Key Points
Protein and DNA analyses reveal that mutation in the immunoglobulin κ light-chain constant region gene may cause hereditary amyloidosis.
Sequencing of immunoglobulin light-chain constant region genes is indicated for patients with AL amyloidosis and no evidence of a plasma cell dyscrasia.
Abstract
Several members of a family died from renal failure as a result of systemic amyloidosis. Extensive studies to detect previously documented gene mutations associated with amyloidosis failed to identify a causative factor. In search of the genetic basis for this syndrome, amyloid fibrils were isolated from renal tissue of a member of the kin who died while on renal dialysis. Amino acid sequencing of isolated amyloid protein identified sequences compatible with the constant region of the immunoglobulin κ light-chain. Isolation and characterization of κ light-chain protein from serum of an affected member of the kindred revealed mutation in the constant region of κ light-chain, with cysteine replacing serine at amino acid residue 131. This mutation (Ser131Cys) was confirmed by DNA analysis, which identified a single-base change of cytosine to guanine at the second position of codon 131 of the κ light-chain gene (TCT131TGT). DNA analysis of members of the extended family revealed transmission of the Ser131Cys mutation and association with systemic amyloidosis. This amyloid light-chain (AL) amyloidosis, which is a hereditary type of amyloidosis and not the result of a monoclonal plasma cell dyscrasia, may be misdiagnosed and lead to inappropriate chemotherapy.
Introduction
Immunoglobulin amyloid light-chain amyloidosis (AL) is the most frequent form of systemic amyloidosis.1 It is a sporadic disease caused by plasma cell dyscrasia, with resulting amyloid fibril formation from monoclonal immunoglobulin light-chain, either κ or λ. There are, however, several forms of systemic amyloidosis that are caused by mutations in other plasma proteins and are expressed with autosomal dominant genetics.2 These inherited forms of amyloidosis may show a predilection for specific organ involvement such as the heart, peripheral nervous system, or kidney, but are all systemic in nature and very often clinically mimic AL amyloidosis. Several of the inherited forms of systemic amyloidosis cause renal manifestations and therefore may be mistaken for AL amyloidosis, which often presents with major renal dysfunction.3
Although plasma cell dyscrasia may be more prevalent in certain families, AL amyloidosis has rarely been reported in first-degree relatives, and no definite inheritance pattern has been identified. Here we report a systemic form of AL that is inherited as an autosomal dominant trait and is associated with a mutation in the constant region of the κ immunoglobulin light-chain.
Methods
The family
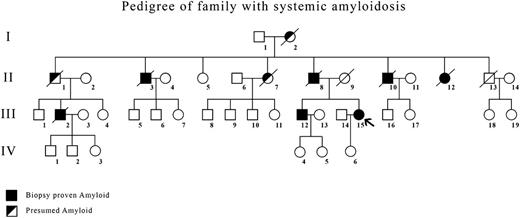
Six members (4 men, 2 women) of a family of 8 died from renal failure between the ages of 53 and 74 years (Figure 1). Five had received renal dialysis. Four (II-3, II-8, II-10, II-12) had the diagnosis of amyloidosis made by renal biopsy. Their mother (I-2) had died at age 58 of renal failure.
Pedigree of family showing members with biopsy-proven amyloidosis (▪) and presumed affected (◩) showed typical autosomal dominant inheritance. The index case is indicated by an arrow.
Pedigree of family showing members with biopsy-proven amyloidosis (▪) and presumed affected (◩) showed typical autosomal dominant inheritance. The index case is indicated by an arrow.
In the next generation, 1 man (III-2) with biopsy-proven renal amyloidosis died at age 74 after several years of treatment with renal dialysis, and 2 cousins (III-12, III-15) have biopsy-proven amyloidosis, one from renal biopsy (III-12) and one from gastric mucosal biopsy (III-15, index case).
Review of their medical histories revealed that 1 man (II-3) who died of renal failure also had amyloid deposition on laryngeal and lung biopsies, verifying that he had a systemic form of amyloidosis. Medical records indicated that immunohistologic analysis of kidney biopsies for 4 individuals showed positive staining for κ light-chain, and laser-dissected mass spectrometry analysis of amyloid identified on a gastric biopsy of a member of the third generation (III-15) also identified κ light-chain constant region peptides.
Amyloid protein characterization
Amyloid fibrils were isolated from formalin-fixed, paraffin-embedded renal tissue of 1 member of the family who died while on dialysis.4 Tissue was solubilized in 8 M guanidine-HCl under reducing conditions, and solubilized protein subjected to amino acid sequencing on an Applied Biosystems Procise 491 cLC protein sequencer using the manufacturer’s standard cycles and methods. Isolated protein was also subjected to tryptic digestion and resultant peptides separated by reverse-phase high-performance liquid chromatography (HPLC) and characterized by amino acid sequencing.5
Immunoglobulin light-chain isolation and characterization
Serum (10 mL) from a family member with biopsy-proven amyloidosis was saturated 33% with ammonium sulfate at room temperature. The precipitated fraction recovered by centrifugation was dissolved in phosphate-buffered saline and chromatographed on a Sepharose CL6B column equilibrated and eluted with phosphate-buffered saline. The major peak, which on sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis in the presence of 2-mercaptoethanol contained 25 kDa and 50 kDa bands (immunoglobulin light-chain and heavy-chain), was dialyzed against distilled water and lyophilized. Dried protein was solubilized in 8 M guanidine hydrochloride, 0.5 M Tris pH 8.2 containing 10 mg dithiothreitol/mL, alkylated with iodoacetic acid, and centrifuged. Chromatography of the supernatant on Sepharose CL6B equilibrated and eluted with 4 M guanidine hydrochloride, 25 mM Tris pH 8.2 yielded 2 major peaks corresponding to immunoglobulin heavy-chains and immunoglobulin light-chains. Fractions containing immunoglobulin light-chains were combined, exhaustively dialyzed against distilled water, and lyophilized. After tryptic digestion, peptides were separated by HPLC on a Beckman Ultrasphere ODS column, and isolated peptides were subjected to amino acid sequencing on an Applied Biosystems Procise 491 cLC protein sequencer using the manufacturer’s standard cycles and methods.
Histochemistry
Tissue biopsy sections (4 μm) were stained with hematoxylin and eosin. Sections were also stained with alkaline Congo red. Immunohistochemistry of biopsy tissues was performed by the indirect immunoperoxidase technique using rabbit anti-κ and anti-λ polyclonal antisera and immunoperoxidase labeled goat anti-rabbit IgG antibody. Sections were developed with diaminobenzidine.
DNA analysis
DNA was isolated from ethylenediaminetetraacetic acid–treated blood and formalin-fixed, paraffin-embedded tissue.6 Polymerase chain reaction amplification of the constant region of the κ light-chain gene used primers 5′ ACCATCCTGTTTGCTTCT 3′ and 5′ CTCTGTGACACTCTCCTG 3′ with standard cycles of 1 minute at 94°, 1 minute at 56°, and 1 minute at 72° for 35 cycles. Sequencing was performed by a Beckman CEQ System.
Results
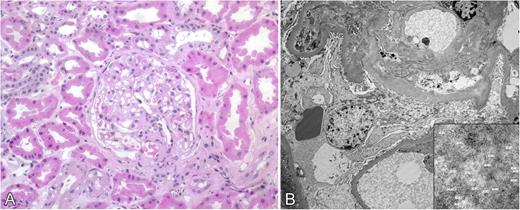
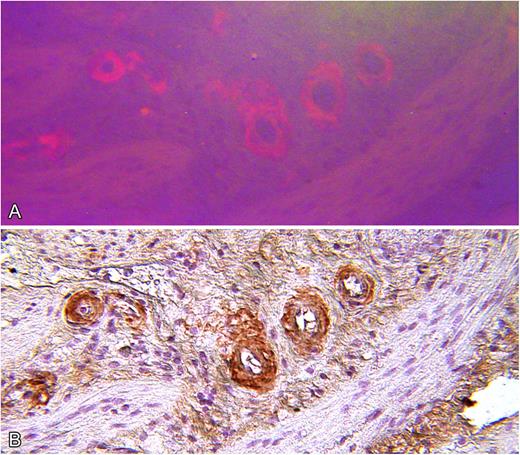
Review of medical records for several members of a family who died from renal failure documented that kidney biopsies demonstrated amyloid deposition in glomerular basement membrane and blood vessel walls. In several cases, positive staining for κ light-chain was described but evaluation for monoclonal gammopathy was negative (Table 1). In the present study, amyloid deposition in a renal biopsy of subject III-12 was identified by routine histology, and electron microscopy demonstrated 7- to 10-μm fibrils consistent with amyloid (Figure 2). Immunohistochemistry for κ light-chain performed on cholecystectomy tissue from the index case (III-15) showed positive staining of blood vessel walls, which coincided with Congo red staining (Figure 3).7
Clinical features of C κ amyloidosis
| Subject . | Clinical features . | Age at death (y) . | Tissue-positive amyloid . | Bone marrow . | Immunoglobulin light-chain . | κ131Cys . |
|---|---|---|---|---|---|---|
| I-2 | Renal failure | 58 | ||||
| II-1 | Renal failure, age 52 | 53 | ||||
| II-3 | Renal failure, age 60 | 66 | Kidney, larynx, lung, bone marrow | Hypocellular | ||
| Dialysis, age 64 | ||||||
| II-7 | Renal failure | 73 | ||||
| Dialysis | ||||||
| II-8 | Renal failure | 54 | Kidney | |||
| Dialysis | ||||||
| II-10 | Renal failure | Postmortem kidney, heart, pancreas, parathyroid | Hypocellular | |||
| Dialysis | 62 | + | ||||
| II-12 | Renal failure, age 60+ | |||||
| Dialysis | ||||||
| III-2 | Renal failure, age 66 | 74 | Kidney | |||
| Dialysis, age 70 | ||||||
| III-8 | Alive, 73 y | – | ||||
| III-9 | Proteinuria, age 64 (stage 3 renal disease) | Increased κ and λ | + | |||
| III-10 | Asymptomatic, age 37 | + | ||||
| III-12 | Proteinuria, age 66 | Kidney | Normal | + | ||
| III-15 | Cholecystitis, age 53 | Stomach, gall bladder | Normal | Normal | + | |
| IV-1 | – | |||||
| IV-2 | – | |||||
| IV-3 | ||||||
| IV-4 | Asymptomatic, age 40 | Normal | + | |||
| IV-5 | Asymptomatic, age 39 | Normal | + | |||
| IV-6 | – |
| Subject . | Clinical features . | Age at death (y) . | Tissue-positive amyloid . | Bone marrow . | Immunoglobulin light-chain . | κ131Cys . |
|---|---|---|---|---|---|---|
| I-2 | Renal failure | 58 | ||||
| II-1 | Renal failure, age 52 | 53 | ||||
| II-3 | Renal failure, age 60 | 66 | Kidney, larynx, lung, bone marrow | Hypocellular | ||
| Dialysis, age 64 | ||||||
| II-7 | Renal failure | 73 | ||||
| Dialysis | ||||||
| II-8 | Renal failure | 54 | Kidney | |||
| Dialysis | ||||||
| II-10 | Renal failure | Postmortem kidney, heart, pancreas, parathyroid | Hypocellular | |||
| Dialysis | 62 | + | ||||
| II-12 | Renal failure, age 60+ | |||||
| Dialysis | ||||||
| III-2 | Renal failure, age 66 | 74 | Kidney | |||
| Dialysis, age 70 | ||||||
| III-8 | Alive, 73 y | – | ||||
| III-9 | Proteinuria, age 64 (stage 3 renal disease) | Increased κ and λ | + | |||
| III-10 | Asymptomatic, age 37 | + | ||||
| III-12 | Proteinuria, age 66 | Kidney | Normal | + | ||
| III-15 | Cholecystitis, age 53 | Stomach, gall bladder | Normal | Normal | + | |
| IV-1 | – | |||||
| IV-2 | – | |||||
| IV-3 | ||||||
| IV-4 | Asymptomatic, age 40 | Normal | + | |||
| IV-5 | Asymptomatic, age 39 | Normal | + | |||
| IV-6 | – |
Kidney biopsy from subject III-12. (A) Hematoxylin and eosin–stained section showing deposits in glomerular basement membrane (original magnification ×100). (B) Electron micrograph showing amyloid deposits with 7- 10-μm fibrils (inset) consistent with amyloid.
Kidney biopsy from subject III-12. (A) Hematoxylin and eosin–stained section showing deposits in glomerular basement membrane (original magnification ×100). (B) Electron micrograph showing amyloid deposits with 7- 10-μm fibrils (inset) consistent with amyloid.
Serial microscopic sections of gallbladder wall from index patient (III-15). (A) Stained with Congo red and viewed by fluorescence microscopy. (B) Stained by indirect immunohistochemistry with polyclonal anti-immunoglobulin κ light-chain antiserum showing specific staining of amyloid deposits (original magnification ×100).
Serial microscopic sections of gallbladder wall from index patient (III-15). (A) Stained with Congo red and viewed by fluorescence microscopy. (B) Stained by indirect immunohistochemistry with polyclonal anti-immunoglobulin κ light-chain antiserum showing specific staining of amyloid deposits (original magnification ×100).
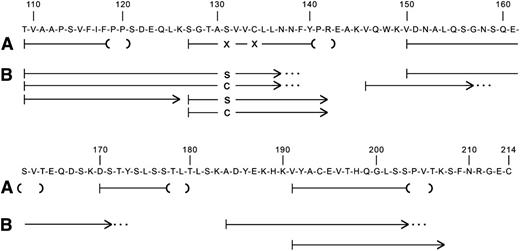
Amino acid sequence analysis of tryptic peptides of amyloid fibril protein isolated from postmortem formalin-fixed, paraffin-embedded kidney tissue gave sequences consistent with immunoglobulin κ light-chain constant region, residues 109 to 120, 127 to 142, 150 to 163, 170 to 179, and 191 to 205 (Figure 4). All numbering is done according to the Kabat numbering system.8 Residue 131, which is normally a serine, and residue 134, which is normally a cysteine, could not be positively identified.
Amino acid sequences of tryptic peptides of (A) amyloid fibril protein isolated from postmortem kidney of patient II-10, and (B) plasma κ light-chain from a member of the next generation (III-15) with biopsy-proven amyloidosis. The normal sequence of the constant region of κ light-chains is shown. Residue numbering is by Kabat et al.8 The lines indicate the sequences obtained by Edman degradation of HPLC-purified tryptic peptides. The parentheses at the ends of the amyloid fibril protein peptides (A) denote residues not completely verified because of decreasingly low Edman degradation yields. The X at residues 131 and 134 denote that no amino acid was identified at these positions. The dots at the ends of some plasma light-chain peptides (B) indicate that the peptide continued but was not analyzed further.
Amino acid sequences of tryptic peptides of (A) amyloid fibril protein isolated from postmortem kidney of patient II-10, and (B) plasma κ light-chain from a member of the next generation (III-15) with biopsy-proven amyloidosis. The normal sequence of the constant region of κ light-chains is shown. Residue numbering is by Kabat et al.8 The lines indicate the sequences obtained by Edman degradation of HPLC-purified tryptic peptides. The parentheses at the ends of the amyloid fibril protein peptides (A) denote residues not completely verified because of decreasingly low Edman degradation yields. The X at residues 131 and 134 denote that no amino acid was identified at these positions. The dots at the ends of some plasma light-chain peptides (B) indicate that the peptide continued but was not analyzed further.
Immunoglobulin light-chain proteins were isolated from the serum of an affected family member and subjected to amino acid sequencing, which identified the entire κ constant region except for amino acid residues 143 to 145, 172 to 183, and 208 to 214 (Figure 4). Of note was identification of separate peptides containing either serine or cysteine at position 131. Position 134 had only the normal cysteine, which is involved in the intramolecular disulfide bridge to the cysteine at position 194.
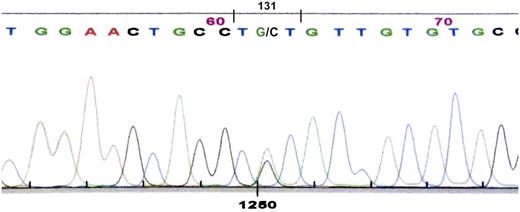
Nucleotide sequencing of the constant region of the κ light-chain gene in the subject described before (III-15), whose κ light-chain serum protein had both serine and cysteine at position 131, showed guanine as well as the normal cytosine in the second position of codon 131 (nucleotide 403). The cytosine-to-guanine transversion gives a sequence-encoding cysteine (TGT) in place of serine (TCT), consistent with the presence of cysteine 131 in the κ light-chain protein (Figure 5).
Nucleotide sequence of polymerase chain reaction product for κ light-chain DNA showing heterozygosity at cDNA position 403 with both cytosine and guanine, giving coding sequence for cysteine (TGT) and serine (TCT) at position 131.
Nucleotide sequence of polymerase chain reaction product for κ light-chain DNA showing heterozygosity at cDNA position 403 with both cytosine and guanine, giving coding sequence for cysteine (TGT) and serine (TCT) at position 131.
Sequencing of DNA isolated from postmortem tissue of the subject whose amyloid fibril protein had been characterized also showed heterozygosity for the Ser131Cys change in the κ light-chain gene. In addition, 2 children of subject II-7 who died with amyloidosis were positive for the Ser131Cys mutation, indicating that their affected parent (II-7) had been an obligate carrier of the Ser131Cys mutation.
Discussion
All forms of hereditary systemic amyloidosis identified thus far are inherited in an autosomal dominant pattern, which is consistent with the structural nature of mutant proteins that form amyloid fibrils.2 Mutation in only 1 allele is sufficient for production of protein that can follow the amyloid fibril–forming pathway.
In the usual type of immunoglobulin light-chain amyloidosis (AL), no hereditary pattern has been identified, and the disease is considered “sporadic.” The only definite requirement for amyloid formation appears to be a plasma cell dyscrasia that produces excess monoclonal amyloid fibril precursor protein. In addition, it has been proposed that certain amino acid residues in the variable region of the light-chain predispose to fibril formation, with certain light-chain structures (eg, λ-6) having more fibril-forming potential.9,10 Although structure of the variable region dictates fibril formation, it is common to find some portions of the light-chain constant region, either κ or λ, in the final fibril product.
The immunoglobulin κ light-chain amyloidosis described here is different. The disease is obviously hereditary and segregates with a Ser131Cys mutation in the constant region of the κ light-chain gene. No significant amount of peptide sequence consistent with any known κ variable region was identified in isolated fibrils. In 1 case subjected to mass spectrometry analysis, minor amounts of κ variable peptide sequences were noted but included κ-I, κ-II, and κ-III, and would not support the presence of a monoclonal immunoglobulin (Figure 6).
Report of LCMS analysis of gastrointestinal biopsy amyloid from index case (III-15).
Report of LCMS analysis of gastrointestinal biopsy amyloid from index case (III-15).
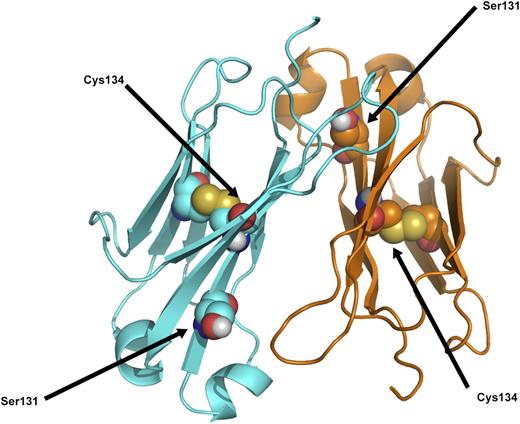
Essentially these are 2 different diseases, one being the result of a monoclonal plasma cell dyscrasia in which the specific variable region of the light-chain is predisposed to amyloid fibril formation when produced in excess. The other is the result of a mutation in the light-chain constant region gene, which, for an individual heterozygous for the mutation, predictably would be present in 50% of serum IgG κ antibodies. Excess production of the mutated constant region peptide is not likely to be a determining factor in amyloid formation, although stimulation of antibody production by repeated immunizations or response to infectious diseases could possibly be a factor in initiation and progression of amyloid formation. A more likely explanation is that age-related metabolic factors in the catabolism of serum proteins, as with other forms of hereditary amyloidosis, dictate the late-onset of amyloid formation from a protein that has been present from birth. Review of the published tertiary structure of the κ light-chain constant region obtained by X-ray diffraction indicates that the cysteine at position 131 may cause a marked change in protein folding, but it is not likely to interfere with the normal intramolecular disulfide bridge from 134Cys to 194Cys (Figure 7).11
Ribbon diagram of κ light-chain molecular structure to show position of the Ser131 residue in relation to the Cys134, which is part of the normal intramolecular disulfide bridge. Modified from Roussel et al.11
Ribbon diagram of κ light-chain molecular structure to show position of the Ser131 residue in relation to the Cys134, which is part of the normal intramolecular disulfide bridge. Modified from Roussel et al.11
There are 2 reports of cases in which mutation in the κ constant gene was associated with systemic amyloidosis.12,13 Both had a κ light-chain mutation (Ser177Asn) identified in amyloid fibril isolates, and both were from patients with plasma cell dyscrasias. In the case reported by Solomon et al,12 nucleotide sequencing of DNA from family members of the patient with light-chain amyloidosis was consistent with the Ser177Asn κ constant region change being the result of a germ-line rather than a somatic mutation. A similar conclusion was proposed by the studies of Wally et al,13 with the suggestion that the Ser177Asn did not cause but contributed to the generation of amyloid formation. In both of the reported cases,12,13 rapid progression of clinical disease was noted. Although it has been proposed that Ser177Asn is a κ light-chain polymorphism, as are Ala153Val and Val191Leu, additional studies are needed to support this hypothesis. Because there are no reports identifying the κ light-chain constant region Ser131Cys mutation in published studies, it is unlikely to meet the designation of a genetic polymorphism.
There have also been reports of AL amyloidosis in first-degree relatives. Miliani et al reported 3 siblings with AL amyloidosis.14 Gertz et al reported AL amyloidosis in 3 families.15 Enqvist et al reported a father and son with AL amyloidosis.16 In all of these cases, an underlying plasma cell dyscrasia was identified. Extensive searches for plasma cell dyscrasia in several of the patients in the present report of κ light-chain amyloidosis were negative.
This is the first report of AL amyloidosis caused by an identified mutation in the constant region of an immunoglobulin light-chain gene in the absence of a monoclonal plasma cell dyscrasia. In this family, the high incidence of affected individuals makes the hereditary nature of the disease obvious, and this probably has protected them from inappropriate treatment with chemotherapy designed to treat plasma cell dyscrasia. One can only speculate as to whether other mutations in the constant region of light-chain genes, κ or λ, cause systemic amyloidosis without the presence of a plasma cell dyscrasia. If no obvious family history of amyloidosis is present, a misdiagnosis and inappropriate treatment with chemotherapy might result. It is also possible that amyloidosis could result from a somatic mutation in the constant region of the light-chain gene. In such a case, there would be no family history of amyloidosis. In the small but significant number of patients with AL amyloidosis who do not have a detectible plasma cell dyscrasia by present testing methods, nucleotide sequencing of the immunoglobulin light-chain constant region genes should be considered as part of the diagnostic evaluation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Thomas D. Hurley for analysis of X-ray structure data and Dr Jill R. Murrell for DNA sequencing. Dr M. Carney Taylor provided clinical data on members of the family. Dr Reinhold Linke provided expert histochemistry assistance. Members of the κ light-chain (C-κ) family provided historical medical records and participated in the genetic studies.
This study was supported in part by the Indiana University Foundation Accounts of the Machado Family Amyloid Research Fund, the Aldo Family Amyloid Research Fund, and the Jacobson Family Amyloid Research Fund.
Authorship
Contribution: M.D.B. performed clinical evaluations, designed experiments, and analyzed data; J.J.L. isolated proteins and performed amino acid sequencing; and B.K.-B. performed immunohistochemistry and supervised DNA analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Merrill D. Benson, Indiana University School of Medicine, Department of Pathology and Laboratory Medicine, 635 Barnhill Dr., MS-128, Indianapolis, IN 46202-5126; e-mail: mdbenson@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal