Key Points
Human FRβ-specific CAR T cells target AML in vitro and in vivo without toxicity against healthy bone marrow HSCs.
Combination with ATRA-mediated receptor upregulation may augment FRβ-directed CAR therapy of AML.
Abstract
T cells expressing a chimeric antigen receptor (CAR) can produce dramatic results in lymphocytic leukemia patients; however, therapeutic strategies for myeloid leukemia remain limited. Folate receptor β (FRβ) is a myeloid-lineage antigen expressed on 70% of acute myeloid leukemia (AML) patient samples. Here, we describe the development and evaluation of the first CARs specific for human FRβ (m909) in vitro and in vivo. m909 CAR T cells exhibited selective activation and lytic function against engineered C30-FRβ as well as endogenous FRβ+ AML cell lines in vitro. In mouse models of human AML, m909 CAR T cells mediated the regression of engrafted FRβ+ THP1 AML in vivo. In addition, we demonstrated that treatment of AML with all-trans retinoic acid (ATRA) enhanced FRβ expression, resulting in improved immune recognition by m909 CAR T cells. Because many cell surface markers are shared between AML blasts and healthy hematopoietic stem and progenitor cells (HSCs), we evaluated FRβ expression and recognition of HSCs by CAR T cells. m909 CAR T cells were not toxic against healthy human CD34+ HSCs in vitro. Our results indicate that FRβ is a promising target for CAR T-cell therapy of AML, which may be augmented by combination with ATRA.
Introduction
Acute myeloid leukemia (AML) remains a disease with a dismal clinical prognosis. Although induction chemotherapy generates remission in the vast majority of patients, nearly all of them relapse and require intense consolidation chemotherapy or hematopoietic stem cell transplant. The majority of patients will eventually die of their disease, and the 5-year survival rate remains below 50%.1 Therefore, the development of new, more effective therapies for AML is essential.
Chimeric antigen receptor (CAR) T-cell therapy is an innovative new treatment that has recently achieved groundbreaking clinical success in treating therapy-refractory lymphocytic leukemia patients. By linking the single-chain variable fragment (scFv) of a conventional monoclonal antibody to intracellular T-cell receptor signaling domains to produce a chimeric T-cell receptor with antibody-like affinity,2 a patient’s own T cells are genetically redirected to target antigen-positive tumor cells. In the case of acute lymphoblastic leukemia (ALL) patients, CD19-redirected CAR T cells are generating complete remissions in as high as 90% of patients.3-7 One major challenge in translating the astonishing clinical success of CAR T cells in ALL to other types of cancer, including AML, is finding an appropriate tumor cell target.
The folate receptor (FR) family is a group of folate-binding protein receptors comprising 4 known members (α, β, γ, and δ). FRα and FRβ are bound to the cell membrane via glycosyl phosphatidylinositol (GPI) linkages8 and share ∼70% homology, similar affinity for folate, and a common mechanism of receptor endocytosis–mediated folate uptake. However, these receptors differ in tissue distribution: FRα is expressed on epithelial tissues, whereas FRβ is primarily found on myeloid-lineage hematopoietic cells.9 Interestingly, both receptors are commonly upregulated in the setting of malignancy.10-12 FRα-specific CAR T cells were developed more than 20 years ago,13-16 with ongoing optimization and new clinical trials currently being designed to evaluate 4-1BB co-stimulated CAR T cells in ovarian cancer patients.17 However, CAR therapy has not yet been expanded to target FRβ+ malignancies. FRβ is expressed on ∼70% of primary AML patient tumors,12,18 thus making it an attractive target for CAR T-cell therapy. In addition, FRβ expression can be enhanced on AML blasts by treatment with all-trans retinoic acid (ATRA), a drug already approved by the US Food and Drug Administration for subclass M3 AML.19,20 In preclinical models, the efficacy of folate-conjugated drug therapy for targeting FRβ+ AML is improved when combined with ATRA treatment.18 Given the presence of FRβ in AML, its limited expression in normal tissues, and its inducibility by clinically approved drugs, we sought to develop the first CAR to target FRβ. Here, we generated and characterized fully human FRβ-specific CAR constructs containing the m909 scFv,21 previously validated for recognition of human FRβ.
Materials and methods
CAR construction
The m909 scFv21 was polymerase chain reaction (PCR)-amplified using the following primers: 5′-TATTGATCAGCCGAAGTGCAGCTGGTGCAGTCTGG-3′ (BclI) and 5′-TATGCTAGCCTGGCCTAGGACGGTCAGCTTGGTC-3′ (NheI). The PCR product was digested and ligated into third-generation pELNS-GFP-2A lentiviral vectors containing CD3ζ or CD28-CD3ζ signaling domains (pELNS is previously described by Song et al16 and Lanitis et al22 ). Resulting constructs were designated pELNS-GFP-2A-m909-Z/28Z. Vectors encoding green fluorescent protein (GFP), MOV19-Z/28Z (specific for FRα), or CD19-28Z have been previously described.16,22
Lentiviral vector production and T-cell transduction
Lentiviral vectors were produced in 293T cells as previously described.22 Primary human T cells were activated with (alpha) CD3/CD28 beads (Invitrogen) and transduced (multiplicity of infection 5-10) at 20 hours postactivation. T cells were expanded in complete medium (CM) (RPMI 1640–GlutaMAX, 10% fetal bovine serum, 100 U/mL of penicillin, 100 μg/mL of streptomycin) with 50 IU/mL of recombinant human interleukin-2 (IL2). Rested T cells were adjusted to normalize transgene expression before functional assays (full details in supplemental Methods, available on the Blood Web site).
Cell lines
293T cells were purchased from American Type Culture Collection. FR-negative human ovarian cancer cell line C3023 was kindly provided by Dr George Coukos.24 C30 was transduced with lentiviral vectors encoding human FRβ complementary DNA (cDNA) (OriGene) to generate C30-FRβ. Human AML cell lines THP1, MV411, and HL60 were kindly provided by Dr Gwenn Danet-Desoyners (University of Pennsylvania). All cells were grown at 37°C in CM. C30, C30-FRβ, and THP1 were transduced with lentiviral firefly luciferase (fLuc).
T-cell activation and cytokine release assays
CAR+ T cells (1 × 105) were cocultured with 1 × 105 targets in triplicate in 200 μL of CM. After 24 hours, supernatants were assayed for the presence of interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) (BioLegend). IL-2, IFN-γ, tumor necrosis factor α, and macrophage inflammatory protein 1α were measured by flow cytometry with Cytometric Bead Array (BD Biosciences). Cell pellets were labeled for CD3 and CD69 and assessed by flow cytometry. Live, CD3+ gates were used for analysis. In some cases, cell pellets were labeled for FRβ expression.
T-cell proliferation
T cells were labeled with 2.5 μM PKH26 (Sigma-Aldrich) according to the manufacturer’s protocol. T cells were cocultured with targets at a 1:1 ratio in the absence of exogenous IL-2. After 5 days, cells were labeled for CD3 and analyzed for PKH26 dilution. Live, CD3+ gates were used for analysis.
Degranulation
CAR+ T cells (1 × 105) were cocultured with 1 × 105 targets in triplicate in 200 μL of CM with anti-CD107a and anti-CD107b antibodies (or control IgG1) and monensin (BD Biosciences). After 5 to 6 hours, cells were labeled for CD3 and analyzed by flow cytometry. Live, CD3+ gates were used for analysis.
Cytotoxicity
fLuc-transduced targets were plated at 1 × 104 per well in triplicate. CAR+ T cells were added at the indicated effector-to-target (E:T) ratios. Cocultures were incubated overnight in phenol-free CM. The Extended-Glow Bioluminescent Reporter Gene Assay (Applied Biosystems) was used to measure residual luciferase activity from remaining targets, and lysis was calculated as follows: percent lysis = 100 – {[(average signal from T cell–treated wells)/(average signal from untreated target wells)] × 100}. Monocyte lysis was assessed after 4-hour coculture at the indicated E:T ratios plated in triplicate wells. Total cells were labeled for CD3, CD14, and 7AAD. Flow cytometry with CountBright beads (Life Technologies) was used to determine the total number of live CD3−, CD14+ monocytes per well (N). Lysis was calculated as follows: percent lysis = 100 – {[(average N in treated wells)/(average N in untreated wells)] × 100}.
CFU
Bone marrow CD34+ hematopoietic stem and progenitor cells (HSCs) were isolated from healthy donors by magnetic bead selection by the University of Pennsylvania Stem Cell and Xenograft Core. A total of 2000 CD34+ cells were cultured with 2000 CAR+ T cells in V-bottom plates. After 4 hours, wells were diluted in methylcellulose and plated in duplicate. After 14 days, colonies were counted and scored as colony-forming unit (CFU)–granulocyte/erythrocyte/monocyte/megakaryocyte, granulocyte/monocyte, granulocyte, monocyte, or erythroid blast forming unit. Untreated CD34+ cells were cultured in the absence of T cells.
Quantitative RT-PCR
Briefly, human FRβ messenger RNA (mRNA) copy number was calculated using the standard curve method and the ViiA7 real-time (RT)-PCR system (Applied Biosystems). Total RNA was extracted from AML cell lines. cDNA was generated using the High-Capacity-RNA-to-cDNA kit. cDNA template was added to SYBR Green PCR Master Mix and PrimeTime qPCR Primers (Integrated DNA Technologies) specific for hFOLR2 in 5 replicate wells. Known quantities of plasmid-FRβ cDNA were used to construct a 6-point standard curve, with relative mRNA copy numbers represented (full details in supplemental Methods).
ATRA
For pretreatment, cells were cultured in CM with 10 nM ATRA. On day 5, cells were washed and then stained for FRβ surface expression by flow cytometry, processed for RNA extraction, or used in cocultures for T-cell functional assays. In ATRA cotreatment assays, cells were prepared as above (for cytokine release), and 10 nM ATRA was included fresh in the culture media. Three-day supernatants were analyzed for IFN-γ by ELISA.
Flow cytometry
All samples for flow cytometry were labeled in 100 μL of staining buffer (phosphate-buffered saline, 2% fetal bovine serum) at 4°C. Cells were processed on a BD FACSCanto flow cytometer, and results were analyzed with FlowJo 7.6.5 software. Surface expression of FRβ was detected using biotinylated m909-IgG antibody (courtesy of D.S.D.) (see antibody details in supplemental Methods). For in vivo T-cell quantification, 50 μL of blood was obtained from treated mice via retroorbital bleeding and labeled for human CD45, CD3, and CD8. Cell numbers were quantified with BD TruCount Tubes per the manufacturer’s instructions. CD4+ subsets were calculated by subtracting CD8+ from total CD3+. m909 CAR expression was detected by biotin-labeled rabbit-anti-human IgG (H+L) (Jackson ImmunoResearch), MOV19 CAR by biotin-recombinant FRα (R&D Systems), and CD19 CAR by biotin-protein L (GenScript). Secondary labeling with streptavidin-allophycocyanin was used for all CARs.
Xenograft model of AML
(NOD/SCID)/γ-chain−/− mice were obtained from the University of Pennsylvania Stem Cell and Xenograft Core. Female mice 6 to 12 weeks old were bred, treated, and maintained under pathogen-free conditions in-house under protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. A total of 5 × 106 THP1-fLuc tumor cells were inoculated subcutaneously or intravenously. Five mice per group were injected intraperitoneally or by IV injection with 5 × 106 CAR+ T cells at the indicated time points. Tumor growth was assessed by weekly imaging and/or caliper measurements. Tumor volumes were calculated using the following formula: volume = [length × (width)2]/2, where length is greatest longitudinal diameter and width is greatest transverse diameter.
Bioluminescence imaging
Bioluminescence imaging of fLuc+ tumor cells was performed with the Xenogen IVIS imaging system and quantified with the Living Image software (PerkinElmer). Mice were injected intraperitoneally with D-luciferin (150 mg/kg) and imaged under isoflurane anesthesia. Images were recorded until 2 consecutive images showed decreasing signal. Peak signal was determined for each mouse at each designated time point. Pseudocolor images (scale 1 × 106-108) representing light intensity were generated with Living Image.
Statistical analysis
The data are reported as mean ± standard error of the mean (SEM) unless otherwise noted. Statistical analysis was performed using unpaired 2-tailed Student t test. GraphPad Prism 6.0 software was used for statistical calculations. P < .05 was considered significant.
Results
Generation of anti-FRβ CAR
The m909 scFv21 was cloned into previously validated lentiviral constructs containing CD8α hinge and transmembrane domains with intracellular CD3ζ alone or with the CD28 signaling domain in tandem, referred to as m909-Z and m909-28Z, respectively.16,22 Both constructs included GFP separated by a viral 2A peptide to identify transduced T cells (Figure 1A). MOV19 CAR T cells with specificity for human FRα, CD19 CAR T cells with specificity for human CD19, or GFP-transduced T cells were used as controls (not shown). Transduction of human T cells with m909 CAR constructs was reproducibly achieved at efficiencies of 70% to 80%, as measured by both GFP and surface CAR expression (Figure 1B). Transduction efficiencies of m909-Z and m909-28Z were virtually identical. After 2 weeks of expansion, CAR expression was maintained in both CD4+ and CD8+ T cells with a usual CD4:CD8 ratio of 30:70 (Figure 1C).
FRβ CAR construction and expression in primary human T cells. (A) Schematic of lentiviral CAR expression vectors containing the anti-human FRβ scFv m909 linked to either intracellular signaling domains from CD3-ζ alone (m909-Z) or CD28 and CD3-ζ in tandem (m909-28Z). Both constructs also encode GFP separated by a viral T2A (2A) ribosomal skipping peptide. (B) CAR expression in primary human T cells. Expression of m909 CAR in primary human T cells was confirmed by GFP, and surface expression was confirmed by labeling with a rabbit anti-human IgG antibody that binds the human m909 scFv portion of the CAR. Upper and lower rows show results from 2 representative donors. (C) After 13 days of expansion, m909 CAR–transduced T-cell populations comprise ∼70% CD8+ and 30% CD4+. Upper and lower rows show results from 2 representative donors. L, linker; TM, transmembrane domain; UN, untransduced T cells; VH, variable heavy chain; VL, variable light chain.
FRβ CAR construction and expression in primary human T cells. (A) Schematic of lentiviral CAR expression vectors containing the anti-human FRβ scFv m909 linked to either intracellular signaling domains from CD3-ζ alone (m909-Z) or CD28 and CD3-ζ in tandem (m909-28Z). Both constructs also encode GFP separated by a viral T2A (2A) ribosomal skipping peptide. (B) CAR expression in primary human T cells. Expression of m909 CAR in primary human T cells was confirmed by GFP, and surface expression was confirmed by labeling with a rabbit anti-human IgG antibody that binds the human m909 scFv portion of the CAR. Upper and lower rows show results from 2 representative donors. (C) After 13 days of expansion, m909 CAR–transduced T-cell populations comprise ∼70% CD8+ and 30% CD4+. Upper and lower rows show results from 2 representative donors. L, linker; TM, transmembrane domain; UN, untransduced T cells; VH, variable heavy chain; VL, variable light chain.
m909 CAR T cells exhibit antigen-specific reactivity against engineered C30-FRβ
To evaluate FRβ-specific reactivity of m909 CAR T cells, we engineered C30, an FR-negative human ovarian cancer cell line, to constitutively overexpress human FRβ (C30-FRβ; Figure 2A). After overnight coculture of CAR T cells with C30 or C30-FRβ, supernatants were assayed for release of proinflammatory cytokines. m909-Z and m909-28Z CAR T cells selectively secreted IFN-γ in response to C30-FRβ (Figure 2B). m909 CAR T cells also produced tumor necrosis factor α, IL-2, and macrophage inflammatory protein 1α (supplemental Figure 1). m909 CAR+ (GFP+) T cells specifically upregulated surface expression of activation marker CD69 in the presence of C30-FRβ but not C30 (Figure 2C). Because increased in vivo expansion of CAR T cells16 and persistence of transferred T cells in melanoma patients25 correlate with antitumor efficacy, we tested the ability of m909 CAR T cells to proliferate in vitro in response to cell surface FRβ. T cells were labeled with PKH26, and dye dilution by proliferating cells was measured by flow cytometry after 5 days in coculture with the indicated targets. Both m909-Z and m909-28Z exhibited specific proliferation in response to C30-FRβ but not C30 (Figure 2D). Degranulation, as quantified by increased cell surface CD107 expression, is an established surrogate for T-cell lytic function.26 After 6-hour coculture, we observed specific degranulation by m909 CAR+ (GFP+) T cells only in the presence of C30-FRβ (Figure 2F). To evaluate the true lytic capability of m909 CAR T cells, we cocultured C30-FRβ-fLuc with CAR T cells. After overnight incubation, both m909-Z and m909-28Z CAR T cells showed high, dose-dependent lysis of C30-FRβ (Figure 2E).
m909 CAR T cells are reactive against cell surface FRβ on engineered C30-FRβ cell line. To first test the functionality of m909 CARs, the antigen-negative ovarian cancer cell line C30 was transduced to stably overexpress human FRβ cDNA. Cocultures were performed at an E:T ratio of 1:1 unless otherwise noted. Control MOV19-28Z CAR T cells are specific for FRα and do not express GFP. Control GFP T cells express only GFP. Error bars represent mean ± SEM. (A) FRβ expression on engineered C30-FRβ was detected by flow cytometry using biotinylated m909-IgG (black histogram). For comparison, the unmodified parental C30 cells were used as a control (gray histogram). (B) Antigen-specific IFN-γ (IFNg) production by m909 CAR T cells as detected by ELISA from 24-hour coculture supernatants. (C) m909 CAR+ T cells upregulate surface CD69 expression following 24-hour exposure to C30-FRβ. The m909 CAR+ cells are identified by GFP expression (y-axis). (D) m909-Z and m909-28Z CAR T cells proliferate in response to C30-FRβ. PKH26 dilution in labeled T cells was measured by flow cytometry after 5 days in coculture. Percentage of CD3+ cells proliferating (diluted PKH26 compared to day 0) is quantified. P values represent significant differences compared to MOV19-28Z CAR T cells. (E) m909-Z and m909-28Z exhibit specific lysis of C30-FRβ. Target cells were transduced to express fLuc and cocultured with CAR T cells at E:T ratios of 10:1, 3:1, and 1:1. Residual luciferase signal was determined after 18 hours. Percent lysis was determined by luminescence comparison with untreated target wells. (F) m909-Z and m909-28Z exhibit degranulation on coculture with C30-FRβ. CD107a/b surface expression was measured after 5 hours of coculture. CAR+ cells are identified by GFP expression (y-axis). Percentage of CAR+ cells with positive staining for CD107a/b is quantified to the right. P values represent significant increases compared to MOV19-28Z control T cells. *P < .05; **P < .01; ***P < .001.
m909 CAR T cells are reactive against cell surface FRβ on engineered C30-FRβ cell line. To first test the functionality of m909 CARs, the antigen-negative ovarian cancer cell line C30 was transduced to stably overexpress human FRβ cDNA. Cocultures were performed at an E:T ratio of 1:1 unless otherwise noted. Control MOV19-28Z CAR T cells are specific for FRα and do not express GFP. Control GFP T cells express only GFP. Error bars represent mean ± SEM. (A) FRβ expression on engineered C30-FRβ was detected by flow cytometry using biotinylated m909-IgG (black histogram). For comparison, the unmodified parental C30 cells were used as a control (gray histogram). (B) Antigen-specific IFN-γ (IFNg) production by m909 CAR T cells as detected by ELISA from 24-hour coculture supernatants. (C) m909 CAR+ T cells upregulate surface CD69 expression following 24-hour exposure to C30-FRβ. The m909 CAR+ cells are identified by GFP expression (y-axis). (D) m909-Z and m909-28Z CAR T cells proliferate in response to C30-FRβ. PKH26 dilution in labeled T cells was measured by flow cytometry after 5 days in coculture. Percentage of CD3+ cells proliferating (diluted PKH26 compared to day 0) is quantified. P values represent significant differences compared to MOV19-28Z CAR T cells. (E) m909-Z and m909-28Z exhibit specific lysis of C30-FRβ. Target cells were transduced to express fLuc and cocultured with CAR T cells at E:T ratios of 10:1, 3:1, and 1:1. Residual luciferase signal was determined after 18 hours. Percent lysis was determined by luminescence comparison with untreated target wells. (F) m909-Z and m909-28Z exhibit degranulation on coculture with C30-FRβ. CD107a/b surface expression was measured after 5 hours of coculture. CAR+ cells are identified by GFP expression (y-axis). Percentage of CAR+ cells with positive staining for CD107a/b is quantified to the right. P values represent significant increases compared to MOV19-28Z control T cells. *P < .05; **P < .01; ***P < .001.
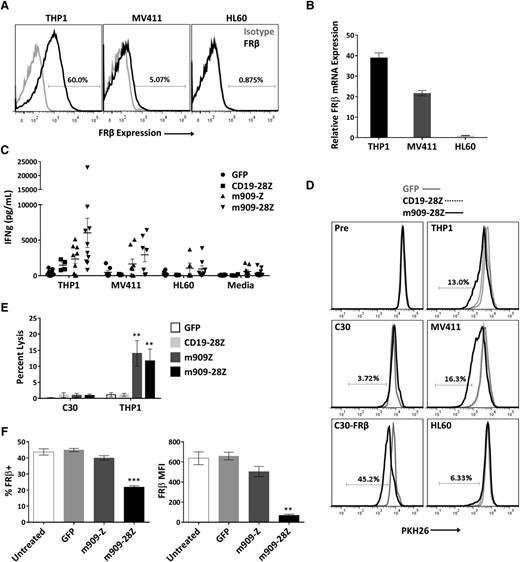
m909 CAR T cells exhibit specific reactivity against endogenous FRβ on human AML
After establishing antigen-specific reactivity for cell surface human FRβ, we next evaluated m909 CAR T-cell function against physiologically relevant levels of antigen in myeloid tumor cells. We acquired 3 human AML cell lines with high, medium, and low (undetectable) surface expression of FRβ (THP1, MV411, and HL60, respectively). Surface protein expression was assessed by labeling with m909-IgG using flow cytometry (Figure 3A), and mRNA expression was confirmed with quantitative RT-PCR (Figure 3B). For all AML experiments, CD19-28Z CAR T cells were used as a control because none of the AML cells expressed CD19 (supplemental Figure 2). After overnight coculture with AML targets, IFN-γ secretion was measured by ELISA (Figure 3C). Because not all CAR T-cell donors respond comparably, we conducted 10 independent experiments using 10 distinct T-cell donors. m909 CAR T cells from most donors produced significantly higher IFN-γ in response to FRβ+ AML targets as compared to CD19-28Z control T cells.
m909-28Z CAR T cells are reactive against endogenous FRβ on human AML cell lines in vitro. To test m909 CAR T-cell reactivity against clinically relevant targets, we acquired 3 human AML cell lines with varying levels of FRβ expression. Cocultures were performed at an E:T ratio of 1:1 unless otherwise noted. Control CD19-28Z CAR T cells are specific for human CD19 and do not express GFP. In media controls, T cells were plated without target cells. Error bars represent mean ± SEM. (A) Surface expression of FRβ on AML cell lines THP1, MV411, and HL60 was determined by flow cytometry using m909-IgG (black) and human IgG isotype control (gray). Percentages represent the proportion of cells with a positive fluorescence signal compared to isotype. (B) Relative FRβ mRNA expression was confirmed using quantitative RT-PCR. Indicated mRNA expression is shown relative to HL60. (C) Antigen-specific IFN-γ (IFNg) secretion was quantified by ELISA after overnight coculture. Each data point represents the mean value of triplicate wells from independent experiments. Represented are n = 10 different normal T-cell donors. (D) m909-28Z CAR T cells proliferate in response to THP1 and MV411, but not HL60, compared to control T cells. PKH26 dilution was measured via flow cytometry before (Pre) and after 5 days in coculture. Overlaying histograms display day-5 PKH26 fluorescence in GFP (gray line), CD19-28Z (dotted black line), and m909-28Z (solid black line) T-cell cocultures with the indicated cell targets. A live, CD3+ gate was used. Percentages represent the proportion of m909-28Z T cells with diluted PKH26 compared to CD19-28Z CAR T cells. (E) m909 CAR T cells exhibit specific lysis of THP1. Luciferase-expressing target cells were cocultured with CAR T cells at an E:T ratio of 1:1. Residual luciferase signal was determined after 24 hours. Percent lysis was determined by luminescence comparison with untreated target wells. Data shown are mean ± SEM of n = 9 independent T-cell donors. P values are calculated compared to CD19-28Z control treated wells. (F) Decreased FRβ expression on THP1 cells surviving overnight coculture with m909 CAR T cells. FRβ surface expression was determined by flow cytometry using m909-IgG and human IgG isotype control. A live, CD3− gate was used to distinguish surviving THP1 cells. The percent of cells showing positive FRβ staining compared to isotype (left) and the FRβ median fluorescence intensity (MFI; right) were determined for triplicate wells (n = 3). P values were determined compared to control GFP T cell–treated wells. **P < .01; ***P < .001.
m909-28Z CAR T cells are reactive against endogenous FRβ on human AML cell lines in vitro. To test m909 CAR T-cell reactivity against clinically relevant targets, we acquired 3 human AML cell lines with varying levels of FRβ expression. Cocultures were performed at an E:T ratio of 1:1 unless otherwise noted. Control CD19-28Z CAR T cells are specific for human CD19 and do not express GFP. In media controls, T cells were plated without target cells. Error bars represent mean ± SEM. (A) Surface expression of FRβ on AML cell lines THP1, MV411, and HL60 was determined by flow cytometry using m909-IgG (black) and human IgG isotype control (gray). Percentages represent the proportion of cells with a positive fluorescence signal compared to isotype. (B) Relative FRβ mRNA expression was confirmed using quantitative RT-PCR. Indicated mRNA expression is shown relative to HL60. (C) Antigen-specific IFN-γ (IFNg) secretion was quantified by ELISA after overnight coculture. Each data point represents the mean value of triplicate wells from independent experiments. Represented are n = 10 different normal T-cell donors. (D) m909-28Z CAR T cells proliferate in response to THP1 and MV411, but not HL60, compared to control T cells. PKH26 dilution was measured via flow cytometry before (Pre) and after 5 days in coculture. Overlaying histograms display day-5 PKH26 fluorescence in GFP (gray line), CD19-28Z (dotted black line), and m909-28Z (solid black line) T-cell cocultures with the indicated cell targets. A live, CD3+ gate was used. Percentages represent the proportion of m909-28Z T cells with diluted PKH26 compared to CD19-28Z CAR T cells. (E) m909 CAR T cells exhibit specific lysis of THP1. Luciferase-expressing target cells were cocultured with CAR T cells at an E:T ratio of 1:1. Residual luciferase signal was determined after 24 hours. Percent lysis was determined by luminescence comparison with untreated target wells. Data shown are mean ± SEM of n = 9 independent T-cell donors. P values are calculated compared to CD19-28Z control treated wells. (F) Decreased FRβ expression on THP1 cells surviving overnight coculture with m909 CAR T cells. FRβ surface expression was determined by flow cytometry using m909-IgG and human IgG isotype control. A live, CD3− gate was used to distinguish surviving THP1 cells. The percent of cells showing positive FRβ staining compared to isotype (left) and the FRβ median fluorescence intensity (MFI; right) were determined for triplicate wells (n = 3). P values were determined compared to control GFP T cell–treated wells. **P < .01; ***P < .001.
To evaluate the proliferative potential of m909 CAR T cells in response to FRβ+ AML, T cells were labeled with PKH26, and dye dilution was measured by flow cytometry after coculture with targets (Figure 3D). Overlaying histograms represent PKH26 fluorescence of GFP, CD19-28Z, or m909-28Z CAR T cells before and after 5-day exposure to the indicated targets. m909-28Z CAR T cells proliferated in response to FRβ+ C30-FRβ, THP1, and MV411 but not FRβ− C30 or HL60; control T cells did not proliferate under any condition. In 3 of 4 T-cell donors evaluated, response to FRβlow MV411 was slightly greater than for FRβhigh THP1. To assess whether this could be due to different levels of non–antigen-specific stimulation by target cells, we evaluated HLA and co-stimulatory ligand expression on the AML cell lines (supplemental Figure 3). Indeed, we found that MV411 expresses more CD86, 41BB-L, and HLA class II compared to THP1.
The lytic capability of m909 CAR T cells against AML was evaluated using THP1-fLuc (Figure 3E). m909 CAR T cells exhibited specific lysis of THP1, but not C30, compared to control CD19-28Z CAR T cells. Lastly, surface FRβ expression was measured on remaining THP1 after overnight coculture (Figure 3F). Cells surviving coculture with m909-28Z CAR T cells had significantly reduced expression as measured by both FRβ median fluorescence intensity and percentage FRβ positive, suggesting that m909 CAR T-cell elimination of THP1 was antigen dependent.
To validate the applicability of m909 CAR T cells to primary AML, we cocultured m909 CAR T cells with peripheral blood cells from 2 patients with validated FRβ expression on blasts. We observed significantly higher IFN-γ secretion from m909-28Z CAR T cells compared to control GFP T cells after overnight coculture (supplemental Figure 4), suggesting that m909 CAR T cells are capable of recognizing FRβ+ primary patient tumor cells in addition to FRβ+ AML cell lines.
Antigen upregulation by ATRA enhances m909 CAR T-cell recognition of AML
We hypothesized that the level of FRβ expression by some AML cells represents a possible limitation to m909 CAR T-cell recognition. ATRA has been reported to increase FRβ expression on AML19 and improve FRβ-targeted liposomal drug delivery in vivo.18 Therefore, we sought to determine whether ATRA-treated AML is more susceptible to targeting with m909 CAR T cells. First, elevated cell surface FRβ expression by THP1 and MV411 AML was observed over a 5-day time course in the presence of 10 nM ATRA (Figure 4A). Increased production of FRβ mRNA was confirmed after 3 and 5 days of ATRA treatment (Figure 4B). Consistent with previous reports,19,27 we observed enhanced expression in FRβ+ THP1 and MV411 but not FRβ− HL60. To assess whether ATRA-induced FRβ expression on target cells sensitized them to m909 CAR T-cell attack, AML cells that had been pretreated for 5 days with ATRA were washed, plated in fresh media, and cocultured with m909 CAR or control GFP T cells. m909-Z and m909-28Z CAR T cells secreted significantly more IFN-γ when cultured with ATRA-pretreated THP1 but not MV411 (Figure 4C). Cytokine secretion in response to ATRA-pretreated HL60 was slightly increased, although receptor levels were not detectably upregulated. To assess whether ATRA-treated AML is more susceptible to CAR T cell–mediated lysis, we cultured untreated or ATRA-pretreated THP1-fLuc with m909 CAR T cells. In addition to IFN-γ secretion, m909 CAR T cells displayed significantly increased lytic activity against ATRA-pretreated THP1 (Figure 4D).
ATRA increases FRβ expression and m909 CAR T-cell recognition of AML cell lines. (A) AML cell lines were treated with (dotted black line) and without (solid black line) 10 nM ATRA for 5 days (d1-d5). Surface FRβ expression was determined each day by flow cytometry with m909-IgG (black) or human IgG isotype control (gray). (B) FRβ mRNA expression was determined before (untreated) and after 3 days and 5 days of 10 nM ATRA treatment. Relative mRNA is shown compared to untreated HL60. Bars represent mean ± SEM of n = 5 replicate wells. P values were calculated for each cell line compared to untreated controls. (C) m909 CAR T cells secrete higher IFN-γ (IFNg) in response to THP1 cells pretreated for 5 days with 10 nM ATRA (gray bars) compared to untreated cells (black bars) in overnight cocultures. (D) THP1-fLuc cells were pretreated with (THP1-ATRA) or without (THP1) 10 nM ATRA for 5 days before coculture with m909 CAR or GFP control T cells at an E:T ratio of 1:1. Percent lysis was determined by residual luciferase activity after overnight coculture. (E) m909 CAR T cells secrete higher IFN-γ after 3 days of coculture in the presence of 10 nM ATRA (gray bars) and AML target cell lines compared to cultures without ATRA (black bars). No significant differences in IFN-γ secretion were observed for m909 T cells activated in the presence of C30-FRβ with or without ATRA. In panels C-E, graphs represent mean ± SEM from n = 3 independent experiments using 3 distinct T-cell donors. P values were calculated for each T-cell subset to compare between untreated and ATRA-treated cell lines. *P < .05; **P < .01; ***P < .001. ns, P > .05.
ATRA increases FRβ expression and m909 CAR T-cell recognition of AML cell lines. (A) AML cell lines were treated with (dotted black line) and without (solid black line) 10 nM ATRA for 5 days (d1-d5). Surface FRβ expression was determined each day by flow cytometry with m909-IgG (black) or human IgG isotype control (gray). (B) FRβ mRNA expression was determined before (untreated) and after 3 days and 5 days of 10 nM ATRA treatment. Relative mRNA is shown compared to untreated HL60. Bars represent mean ± SEM of n = 5 replicate wells. P values were calculated for each cell line compared to untreated controls. (C) m909 CAR T cells secrete higher IFN-γ (IFNg) in response to THP1 cells pretreated for 5 days with 10 nM ATRA (gray bars) compared to untreated cells (black bars) in overnight cocultures. (D) THP1-fLuc cells were pretreated with (THP1-ATRA) or without (THP1) 10 nM ATRA for 5 days before coculture with m909 CAR or GFP control T cells at an E:T ratio of 1:1. Percent lysis was determined by residual luciferase activity after overnight coculture. (E) m909 CAR T cells secrete higher IFN-γ after 3 days of coculture in the presence of 10 nM ATRA (gray bars) and AML target cell lines compared to cultures without ATRA (black bars). No significant differences in IFN-γ secretion were observed for m909 T cells activated in the presence of C30-FRβ with or without ATRA. In panels C-E, graphs represent mean ± SEM from n = 3 independent experiments using 3 distinct T-cell donors. P values were calculated for each T-cell subset to compare between untreated and ATRA-treated cell lines. *P < .05; **P < .01; ***P < .001. ns, P > .05.
Numerous reports suggest direct modulatory effects of ATRA on T cells. In mice, vitamin A deficiency leads to excessive Th1 and impaired Th2 responses,28 and treatment with vitamin A ex vivo decreases Th1 cytokine secretion by activated peripheral blood mononuclear cells,29 suggesting a role for retinoids in negatively regulating Th1 development. Retinoic acid was also shown to impair IFN-γ production in human cells.30 In contrast, others report ATRA enhancement of IL-2–mediated T-cell activation, proliferation, and survival.31-33 To address the possible effects of ATRA on CAR T cells, we cocultured (previously untreated) targets and T cells with or without 10 nM ATRA continually present in the media. Because elevated surface FRβ was not measureable until 2 to 3 days in treatment with ATRA (Figure 4A), 3-day cocultures were performed. IFN-γ was significantly elevated in m909 CAR co-cultures with THP1 and MV411 with ATRA compared to those without ATRA (Figure 4E), indicating maintenance of a Th1 cytokine profile. Because ATRA acts in target cells at the endogenous FRβ promoter,20 C30-FRβ (with FRβ expression driven from the EF1α promoter) is not susceptible to ATRA-induced upregulation. Accordingly, the presence of ATRA did not impact IFN-γ secretion from m909 CAR T cells activated by C30-FRβ, suggesting that direct effects of ATRA on CAR T cells are likely not responsible for their improved reactivity against AML.
m909-28Z CAR T cells reduce AML tumor growth in vivo
After confirming m909 CAR T-cell reactivity against human AML in vitro, we investigated their antitumor activity in vivo. In previous studies, inclusion of the CD28 activation domain in CAR constructs enhanced in vivo performance and persistence of engineered T cells in mice, as well as in patients receiving CD19 CAR T cells.34,35 Therefore, m909-28Z CAR T cells were tested for in vivo reactivity against THP1. GFP and CD19-28Z CAR control T cells were tested in parallel. Immunocompromised (NOD/SCID)/γ-chain−/− mice were inoculated with 5 × 106 THP1-fLuc subcutaneously. Bioluminescent imaging was used to confirm engraftment of tumor cells. On days 8 and 10 after tumor inoculation, mice received 5 × 106 CAR+ (or GFP+) T cells via intraperitoneal injection. Tumor progression was evaluated by luminescence (Figure 5A-B) and by caliper measurements (Figure 5C). Treatment with m909-28Z CAR T cells mediated tumor regression and significantly inhibited THP1 outgrowth. To investigate in vivo expansion/persistence of m909-28Z CAR T cells, we evaluated human CD3+ cells in the peripheral blood of treated mice. Consistent with a productive antitumor response, mice treated with m909-28Z CAR T cells exhibited significantly increased peripheral blood T cells (comprising both CD8+ and CD4+) compared to controls at 4 weeks post–T-cell treatment (Figure 5D). In addition, we tracked CAR expression during treatment and showed that m909-28Z CAR+ T cells persist long-term in THP1-treated mice (supplemental Figure 5). These data suggest that m909-28Z CAR T cells expanded peripherally on specific recognition of FRβ+ tumor in vivo. Because AML is a disseminated systemic disease in humans, we also evaluated m909-28Z CAR T-cell activity against disseminated THP1, delivered by IV injection. m909-28Z CAR T cells also significantly inhibited systemic AML tumor growth in vivo compared to control T cells (supplemental Figure 6A-B). To begin to assess the impact of ATRA on FRβ-directed CAR T cells in vivo, we provided ATRA by intraperitoneal injection during the course of T-cell treatment. In initial results from one study, ATRA did not impact THP1 growth in untreated or T cell–treated mice (supplemental Figure 6C-F), nor did it affect T-cell phenotype when assessed 17 days posttransfer (supplemental Figure 6H-M).
m909-28Z CAR T cells prevent THP1 AML tumor growth in vivo. THP1-fLuc cells (5 × 106) were injected into (NOD/SCID)/γ-chain−/− mice subcutaneously on day (d)0. CAR+ T cells (5 × 106) were given intraperitoneally on days 8 and 10. Tumor growth was monitored by luminescence (A-B) and by caliper measurement (C). Graphs represent mean ± SEM of n = 5 mice per experiment. P values were calculated compared to CD19-28Z–treated control mice. Differences between GFP and CD19-28Z groups did not reach statistical significance at any time point. (D) Preferential expansion and survival of peripheral human T cells in m909-28Z–treated mice compared to control T cells. Peripheral blood was collected on day 38 (4 weeks post–T-cell injection), and absolute numbers of human CD3+ (left), CD8+ (middle), and CD4+ (right) T cells were quantified by flow cytometry and are reported in total cells per microliter of blood. *P < .05; **P < .01. ns, P > .05.
m909-28Z CAR T cells prevent THP1 AML tumor growth in vivo. THP1-fLuc cells (5 × 106) were injected into (NOD/SCID)/γ-chain−/− mice subcutaneously on day (d)0. CAR+ T cells (5 × 106) were given intraperitoneally on days 8 and 10. Tumor growth was monitored by luminescence (A-B) and by caliper measurement (C). Graphs represent mean ± SEM of n = 5 mice per experiment. P values were calculated compared to CD19-28Z–treated control mice. Differences between GFP and CD19-28Z groups did not reach statistical significance at any time point. (D) Preferential expansion and survival of peripheral human T cells in m909-28Z–treated mice compared to control T cells. Peripheral blood was collected on day 38 (4 weeks post–T-cell injection), and absolute numbers of human CD3+ (left), CD8+ (middle), and CD4+ (right) T cells were quantified by flow cytometry and are reported in total cells per microliter of blood. *P < .05; **P < .01. ns, P > .05.
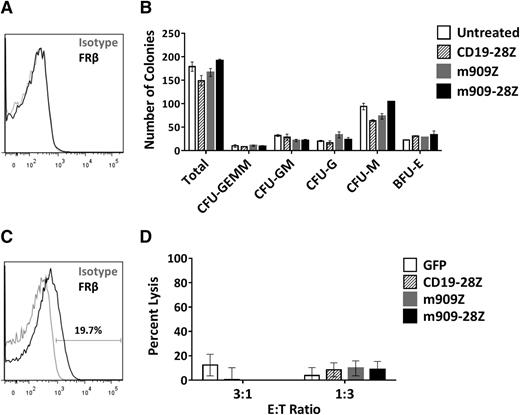
m909 CAR T cells do not inhibit CD34+ colony formation or eliminate FRβ-low healthy monocytes
Many surface markers, including others exploited for CAR therapy of AML (eg, CD123, CD33, and CD38), are shared between AML blasts and normal HSCs. One concern in the development of AML-directed CAR therapies is the potential for depletion of healthy bone marrow progenitors.36-39 FRβ has been reported at low levels on human CD34+ bone marrow HSCs, although this receptor was nonfunctional and unable to bind folate.40 To investigate whether m909 CAR T cells recognize healthy HSCs, we first assessed the binding potential of m909-IgG to normal human bone marrow CD34+ cells. We were unable to detect surface FRβ protein in any of 3 healthy donors by flow cytometry (representative donor, Figure 6A). To test for functional reactivity of m909 CAR T cells against hematopoietic progenitors, we conducted CFU assays after coculture of CD34+ HSCs and CAR T cells. After 4-hour co-incubation, each well was diluted in methylcellulose and cultured for 14 days. Colonies were counted and scored for CFU-granulocyte/erythrocyte/monocyte/megakaryocyte, granulocyte/monocyte, granulocyte, monocyte, or erythroid blast forming unit. Unlike other CARs targeting AML,36-39 neither m909-Z nor m909-28Z CAR T-cell pretreatment inhibited colony formation (Figure 6B). There were no significant differences in the number of total or lineage-specific colonies compared to untreated controls. Furthermore, 5-day ATRA treatment did not induce FRβ in HSCs (2 pools representing 7 normal CD34+ donors were tested) (supplemental Figure 7).
m909 CAR T cells do not inhibit CD34+ HSC colony formation or eliminate FRβ-low healthy monocytes in vitro. (A) After healthy adult human bone marrow CD34+ HSCs were isolated, they were stained for FRβ expression using m909-IgG (black) or human IgG isotype control (gray). One representative donor is shown. (B) Isolated CD34+ HSCs were cocultured with CAR T cells at an E:T ratio of 1:1 for 4 hours. Wells were diluted in methylcellulose and cultured for 14 days. Total colonies were counted and scored for CFU-GEMM, CFU-GM, CFU-G, CFU-M, and BFU-E. There were no significant differences between total or lineage-specific colonies for any of the treated groups compared to untreated CD34+ HSCs. Bar graphs represent mean + standard deviation for n = 2 wells per condition. Results are representative of 4 independent experiments and 3 normal bone marrow donors. (C) Low surface expression of FRβ on normal human monocytes detected by flow cytometry using m909-IgG (black) or human IgG isotype control (gray). One representative of 7 normal donors is shown. (D) CD14+ human monocytes were cocultured with indicated engineered T cells at E:T ratios of 3:1 and 1:3 for 4 hours, after which the total number of live CD3−, CD14+ monocytes per well was quantified by bead-based flow cytometry. Data incorporates results using 3 different CAR T-cell donors and 4 different monocyte donors as target cells. BFU-E, erythroid blast forming unit; G, granulocyte; GEMM, granulocyte/erythrocyte/monocyte/megakaryocyte; GM, granulocyte/monocyte; M, monocyte.
m909 CAR T cells do not inhibit CD34+ HSC colony formation or eliminate FRβ-low healthy monocytes in vitro. (A) After healthy adult human bone marrow CD34+ HSCs were isolated, they were stained for FRβ expression using m909-IgG (black) or human IgG isotype control (gray). One representative donor is shown. (B) Isolated CD34+ HSCs were cocultured with CAR T cells at an E:T ratio of 1:1 for 4 hours. Wells were diluted in methylcellulose and cultured for 14 days. Total colonies were counted and scored for CFU-GEMM, CFU-GM, CFU-G, CFU-M, and BFU-E. There were no significant differences between total or lineage-specific colonies for any of the treated groups compared to untreated CD34+ HSCs. Bar graphs represent mean + standard deviation for n = 2 wells per condition. Results are representative of 4 independent experiments and 3 normal bone marrow donors. (C) Low surface expression of FRβ on normal human monocytes detected by flow cytometry using m909-IgG (black) or human IgG isotype control (gray). One representative of 7 normal donors is shown. (D) CD14+ human monocytes were cocultured with indicated engineered T cells at E:T ratios of 3:1 and 1:3 for 4 hours, after which the total number of live CD3−, CD14+ monocytes per well was quantified by bead-based flow cytometry. Data incorporates results using 3 different CAR T-cell donors and 4 different monocyte donors as target cells. BFU-E, erythroid blast forming unit; G, granulocyte; GEMM, granulocyte/erythrocyte/monocyte/megakaryocyte; GM, granulocyte/monocyte; M, monocyte.
We recently published a study highlighting the presence of FRβ on a subset of peripheral blood monocytes in healthy donors.41 This finding was confirmed in the present study, with FRβ expression from 1 representative donor shown in Figure 6C. Five-day ATRA treatment did not enhance FRβ expression in healthy monocytes from any of the 3 donors evaluated (data not shown). To assess the potential for myeloid toxicity with FRβ-directed CAR T cells, we cocultured m909 or control T cells with CD14+ monocytes isolated from healthy donors. Four-hour lysis of CD14+ monocytes was assessed using flow cytometry. We did not observe any significant lysis of monocytes by m909 CAR T cells compared to controls (Figure 6D). Together, our findings suggest that FRβ can be safely pursued as a target for CAR T-cell therapy of AML without harming essential healthy HSCs or normal monocytes expressing low levels of FRβ.
Discussion
Here, we describe the first CAR specific for human FRβ for the targeting of AML. m909 CAR constructs are fully human in composition, addressing the issue of transgene immunogenicity reported elsewhere with CARs using mouse scFvs.14,42,43 Our initial data using C30 and C30-FRβ, a cell line engineered for FRβ expression, confirm the feasibility of targeting cell surface human FRβ with CAR T cells. This model provides a robust positive control for CAR T-cell specificity by providing high levels of antigen for CAR stimulation in a true negative epithelial cell line. The m909 CAR platform allows for efficient and specific targeting, as demonstrated by in vitro coculture assays resulting in cytokine production, activation marker upregulation, proliferation, and target cell lysis when high levels of antigen are present. In the presence of human AML cells expressing endogenous levels of FRβ, m909 CAR T cells maintained specific activation in the presence of antigen. However, CAR activity decreased with lower surface levels of FRβ, as demonstrated by reduced output of IFN-γ and cytolysis. In addition, it was clear that m909 CAR T cells specifically eliminated THP1 target cells displaying the highest antigen expression.
Despite only moderate activity against THP1 in vitro, m909-28Z CAR T cells did significantly inhibit subcutaneous and disseminated THP1 tumor growth in vivo, suggesting that systemic delivery of m909-28Z CAR T cells to tumor-bearing mice resulted in efficient trafficking, activation, and lysis at sites of tumor growth. When m909-28Z CAR T cells were delivered to mice bearing large established subcutaneous THP1 tumors (∼3 weeks post–tumor injection), they were unable to control tumor growth (data not shown). These data suggest that m909-based CARs may be effective at overcoming only small tumor burden. However, >90% of AML patients reach remission through chemotherapy but eventually relapse due to minimal residual disease that is often undetectable.1 Therefore, m909-28Z CAR T cells could be used as an effective treatment of patients with chemotherapy-induced remission or minimal residual disease conditions.
Because m909 CAR T cells displayed decreased lysis against targets with lower FRβ expression, outgrowth of FRβ-low leukemic clones remains a potential concern for m909 CAR-based therapy. However, we and others have established that ATRA specifically upregulates FRβ expression in FRβ+ AML. Tumor cells with surface antigen expression under the threshold for m909 CAR recognition can potentially be induced to levels high enough to stimulate T-cell activation. Indeed, IFN-γ release and lytic activity from m909 CAR T cells was increased after coculture with ATRA-pretreated AML. We also observed small increases in IFN-γ secretion of control GFP T cells and m909 CAR T cells against FRβ− HL60, suggesting that other effects of ATRA on target cells may slightly enhance T-cell recognition of AML. Notably, ATRA is known to induce differentiation of THP1 and HL6044,45 as measured by greater cytokine production and co-stimulatory molecule expression.46 ATRA-mediated differentiation of AML cells may have heightened the allogeneic T-cell response. However, the largest differences in reactivity were observed when m909 CAR T cells were incubated with ATRA-treated FRβ+ AML, suggesting that greater antigen density played the dominant role in mediating increased m909 CAR T-cell reactivity. Although a pilot experiment with the addition of ATRA in vivo did not provide augmentation of m909 CAR T-cell performance, we also did not observe any reduction in antitumor response. Although further optimization of dosing and treatment regimen will be necessary, these preliminary results suggest that ATRA can safely be combined without adverse effects on CAR T-cell function in vivo. In addition to ATRA alone, dual treatment with histone deacetylase (HDAC) inhibitors has been shown to stimulate FRβ expression in AML even further in vitro.27 Optimized combinations of ATRA and other FRβ-inducing agents present an opportunity for additional augmentation of m909 CAR T-cell efficacy. Importantly, ATRA did not impact FRβ expression in healthy HSCs or monocytes, suggesting that ATRA induction of FRβ in AML could be applied without increasing the capacity for healthytissue recognition by m909 CAR T cells.
Of note, neither ATRA or HDAC inhibition, or a combination of both, is capable of inducing FRβ in nonexpressing cells of myeloid origin (eg, HL60) or epithelial origin (eg, 293T),27 suggesting that their effects are not potent enough to overcome the genetic program responsible for maintaining tissue specificity. Therefore, de novo induction of FRβ expression in negative tissues is not a major concern. Similarly, AML patients would need to be prescreened for FRβ because ATRA will not induce FRβ without baseline expression present. Although previous studies have identified FRβ on all classes of AML, incidence does increase with myeloid/monocytic distinctions (M4 and M5),18 and these patients may benefit the most from FRβ-directed CAR therapy. Generally, new cancer therapeutics are moving toward a more personalized approach and need not necessarily be applicable to all patients across a broad and complex disease indication to be clinically beneficial.
Beyond pharmacologic upregulation of FRβ antigen, the modest activity of m909 CAR T cells against FRβ-low AML targets may also be overcome by CAR platform optimization. For example, Hudecek and colleagues were able to greatly increase the activity of an ROR1-specific CAR by modifying the hinge length and improving the scFv affinity.47 It remains possible that the monovalent affinity of the m909 scFv (KD = 57 nM) may be suboptimal for interaction with FRβ expressed at low levels. We anticipate that modifying the FRβ CAR T-cell platform by introducing higher-affinity scFvs could improve overall antitumor efficacy. However, higher-affinity activity could result in increased toxicity against normal cells expressing low levels of FRβ. Further toxicology and other preclinical evaluation of m909 and variants could help identify platforms with optimal affinity for tumor cell destruction while sparing normal tissues.
In addition to leukemia, FRβ expression is also reported on some normal myeloid-lineage cells and can be induced on macrophage activation.48 Although we did not observe lysis of peripheral blood monocytes with m909 CAR T cells, healthy myeloid tissues remain a potential target for off-tumor toxicity by FRβ-specific CAR T cells. Recent innovations in the field have the potential to mitigate these risks by restricting CAR T-cell persistence via transient expression through RNA electroporation49 or by combining CAR delivery with an inducible suicide gene.50 CD34+ HSCs continuously give rise to peripheral myeloid-lineage immune cells. Despite previous reports of FRβ expression in HSCs, we did not observe any toxicity against CD34+ bone marrow progenitors, suggesting that m909 CAR T cells may be applied with reduced risk to HSCs. Therefore, by providing transient expression of the m909 CAR platform, as described earlier, CAR T-cell elimination after tumor clearance could allow for restoration of affected healthy myeloid populations from normal HSCs.
A successful FRβ CAR T-cell platform has the potential for therapeutic benefit in a wide variety of diseases beyond AML. FRβ has also been described in chronic myelogenous leukemia.9,11,12 In addition to leukemia, FRβ is increased on the surface of macrophages associated with various pathological conditions. Tumor-associated macrophages display high levels of FRβ in solid tumors from diverse tissue origins.51 Because tumor-associated macrophages correlate with worse prognosis across multiple types of cancer,52 FRβ CAR T cells could potentially be used to improve the treatment of solid tumors by eliminating immunosuppressive, protumorigenic macrophages. FRβ is also highly expressed on macrophages at sites of ongoing inflammation53 and has been effectively exploited for imaging and targeting of pathological macrophages in rheumatoid arthritis54 and atherosclerosis.55 FRβ-specific immunotoxins have successfully depleted macrophages in mouse models of glioma,56 atherosclerosis,57 collagen-induced arthritis,58 and fibrosis.59 Given their potent effector function and ability to persist after infusion, FRβ-specific CAR T cells have the potential to improve on antibody-directed toxicity and may present an exciting new way to expand the use of CAR T cells in inflammatory diseases, as well as in cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health National Cancer Institute (NCI) grant RO1-CA168900 (D.J.P.), National Institute of Allergy and Infectious Diseases grant T32-AI070099 (R.C.L.), Joint Fox Chase Cancer Center and University of Pennsylvania Ovarian Cancer Specialized Program of Research Excellence NCI grant P50 CA083638 (D.J.P.), the Intramural Research Program at the NCI Center for Cancer Research (D.S.D.), and NCI grant CA016520, which supported imaging at the University of Pennsylvania Small Animal Imaging Facility Optical/Bioluminescence Core.
Authorship
Contribution: R.C.L. and D.J.P. designed the experiments, formatted the figures, and wrote the manuscript; R.C.L. performed the experiments and analyzed the data; M.P. performed the in vivo bioluminescent imaging; A.K. counted/scored the CFU colonies; Y.F., P.S.L., and D.S.D. isolated the m909 scFv; and D.S.D. and P.S.L. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel J. Powell Jr, Perelman School of Medicine, University of Pennsylvania, 3400 Civic Center Blvd, Building 421, Smilow Center, Room 08-103, Philadelphia, PA 19104-5156; e-mail: poda@mail.med.upenn.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal