Key Points
Podoplanin and CLEC-2 critically drive the formation and integrity of developing cerebral blood vessels.
Loss of cerebrovascular integrity is influenced by the loss of αIIb-mediated platelet aggregation and platelet secretion.
Abstract
Mice with a constitutive or platelet-specific deletion of the C-type-lectin-like receptor (CLEC-2) exhibit hemorrhaging in the brain at mid-gestation. We sought to investigate the basis of this defect, hypothesizing that it is mediated by the loss of CLEC-2 activation by its endogenous ligand, podoplanin, which is expressed on the developing neural tube. To induce deletion of podoplanin at the 2-cell stage, we generated a podoplaninfl/fl mouse crossed to a PGK-Cre mouse. Using 3-dimensional light-sheet microscopy, we observed cerebral vessels were tortuous and aberrantly patterned at embryonic (E) day 10.5 in podoplanin- and CLEC-2-deficient mice, preceding the formation of large hemorrhages throughout the fore-, mid-, and hindbrain by E11.5. Immunofluorescence and electron microscopy revealed defective pericyte recruitment and misconnections between the endothelium of developing blood vessels and surrounding pericytes and neuro-epithelial cells. Nestin-Cre-driven deletion of podoplanin on neural progenitors also caused widespread cerebral hemorrhaging. Hemorrhaging was also seen in the ventricles of embryos deficient in the platelet integrin subunit glycoprotein IIb or in embryos in which platelet α-granule and dense granule secretion is abolished. We propose a novel role for podoplanin on the neuro-epithelium, which interacts with CLEC-2 on platelets, mediating platelet adhesion, aggregation, and secretion to guide the maturation and integrity of the developing vasculature and prevent hemorrhage.
Introduction
Thrombocytopenia is the most common risk factor of intraventricular hemorrhage (IVH) in premature infants, which effects >12 000 infants every year.1 IVH in neonates causes substantial morbidity and mortality, the onset of which can be immediate or significantly delayed into adulthood. Strikingly, there has been no change in mortalities resulting from IVH over the last 3 decades, and although diagnostics are improving, no preventative therapeutic strategies currently exist.2,3 This relies on a better understanding of the molecular mechanisms that regulate cerebral vascular integrity during development.
The cardiovascular system is the first functional organ system to develop in the mammalian embryo with angioblasts emerging around E7.0 to form the initial primitive vascular plexuses through vasculogenesis.4 The perineural vascular plexus (PNVP) develops around the neural tube between E8.5 and E9.5 and provides essential nutrients and oxygen to developing neural tissue.5 Uniquely, the entire vascularization of the neural tube subsequent to the formation of the PNVP is derived through angiogenesis. Blood vessels invade the neural tube at E10.0 in response to vascular endothelial growth factor (VEGF) released by cells of the neuroepithelium and migrate along a preformed lattice network of neuroepithelial cells.4,6 Capillary stabilization, branching, and remodeling are aided by the recruitment of a wide range of extracellular matrix (ECM) proteins and their intimate association with surrounding neurons, glial cells, and pericytes to form multicellular complexes termed neurovascular units (NVUs). The NVUs provide the anatomical basis for the formation of the blood-brain barrier, a tightly regulated interface between the central nervous system and the circulation.6 Within the NVUs, tight junctions between endothelial cells restrict movement of molecules. Many studies have shown that impaired development of NVUs through the loss of key molecules or signaling pathways leads to fatal hemorrhaging in mid-gestation as a result of abnormal vascular patterning and aberrant associations with pericytes and ECM components.7-11
CLEC-2 is a C-type lectin-like receptor, which is expressed at high levels on megakaryocytes and platelets, with no evidence for significant expression on any other circulating hematopoietic cells during development. The only known endogenous ligand for CLEC-2 is the transmembrane protein podoplanin, which is expressed on a wide variety of cell types outside of the vasculature. In addition, podoplanin is itself a receptor, which signals through the ezrin, radixin, and moesin (ERM) family of actin-binding proteins. Thus, binding of podoplanin to CLEC-2 generates reciprocal signals that regulate the function of both of the interacting cells.12
The binding of podoplanin to CLEC-2 on platelets activates a Src and Syk tyrosine kinase-dependent signaling cascade that regulates phospholipase C (PLC)γ2 and platelet activation.13 Mice deficient in podoplanin, CLEC-2, and other key signaling proteins, including Syk and PLCγ2, exhibit blood-lymphatic shunts at mid-gestation and are embryonic lethal.14-19 This is thought to be mediated by a combination of lymphatic-venous connections and retrograde flow through the thoracic duct.20-22 They additionally have a number of other developmental defects, including hemorrhaging in the brain at E12.5 and the absence of lymph nodes.18,23,24 These developmental defects are believed to be due to loss of platelet activation, as they are seen in mice with a megakaryocyte/platelet-specific deletion of CLEC-2 or its signaling kinase Syk.18,21,25 However, the neurovascular defects are believed to be unrelated to defects in lymphatic development because the lymphatic system is absent in the brain, and cerebral hemorrhages are observed before the presence of blood-lymphatic mixing.
In the characterization of CLEC-2- and Syk-deficient mice, we localized brain hemorrhages to the ventricles and parenchyma at E12.5 and reported that hemorrhages in Clec-2fl//flPF4-Cre mice were restricted to the parenchyma.18 Although cerebral hemorrhages have not been reported in podoplanin-deficient mice, the loss of T-synthase, a key glycosyltransferase enzyme responsible for glycosylation of the podoplanin extracellular domain, results in the formation of a disorganized microvasculature network, with the defective recruitment of ECM and pericytes, leading to hemorrhaging throughout the brain by E12.0 and lethality by E14.0.26 However, hemorrhages were not observed in endothelial specific T-synthase-deficient mice, which exhibited blood-lymphatic mixing, consistent with the neurovascular defect being independent of the defect in lymphatic development.27
In the present study, we investigated the mechanism of developmental defects within the central nervous system of CLEC-2-deficient mice. We generated a novel podoplanin-floxed mouse and crossed this to mice expressing PGK-Cre to delete the transmembrane protein at the 2-cell stage. We demonstrate that constitutive deletion of CLEC-2 or podoplanin induce a similar pattern of hemorrhaging from E11.5 in association with defective angiogenesis of cerebral blood vessels. A similar defect is also observed following nestin-Cre (Nes-Cre)-driven deletion of podoplanin specifically on neuro-epithelial cells. We also show the presence of hemorrhages in the ventricles of mice deficient in the subunit αIIb of the major platelet integrin αIIbβ3 and in mice where platelet α-granule and dense-granule secretion is abolished. This leads us to propose a model in which expression of podoplanin on the neuro-epithelium interacts with CLEC-2 on platelets to regulate the maturation and integrity of cerebral blood vessels. During the development of these nascent, fragile vessels, we propose that CLEC-2-induced αIIbβ3-dependant aggregation drives the formation of small platelet aggregates to plug the vessel wall to prevent hemorrhage and maintain vascular integrity. Meanwhile, platelet-secreted molecules may function to recruit mural cells to the endothelium to aid blood vessel maturation and integrity, as well as to reinforce αIIbβ3-dependent platelet aggregation.
Methods
An expanded methods section is available in the supplemental Materials available on the Blood Web site.
Mouse strains
All animal experimentation was performed under an approved license from the UK Home Office. Clec-2−/− mice have been previously described.28 Podoplaninfl/fl(Pdpnfl/fl) mice were generated at Taconic Artemis by insertion of loxP sites flanking exon 3 of the podoplanin allele using standard methods and back-crossed to C57BL/6 mice. Pdpnfl/fl mice were crossed to PGK-Cre, Nes-Cre, or Tie2-Cre mice for constitutive, neural-, or endothelial-specific deletion of the podoplanin allele, respectively.29-31 PGK-Cre mice were purchased from Harlan Laboratories. Nes-Cre mice were obtained from Cancer Research UK (London, UK). Tie2-Cre mice were purchased from Jackson Laboratories. Nbeal-2−/−, Unc13d−/−, and Nbeal-2−/−Unc13d−/− embryos were provided by Bernhard Nieswandt32,33 (Deppermann et al, unpublished data). Sphk1fl/flSphk2−/− and Sphk1fl/flSphk2−/−-PF4-Cre embryos were provided by Eric Camerer (Mariko et al, unpublished data). αIIb−/− mice were provided by John Frampton and have been previously described.34
Immunolabeling and microscopy
Immunohistochemistry and immunofluorescence are described in detail in the supplemental Materials.
Briefly, sections of paraffin-embedded embryos were either processed for hematoxylin and eosin (H&E) staining or for podoplanin staining, whereby sections were boiled in citric acid buffer (pH 6) before permeabilizing, blocking, and incubating with hamster anti-podoplanin (clone eBio8.1.1; eBiosciences), followed by goat anti-hamster Cy3 (ab6969; Abcam) secondary antibody. H&E sections were analyzed using a Zeiss Axiovert Zoom brightfield microscope. Confocal imaging was performed using a Leica SP2 confocal microscope.
For immunofluorescence staining of frozen embryo tissue, sections were quenched with ammonium chloride and permeabilized before blocking and incubating with the following primary antibodies: rat anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM-1; clones 5D2.6 and 1G5.1, provided by Dr S. Butz35 ), rabbit anti-NG2 (clone 132.39; Millipore), rat anti-nestin (Rat 401; Developmental Studies Hybridoma Bank), rat anti-TER119 (Cambridge Bioscience), rat anti-mouse CD41 (clone MWReg30; BD Pharmigen), or hamster anti-podoplanin (clone eBio8.1.1; eBiosciences). Sections were then incubated in the appropriate secondary antibodies: goat anti-hamster Cy3 (ab6969; Abcam), goat anti-rat Alexa Fluor 488 (A-11006; Invitrogen), or goat anti-rat Alexa Fluor 647 (A-21247; Invitrogen), and where applicable, further incubated with TO-PRO-3-Iodide (Invitrogen). Confocal imaging was performed using a Leica SP2 confocal microscope.
Whole mount immunofluorescence and ultramicroscopy preparation and imaging have been previously described35 and are detailed in the supplemental Materials. Briefly, embryos were permeabilized, blocked, and immunostained with rat anti-mouse PECAM-1 (clones 5D2.6 and 1G5.1) primary antibodies, followed by goat anti-rat Alexa Fluor 488 secondary antibody. Embryos were embedded in agarose, successively dehydrated, and chemically cleared for imaging using a LaVision Ultramicroscope (La Vision BioTec; Bielefield). Images were analyzed using IMARIS Vantage software (Version 7.6.0; Bitplane).
Electron microscopy
Electron microscopy is described in detail in the supplemental Materials (reagents supplied by Agar Scientific). Briefly, embryos were fixed in 2.5% glutaraldehyde and washed in 0.1 M sodium cacodylate buffer (pH 7.4; Na Cacodylate) before treatment with 1% osmium tetroxide. Samples were washed in Na Cacodylate before successive dehydration and emersion in propylene oxide:resin (1:1), followed by resin only overnight. Samples were polymerized at 80°C before sectioning and staining with lead citrate/uranyl acetate for imaging using a Jeol 2100 200-kV LaB6 TEM.
Statistical analysis
The number of NG2-positive pericytes was quantified using a 1-way analysis of variance with a Tukey posttest, where *P < .05 and **P < .01. Data are means ± standard error of the mean.
Results
As part of the characterization of CLEC-2-deficient mice, we reported extensive hemorrhaging in the fore-, mid-, and hindbrain regions at E12.5, which we hypothesized were mediated by the activation of platelets with the podpolanin-expressing choroid plexus.18 In the present study, we extensively mapped the time of onset of hemorrhaging through investigation of earlier time points in development and found that vascular defects and hemorrhage occur before the development of the choroid plexus. Cerebral hemorrhages were found to develop between E10.5 and E11.5 (supplemental Figure 1A). At this stage of development, the endogenous ligand for CLEC-2, podoplanin, is widely expressed along with the intermediate filament protein, nestin, throughout the neural tube on neuro-epithelial cells (Figure 1A; supplemental Figure 2). By E14.5, podoplanin expression is localized to the ependymal lining of the ventricle wall and to the choroid plexus (Figure 1A).
Podoplanin is expressed throughout the neural tube in development and its loss results in hemorrhaging. (A) Immunostaining shows podoplanin in the neuro-epithelium on frozen sections of wild-type embryonic mouse heads at E11.5 (n > 4) and E14.5 (n = 3); white boxes in the left panel highlight magnified areas in the right panel. NE, neuroepithelium; V, ventricle; CP, choroid plexus. Scale bars, 100 μm. (B) Pdpnfl/fl mice were generated on a C57BL/6 background at Taconic Artemis by insertion of loxP sites flanking exon 3 of the podoplanin gene. Pdpnfl/fl mice were crossed to mice expressing PGK-cre recombinase resulting in constitutive deletion of exon 3 creating a nonfunctional podoplanin gene. Gray arrows mark primer binding sites. (C) Pdpnfl/flPGK-Cre embryos (E10.5, n = 3; E11.5, n > 10; and E12.5, n > 10) develop hemorrhages in the brain between E10.5 and E11.5 (arrows), whereas Pdpnfl/fl littermates appear normal (E10.5, n = 5; E11.5, n = 7; and E12.5, n > 10). Scale bars, 1 mm. (D) H&E coronal sections from E12.5 embryo heads of Pdpnfl/fl (n = 3) or Pdpnfl/flPGK-Cre (n = 4) embryos. White block or dashed boxes in the left panel show magnified areas in the middle and right panels, respectively. Yellow arrows mark blood vessels closely associated with surrounding neuro-epithelial cells in wild-type embryos compared with dissociations in Pdpnfl/flPGK-Cre mice (middle). Eosin-stained red erythrocytes from large hemorrhages are seen to invade matrix tissue into neighboring vascular beds in Pdpnfl/flPGK-Cre embryos (right). Scale bars, 50 μm.
Podoplanin is expressed throughout the neural tube in development and its loss results in hemorrhaging. (A) Immunostaining shows podoplanin in the neuro-epithelium on frozen sections of wild-type embryonic mouse heads at E11.5 (n > 4) and E14.5 (n = 3); white boxes in the left panel highlight magnified areas in the right panel. NE, neuroepithelium; V, ventricle; CP, choroid plexus. Scale bars, 100 μm. (B) Pdpnfl/fl mice were generated on a C57BL/6 background at Taconic Artemis by insertion of loxP sites flanking exon 3 of the podoplanin gene. Pdpnfl/fl mice were crossed to mice expressing PGK-cre recombinase resulting in constitutive deletion of exon 3 creating a nonfunctional podoplanin gene. Gray arrows mark primer binding sites. (C) Pdpnfl/flPGK-Cre embryos (E10.5, n = 3; E11.5, n > 10; and E12.5, n > 10) develop hemorrhages in the brain between E10.5 and E11.5 (arrows), whereas Pdpnfl/fl littermates appear normal (E10.5, n = 5; E11.5, n = 7; and E12.5, n > 10). Scale bars, 1 mm. (D) H&E coronal sections from E12.5 embryo heads of Pdpnfl/fl (n = 3) or Pdpnfl/flPGK-Cre (n = 4) embryos. White block or dashed boxes in the left panel show magnified areas in the middle and right panels, respectively. Yellow arrows mark blood vessels closely associated with surrounding neuro-epithelial cells in wild-type embryos compared with dissociations in Pdpnfl/flPGK-Cre mice (middle). Eosin-stained red erythrocytes from large hemorrhages are seen to invade matrix tissue into neighboring vascular beds in Pdpnfl/flPGK-Cre embryos (right). Scale bars, 50 μm.
To investigate a role for podoplanin in the development of cerebral hemorrhages, we generated a floxed podoplanin mouse (Figure 1B) and crossed this to a mouse expressing Cre recombinase driven by the PGK promoter (Pdpnfl/flPGK-Cre) for deletion of podoplanin at the 2-cell stage.29 Pdpnfl/flPGK-Cre embryos developed cerebral hemorrhages between E10.5 and E11.5, which were prominent by E12.5 (Figure 1C). Examination of H&E-stained histologic sections of Clec-2−/− and Pdpnfl/flPGK-Cre embryos at E12.5 revealed hemorrhages that extended across vast regions of the neuro-epithelium, displacing surrounding neuro-epithelial cells and protruding into the ECM (Figure 1D; supplemental Figure 1B). Immunofluorescence of wild-type and Clec-2−/− embryos at E14.5 demonstrated that hemorrhages in the parenchyma and in the ventricles were rich in CD41+ platelets (supplemental Figure 3). The overall pattern of hemorrhaging was similar in Clec-2−/− and Pdpnfl/flPGK-Cre embryos.
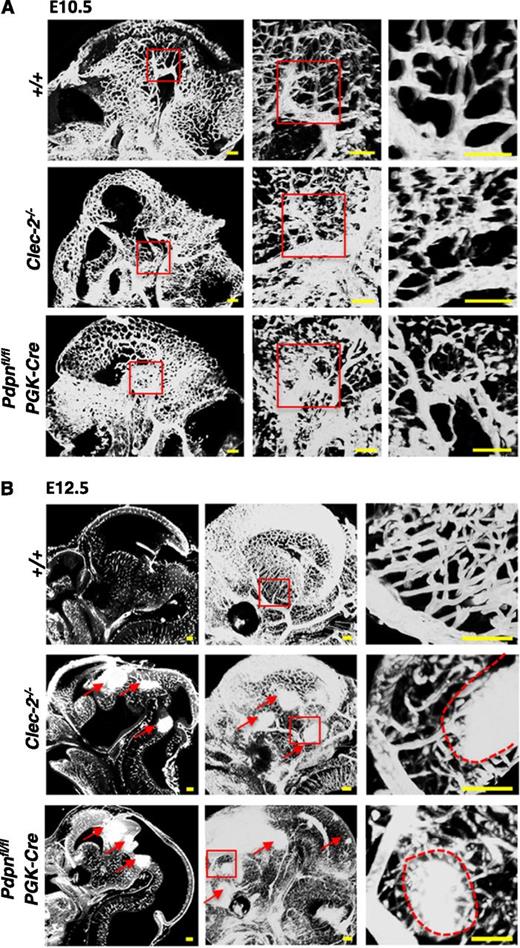
Because of the limitations of 2-dimensional analysis, we used the planar illumination-based microscope modality, ultramicroscopy, to visualize the developing cerebral vasculature in 3 dimensions (3D). Ultramicroscopy creates stacks of optical sections that are digitally analyzed and reconstructed into a single 3D image.35,36 The cerebral vasculature was visualized at E10.5 and E12.5 in Clec-2−/− and Pdpnfl/flPGK-Cre embryos stained with PECAM-1 (Figure 2). Before the appearance of hemorrhages, the vasculature at E10.5 appeared tortuous and abnormally patterned in Clec-2−/− and Pdpnfl/flPGK-Cre embryos compared with the organized, characteristic branching patterns of littermates (Figure 2A). By E12.5, hemorrhages were clearly visible throughout the developing fore-, mid-, and hindbrain in CLEC-2- and podoplanin-deficient mice, as indicated by dense areas of PECAM-1 staining (Figure 2B, red arrows). The size and localization of hemorrhages can only fully be appreciated by a complete spatial reconstruction of the entire embryonic brain that is provided by our ultramicroscopy approach (supplemental Videos 1-3). Higher-magnification images of the vasculature at E12.5 revealed hemorrhages forming among aberrantly patterned vascular networks in Clec-2−/− and Pdpnfl/flPGK-Cre embryos (Figure 2B, right, red dashed lines outline hemorrhages). Developing vessels were discontinuous and appeared prone to hemorrhage compared with the intricately branched sheets of vessels forming in littermate controls (Figure 2B, right). The length of vessels and the number of branch points were quantified using the hindbrain model developed by Fantin et al (supplemental Figure 4).37 Although a trend for increased vessel length and brain points was observed in CLEC-2- and podoplanin-deficient mice, the defect mainly lies in the tortuosity of vessels.
Hemorrhages develop within tortuous vascular networks in CLEC-2- and podoplanin-deficient mice. Whole mount immunostaining of PECAM-1+ blood vessels in whole embryonic heads of Clec-2−/− and Pdpnfl/flPGK-Cre embryos at (A) E10.5 and (B) E12.5 (Clec-2−/−; E10.5, n = 3; E12.5, n = 3; Pdpnfl/flPGK-Cre; E10.5, n = 4; E12.5, n = 3) and littermate controls (E10.5, n = 4; E12.5, n = 5). PECAM-1+ vessels in the whole embryonic head are shown as a sagittal section (z-dimension = 100 μm) taken from a 3-dimensional reconstructed image of optical sections (A, left). Red boxes in the left panel mark areas of higher magnification depicted in the middle panel where a 3-dimensional image shows abnormally patterned, tortuous capillaries branching off a large cerebral vessel in Clec-2−/− and Pdpnfl/flPGK-Cre mice (z-dimension = 650 μm). Red boxes in the second panel depict a further magnified area displayed as a single sagittal section in the third panel (z-dimension = 250 μm). (B) At E12.5, sagittal sections taken from 3-dimensional movies (supplemental Videos 1-3) show hemorrhages located throughout the fore-, mid-, and hindbrain in CLEC-2-deficient and Pdpnfl/flPGK-Cre mice only (left, red arrows). A 3-dimensional reconstruction of optical sections shows a clear well-patterned PECAM-1-stained vascular network in wild-type mice compared with the disorganized vascular network in Clec-2−/− and and Pdpnfl/flPGK-Cre mice (middle, red arrows mark hemorrhages; z-dimension = 1500 μm). Red boxes in the middle panel mark areas of higher magnification depicted in the third panel of cerebral capillaries branching off a major vessel leading to hemorrhage (outlined by a red dashed line) in Clec-2−/− and Pdpnfl/flPGK-Cre mice (z-dimension = 300 μm). Images were processed using Imaris software. Scale bars, 100 μm.
Hemorrhages develop within tortuous vascular networks in CLEC-2- and podoplanin-deficient mice. Whole mount immunostaining of PECAM-1+ blood vessels in whole embryonic heads of Clec-2−/− and Pdpnfl/flPGK-Cre embryos at (A) E10.5 and (B) E12.5 (Clec-2−/−; E10.5, n = 3; E12.5, n = 3; Pdpnfl/flPGK-Cre; E10.5, n = 4; E12.5, n = 3) and littermate controls (E10.5, n = 4; E12.5, n = 5). PECAM-1+ vessels in the whole embryonic head are shown as a sagittal section (z-dimension = 100 μm) taken from a 3-dimensional reconstructed image of optical sections (A, left). Red boxes in the left panel mark areas of higher magnification depicted in the middle panel where a 3-dimensional image shows abnormally patterned, tortuous capillaries branching off a large cerebral vessel in Clec-2−/− and Pdpnfl/flPGK-Cre mice (z-dimension = 650 μm). Red boxes in the second panel depict a further magnified area displayed as a single sagittal section in the third panel (z-dimension = 250 μm). (B) At E12.5, sagittal sections taken from 3-dimensional movies (supplemental Videos 1-3) show hemorrhages located throughout the fore-, mid-, and hindbrain in CLEC-2-deficient and Pdpnfl/flPGK-Cre mice only (left, red arrows). A 3-dimensional reconstruction of optical sections shows a clear well-patterned PECAM-1-stained vascular network in wild-type mice compared with the disorganized vascular network in Clec-2−/− and and Pdpnfl/flPGK-Cre mice (middle, red arrows mark hemorrhages; z-dimension = 1500 μm). Red boxes in the middle panel mark areas of higher magnification depicted in the third panel of cerebral capillaries branching off a major vessel leading to hemorrhage (outlined by a red dashed line) in Clec-2−/− and Pdpnfl/flPGK-Cre mice (z-dimension = 300 μm). Images were processed using Imaris software. Scale bars, 100 μm.
To confirm a role for podoplanin specifically in the developing nervous system, we crossed Pdpnfl/fl mice to mice expressing Cre driven by the nestin promoter (Nes-Cre). The Nes-Cre transgenic line specifically targets neural progenitors from E8.5 before the onset of neural vascularization.30 We confirmed that podoplanin was successfully deleted from the neural tube of Pdpnfl/flNes-Cre embryos by immunofluorescence (supplemental Figure 2B). Pdpnfl/flNes-Cre embryos developed cerebral hemorrhages by E12.5, reminiscent of Pdpnfl/flPGK-Cre and Clec-2−/− embryos (Figure 3). Three-dimensional analysis of the cerebral vasculature in Pdpnfl/flNes-Cre embryos again revealed areas of defective vascular integrity, resulting in hemorrhaging among visibly tortuous blood vessels, resulting in the vast accumulation of blood in the ventricles (supplemental Figure 5; supplemental Video 4). Although hemorrhages were consistent and equally widespread in Pdpnfl/flNes-Cre embryos, the size of parenchymal hemorrhages was often smaller, indicating a slightly milder phenotype than in Clec-2−/− and Pdpnfl/flPGK-Cre embryos. Although the lymphatic system is absent from the brain, we excluded a role for endothelial-derived podoplanin in maintaining cerebral vascular integrity, showing no evidence of hemorrhaging in Pdpnfl/flTie2-Cre embryos at E12.5 (supplemental Figure 6). These results provide strong evidence for a role of podoplanin specifically on neuro-epithelial cells interacting with CLEC-2 to maintain cerebral vascular integrity.
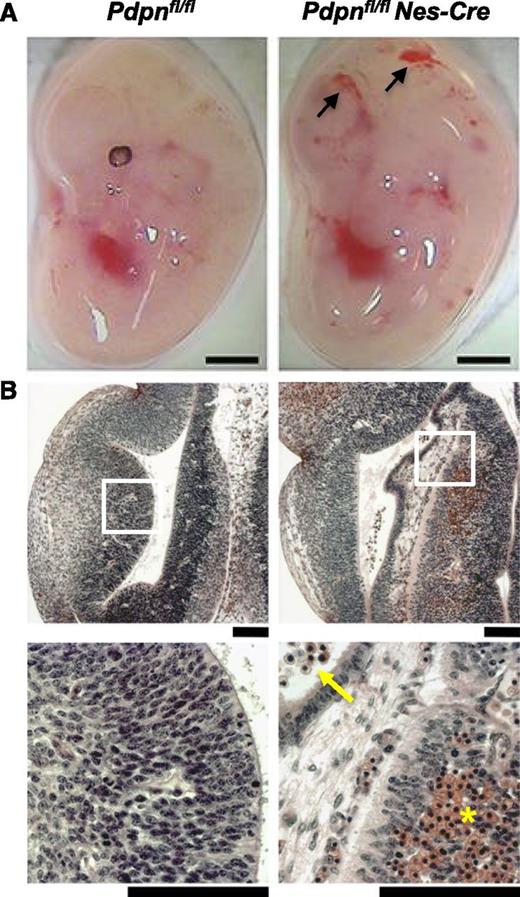
Loss of podoplanin in the neural tube causes cerebral hemorrhages. (A) Pdpnfl/flNes-Cre embryos develop hemorrhages in the brain by E12.5 (n = 4), whereas Pdpnfl/fl littermates appear normal (n = 3). Scale bars, 1 mm. (B) H&E coronal sections from E12.5 embryo heads of Pdpnfl/fl (n = 3) and Pdpnfl/flNes-Cre (n = 4) embryos. White boxes in the middle panel magnify areas in the bottom panel. Yellow arrows indicate reticulated red blood cells in the ventricles, and yellow asterisks (*) marks an area of hemorrhage in the neuro-epithelium. Scale bars, 50 μm.
Loss of podoplanin in the neural tube causes cerebral hemorrhages. (A) Pdpnfl/flNes-Cre embryos develop hemorrhages in the brain by E12.5 (n = 4), whereas Pdpnfl/fl littermates appear normal (n = 3). Scale bars, 1 mm. (B) H&E coronal sections from E12.5 embryo heads of Pdpnfl/fl (n = 3) and Pdpnfl/flNes-Cre (n = 4) embryos. White boxes in the middle panel magnify areas in the bottom panel. Yellow arrows indicate reticulated red blood cells in the ventricles, and yellow asterisks (*) marks an area of hemorrhage in the neuro-epithelium. Scale bars, 50 μm.
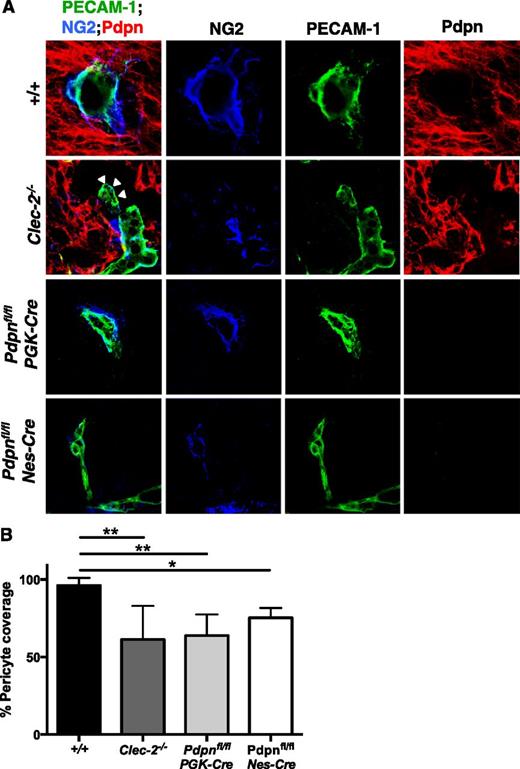
Despite the clear defects in development, blood vessels in CLEC-2- and podoplanin-deficient mice successfully sprout from the PNVP to extend throughout the neuro-epithelium, indicating that the initial stages of angiogenesis, driven primarily by the VEGF and Wnt-β-catenin pathways, are largely unperturbed.4,38,39 In view of this, we focused our attention on the maturation of cerebral vessels, a process by which leaky, nascent angiogenic vessels are transformed into a structurally supported vascular network through the recruitment of ECM proteins, mural cells, and neurons. Among these mural cells are pericytes, which overlay endothelial cell junctions to support smaller arterioles, venules, and capillaries.40 Immunostaining of tissue sections revealed a significant reduction in the number of NG2-positive pericytes recruited to PECAM-1-positive blood vessels in the neuro-epithelium of Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre embryos compared with littermates (Figure 4). Blood vessels were often distended from the surrounding neuro-epithelium (Figure 4A, white arrowheads), similar to that observed on H&E sections (Figure 1D; supplemental Figure 1B).
Altered pericyte recruitment to cerebral blood vessels in CLEC-2- and podoplanim-deficient mice. (A) Immunostaining of frozen sections of embryonic heads at E11.5 show pericytes (NG2, blue) adhering to the surface of blood vessels (PECAM-1, green) within the Pdpn (Pdpn, red) expressing neuro-epithelium in wild-type (+/+, includes Pdpnfl/fl littermates; n = 8), Clec-2−/− (n = 3), Pdpnfl/flPGK-Cre (n = 3), and Pdpnfl/flNes-Cre (n = 3) embryos. White arrowheads in Clec-2−/− embryos indicate where blood vessels are distended from the surrounding neuro-epithelium. Scale bars, 10 μm. (B) A reduction in the total number of pericytes in association with blood vessels was observed in Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre embryos. For each n = 1, a minimum of 4 different sections with 8 images per section were analyzed. Statistical significance was measured by a 1-way analysis of variance with a Tukey posttest, where *P < .05 and **P < .01. Error bars show means ± standard error of the mean.
Altered pericyte recruitment to cerebral blood vessels in CLEC-2- and podoplanim-deficient mice. (A) Immunostaining of frozen sections of embryonic heads at E11.5 show pericytes (NG2, blue) adhering to the surface of blood vessels (PECAM-1, green) within the Pdpn (Pdpn, red) expressing neuro-epithelium in wild-type (+/+, includes Pdpnfl/fl littermates; n = 8), Clec-2−/− (n = 3), Pdpnfl/flPGK-Cre (n = 3), and Pdpnfl/flNes-Cre (n = 3) embryos. White arrowheads in Clec-2−/− embryos indicate where blood vessels are distended from the surrounding neuro-epithelium. Scale bars, 10 μm. (B) A reduction in the total number of pericytes in association with blood vessels was observed in Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre embryos. For each n = 1, a minimum of 4 different sections with 8 images per section were analyzed. Statistical significance was measured by a 1-way analysis of variance with a Tukey posttest, where *P < .05 and **P < .01. Error bars show means ± standard error of the mean.
Ultrastructural analysis of cerebral microvessels at E11.5 by electron microscopy showed the endothelium in wild-type vessels enclosing a lumen containing nucleated fetal red blood cells (Figure 5). The thick, supportive basal lamina noticeable in mature microvessels could not be observed at this stage of development, but tight junctions could be seen to connect adjacent endothelial cells. Pericytes and neuro-epithelial cells were intimately associated with the endothelium, providing structural support for developing vessels. In Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre vessels, endothelial cell junctions are well established, with endothelial cell flaps clearly visible (Figure 5). Despite this, vascular lumens appear expanded, and the endothelium was enriched in vacuoles, appearing fragile, tortuous, and prone to hemorrhage. Large gaps were visible between the endothelial cell layer and the surrounding pericytes and neuro-epithelial cells, precluding their supportive role to the enclosed vessel (Figure 5).
Ultrastructural analysis of Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre microvessels at E11.5 by electron microscopy. In wild-type embryos (+/+, includes Pdpnfl/fl littermates; n = 4), endothelial cells enclose a vascular lumen containing a nucleated fetal erythrocyte and are connected by tight junctions (yellow arrowheads). The endothelium is closely associated with surrounding pericyte foot processes and neuro-epithelial cells. In Clec-2−/− (n = 3), Pdpnfl/flPGK-Cre (n = 4), and Pdpnfl/flNes-Cre (n = 4) microvessels, vascular lumens appear expanded, and their surrounding endothelium is enriched in vacuoles. Inter-endothelial junctions remain tightly closed and secured by endothelial cell flaps (yellow arrowheads). Large gaps between the endothelium and overlying pericytes and neuro-epithelial cells are noticeable. NE, neuro-epithelial cell; BV, blood vessel; RBC, nucleated red blood cell; P, pericyte; E, endothelial cell. Original magnification: (left) ×7500; (right) ×15 000. Scale bar, 5 μm.
Ultrastructural analysis of Clec-2−/−, Pdpnfl/flPGK-Cre, and Pdpnfl/flNes-Cre microvessels at E11.5 by electron microscopy. In wild-type embryos (+/+, includes Pdpnfl/fl littermates; n = 4), endothelial cells enclose a vascular lumen containing a nucleated fetal erythrocyte and are connected by tight junctions (yellow arrowheads). The endothelium is closely associated with surrounding pericyte foot processes and neuro-epithelial cells. In Clec-2−/− (n = 3), Pdpnfl/flPGK-Cre (n = 4), and Pdpnfl/flNes-Cre (n = 4) microvessels, vascular lumens appear expanded, and their surrounding endothelium is enriched in vacuoles. Inter-endothelial junctions remain tightly closed and secured by endothelial cell flaps (yellow arrowheads). Large gaps between the endothelium and overlying pericytes and neuro-epithelial cells are noticeable. NE, neuro-epithelial cell; BV, blood vessel; RBC, nucleated red blood cell; P, pericyte; E, endothelial cell. Original magnification: (left) ×7500; (right) ×15 000. Scale bar, 5 μm.
We previously showed that constitutive deletion of the tyrosine kinase, Syk, also leads to development of hemorrhages in the brain by E12.5. A similar phenotype, albeit less severe, is seen when deleting Syk or CLEC-2 in the megakaryocyte/platelet lineage, supporting a role for CLEC-2-induced platelet activation in this phenotype.18 To dissect the mechanism by which CLEC-2- induced platelet activation safeguards the developing cerebral vasculature, we studied embryos from mice deficient in αIIb, which together with β3 forms the major platelet integrin, as well as mice with secretion defects, including mice deficient in neurobeachin-like 2 protein (Nbeal-2−/−); which lack α-granules, mice deficient in Munc13-4 (Unc13d−/−), in which platelet dense-granule secretion is abolished and platelet α-granule secretion is reduced; and double-deficient Nbeal-2−/−Unc13d−/− mice.
Although there was no evidence of hemorrhaging in Unc13d−/− embryos (data not shown), hemorrhages were observed in 2 of 12 Nbeal-2−/− embryos (supplemental Figure 7) and in 6 of 8 Nbeal-2−/−Unc13d−/− embryos (Figure 6), whereas no hemorrhages were seen in littermate controls (n = 10). A comparable phenotype was observed in αIIb−/− embryos, where blood was visible in the ventricles from E11.5 (data not shown), becoming more prominent by E12.5 in 6 of 6 embryos compared with the absence of significant hemorrhaging in 7 littermate controls (Figure 6). In all cases, hemorrhages were not identifiable by histology in the parenchymal tissue, suggesting a more discrete defect in vascular integrity that leads to the accumulation of blood in the ventricles. Furthermore, hemorrhages were not observed on H&E sections at E14.5 in αIIb−/− (n = 5) and Nbeal-2−/− (n = 10) embryos (data not shown), suggesting they had largely resolved, whereas hemorrhages were seen to persist past E14.5 in Clec-2−/− (n > 10), Pdpnfl/flPGK-Cre (n = 5), and Pdpnfl/flNes-Cre (n = 6) embryos (data not shown).
Evidence for a role for both platelet aggregation and secretion in maintaining cerebral vascular integrity. (A) Mice deficient in the major platelet integrin subinit αIIb present with cerebral hemorrhaging at E12.5 (black arrows; 6 of 6 αIIb−/− embryos). Hemorrhages were also observed in 6 of 8 embryos at E12.5 from mice in which both platelet α-granule and dense-granule secretion is abolished (black arrows; Nbeal-2−/−Unc13d−/−). Scale bars, 1 mm. (B) H&E coronal sections from E12.5 embryo heads show the accumulation of erythrocytes in the ventricles of αIIb−/− (n = 6) and Nbeal-2−/−Unc13d−/− (n = 6 of 8) embryos (red arrows), whereas only a minor hemorrhage was observed in 1 of 17 wild-type littermate controls (+/+). White boxes show magnified areas in the lower panel. Scale bars, 50 μm.
Evidence for a role for both platelet aggregation and secretion in maintaining cerebral vascular integrity. (A) Mice deficient in the major platelet integrin subinit αIIb present with cerebral hemorrhaging at E12.5 (black arrows; 6 of 6 αIIb−/− embryos). Hemorrhages were also observed in 6 of 8 embryos at E12.5 from mice in which both platelet α-granule and dense-granule secretion is abolished (black arrows; Nbeal-2−/−Unc13d−/−). Scale bars, 1 mm. (B) H&E coronal sections from E12.5 embryo heads show the accumulation of erythrocytes in the ventricles of αIIb−/− (n = 6) and Nbeal-2−/−Unc13d−/− (n = 6 of 8) embryos (red arrows), whereas only a minor hemorrhage was observed in 1 of 17 wild-type littermate controls (+/+). White boxes show magnified areas in the lower panel. Scale bars, 50 μm.
In light of the clear redundancy between CLEC-2-induced platelet activation pathways that are involved in maintaining cerebral vascular integrity, we considered a role for the bioactive lipid sphingosine-1 phosphate (S1-P). Platelets store a rapidly deployable pool of S1-P that has been demonstrated to be released on CLEC-2-induced platelet activation, changing local S1-P concentrations and influencing endothelial barrier integrity in lymph nodes.41,42 Furthermore, deletion of the S1-P receptor 1 globally, and specifically on endothelial cells, has been shown to cause extensive hemorrhaging throughout the developing brain, thereby mimicking the phenotype in CLEC-2- and podoplanin-deficient mice.43,44 However, we observed no evidence of cerebral hemorrhage in mice deficient in platelet-derived S1-P (Sphk1fl/flSphk2−/−-PF4-Cre; supplemental Figure 8), suggesting platelet S1-P is not required for maintaining cerebral vascular integrity at this time in embryonic development.
Discussion
In our initial characterization of CLEC-2-deficient mice, we reported that mice deficient in the platelet C-type lectin-like receptor, CLEC-2, or the downstream-signaling tyrosine kinase, Syk, develop cerebral hemorrhages at E12.5 and speculated that this was mediated by the interaction of platelets with podoplanin on the choroid plexus.18 In the present study, we show that hemorrhages develop between E10.5 and E11.5, a critical time point for vascularization of the neural tube and before the choroid plexus has fully formed. We further show through the generation of a mouse model that allows inducible deletion of podoplanin at the 2-cell stage that mice deficient in podoplanin develop cerebral hemorrhages at the same stage of development. Furthermore, using 3D ultramicroscopy, we were able to show a similar location and scale of hemorrhages in Pdpnfl/flPGK-Cre and Clec-2−/− embryos in association with early developmental defects in vessel formation. The pattern of hemorrhaging and vascular defects was indistinguishable in the 2 sets of mutant embryos.
Podoplanin is widely expressed on neuro-epithelial cells in the developing neural tube and was selectively deleted on this cell population using the Nes-Cre transgene. Pdpnfl/flNes-Cre embryos developed hemorrhages and defects in vascular development at the same timescale as Pdpnfl/flPGK-Cre and Clec-2−/− embryos. Although the accumulation of blood in the ventricles of Pdpnfl/flNes-Cre embryos was comparable to Pdpnfl/flPGK-Cre and Clec-2−/− embryos, the size of parenchymal hemorrhages was slightly reduced. There is no evidence for expression of podoplanin outside of the neuro-epithelium in the central nervous system, suggesting the slightly milder phenotype is a result of incomplete efficiency of the Nes-Cre transgene, which becomes active at E8.5 compared with the activation of PGK-Cre from the 2-cell stage.
To further support a role for neuro-epithelial podoplanin, we showed both a reduction in pericyte recruitment and a marked disruption in the association of mural cells to the visibly tortuous endothelium of cerebral vessels in Pdpnfl/flNes-Cre, Pdpnfl/flPGK-Cre and Clec-2−/− embryos. This phenotype is characteristic of a defect in vessel maturation and has been observed in other studies where key signaling pathways that guide mural cell and ECM recruitment are disrupted.7-9,11
Having established that podoplanin on neuro-epithelial cells is critical for the development and integrity of the cerebral vasculature, we sought to investigate the mechanism that governs this process. Previous work by our group showed hemorrhages in the brains of mice with a megakaryocyte/platelet specific deletion of CLEC-2 or the downstream signaling molecule Syk. Taken together with the lack of significant CLEC-2 expression on other circulating hematopoietic cells during development, this provides strong evidence of a causal relationship for neuro-epithelial-expressed podoplanin in regulating platelet activation through CLEC-2 to safeguard the integrity of the developing cerebral vasculature.
With this in mind, we sought to identify the mechanism by which activated platelets guide and maintain vascular integrity during development. Strikingly, we saw a hemorrhagic phenotype in mice deficient in one of the subunits of the major platelet integrin, αIIb, from E11.5. Thus, hemorrhaging is presumably influenced by a loss of integrin αIIbβ3-mediated platelet adhesion and/or aggregation. Although this role of the integrin is a component of classical hemostatic function of platelets, this is the first description to our knowledge of hemorrhaging associated specifically with loss of this hemostatic pathway during development. Remarkably, however, hemorrhages in αIIb-deficient mice appeared to resolve by E14.5, whereas in CLEC-2- and podoplanin-deficient mice, hemorrhages were seen to persist. This suggests that an additional mechanism is influenced by the loss of CLEC-2 activation.
Platelets are powerful secretory cells and release a range of bioactive molecules on activation. In this study, a specific role for α-granule secretion was considered, given their enrichment in growth factors, such as VEGF and various hemostatic proteins including von Willebrand factor. However, the mild hemorrhagic phenotype observed in NBEAL-2-deficient embryos, with <20% exhibiting hemorrhaging, argues against a major role of platelet α-granule secretion in development and is consistent with the mild bleeding diathesis observed in adult NBEAL-2 mice.32 We further considered a role for platelet-dense granules, which release a range of nonprotein molecules that can promote platelet activation, but saw no defect in vascular development in Unc13d−/− embryos.33 However, we did observe hemorrhaging in 75% of embryos where both α-granule and dense-granule secretion was abolished. These data strongly suggest a significant level of redundancy exists between different platelet activation pathways to compensate for the targeted loss of a single pathway.
Recent studies by our group have demonstrated the ability of CLEC-2 to cluster podoplanin on the surface of cells, which is proposed to induce podoplanin signaling through ERM proteins, as well as supporting platelet adhesion.45 A number of in vitro studies have associated podoplanin-ERM signaling with changes in cell motility, adhesion, and shape change, raising the possibility for platelets to induce neuro-epithelial cell behavior and influence their interactions with surrounding mural cells and the endothelium.46-48 This supports the concept of reciprocal signaling between CLEC-2 and podoplanin on platelets and neuro-epithelial cells, respectively, which may account for the enhanced phenotype in CLEC-2- and Syk-deficient platelets relative to platelets deficient in the major platelet integrin or in granule secretion.
In conclusion, we propose that during the initial vascularization of the neural tube, the activation of CLEC-2 by podoplanin is critical in maintaining cerebral vascular integrity. Although platelet aggregates plug the vessel wall to prevent hemorrhage, we propose that secreted molecules function to recruit cells and matrix components to developing vessels. In the absence of this interaction, vessels become tortuous and prone to hemorrhage, as shown in the accompanying model (supplemental Figure 9). It was recently reported that platelet infusions in high-risk preterm infants enhance hemostasis and reduce the risk of IVH.49 In this context, the present observations are of great interest as they provide a potential explanation for IVH and identify new pathways that can be targeted for noninvasive therapeutic intervention of IVH.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The author thank Drs Beata Grygielska, Danai Bem, and Sian Lax for assistance with genotyping, TEM, and animal licensing, respectively; the Centre for Electron Microscopy and the Biomedical Services unit for technical support; and Drs Alice Pollitt, Craig Hughes, Guillaume Desanti, and Leyre Navarro Núñez for valuable discussion.
This work was supported by the British Heart Foundation (RG/13/18/30563) and the Wellcome Trust (088410). K.L.L. was supported by the Medical Research Council and the British Heart Foundation (RG/13/18/30563). S.P.W. holds a British Heart Foundation Chair (Ref CH/03/003)
Authorship
Contribution: K.L.L. and B.A.F. designed the research, performed the research, and analyzed data; K.L.L. wrote the manuscript; R.H. and F.K. assisted with microscopy and provided essential reagents; C.D. and S.L.G. performed experimental work; B.N., E.C., J.F., and C.B. contributed essential reagents; and S.P.W. contributed to experimental design, discussion, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steve Watson, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; e-mail: s.p.watson@bham.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal