.
Conflict-of-interest disclosure: C.R.C. and G.M. have filed intellectual property rights to the University of Florida for prevention of GVHD by MYXV virotherapy. The remaining authors declare no competing financial interests.
Correspondence: Christopher R. Cogle, Division of Hematology and Oncology, Department of Medicine, University of Florida, 1600 SW Archer Rd, Box 100278, Gainesville, FL 32610-0278; e-mail: christopher.cogle@medicine.ufl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal