Key Points
BCR variable-region mannoses in follicular lymphoma are recognized by lectins of common opportunistic bacteria.
Introduction of N-linked sugars into the BCR variable region interferes with antigen recognition.
Abstract
B-cell antigen receptor (BCR) expression is a key feature of most B-cell lymphomas, but the mechanisms of BCR signal induction and the involvement of autoantigen recognition remain unclear. In follicular lymphoma (FL) B cells, BCR expression is retained despite a chromosomal translocation that links the antiapoptotic gene BCL2 to the regulatory elements of immunoglobulin genes, thereby disrupting 1 heavy-chain allele. A remarkable feature of FL-BCRs is the acquisition of potential N-glycosylation sites during somatic hypermutation. The introduced glycans carry mannose termini, which create potential novel binding sites for mannose-specific lectins. Here, we investigated the effect of N-linked variable-region glycosylation for BCR interaction with cognate antigen and with lectins of different origins. N-glycans were found to severely impair BCR specificity and affinity to the initial cognate antigen. In addition, we found that lectins from Pseudomonas aeruginosa and Burkholderia cenocepacia bind and stimulate FL cells. Human exposure to these bacteria can occur by contact with soil and water. In addition, they represent opportunistic pathogens in susceptible hosts. Understanding the role of bacterial lectins might elucidate the pathogenesis of FL and establish novel therapeutic approaches.
Introduction
B-cell antigen receptor (BCR) expression and function is critical during B-cell development1-4 and is maintained in most B-cell lymphomas, including follicular lymphoma (FL).5 One genetic hallmark of FL is the overexpression of the anti-apoptotic BCL-2 protein owing to the t(14;18) translocation.6,7 Despite the disruption of a heavy-chain (HC) allele by this translocation, FL B cells retain BCR expression, suggesting an important role for lymphoma development and survival. Remarkable features of the BCR in FL are N-linked glycosylation sites introduced during somatic hypermutation (SHM) bearing high-mannose–terminated glycans.8-12 N-linked glycosylation occurs cotranslationally in the endoplasmic reticulum at asparagine-X-serine/threonine (N-X-S/T) sequons, with X being any amino acid apart from proline.13 Normally, mannose-terminated glycosylation is restricted to glycoproteins present in the endoplasmic reticulum, whereas plasma membrane glycoproteins carry branched complex oligosaccharides. FL receptors display fully processed sugars in the constant regions and the high-mannose type in the variable (V) region.12
N-linked glycosylation of the antigen-binding groove was recently discovered as a mechanism to mask self-antigen binding sites,14 suggesting that glycosylation might represent an alternative pathway (in addition to clonal deletion, receptor editing, and anergy) to avoid the activation of self-reactive mature B cells.
Available data suggest an important role of the tumor microenvironment for survival and proliferation of FL cells.15-22 Lymphoma cells reside and proliferate in follicular structures potentially interacting with T-helper and follicular dendritic cells, as is the case for normal germinal center (GC) B cells.22 Because BCR expression is an important feature of FL B cells, interactions with surrounding cells are likely to occur through the BCR. Accordingly, we previously showed that the mannosylated V regions of FL immunoglobulins bind to recombinant lectin domains of the mannose receptor and dendritic-cell–specific intercellular adhesion molecule-3–grabbing nonintegrin (DC-SIGN), leading to stimulation of FL cells.23
We now investigated in more detail the role of N-linked V-region glycosylation in conventional antigen binding and in the interaction with endogenous and exogenous lectins. We found that V-region mannosylation conferred the ability of B cells to be activated by soluble bacterial lectins from common opportunistic pathogens such as Pseudomonas aeruginosa or Burkholderia cenocepacia while disrupting the initial receptor specificity for potential autoantigens.
Materials and methods
Patient samples
Frozen samples of lymph nodes, peripheral blood, and bone marrow were obtained from the University Medical Center Freiburg and Leiden University Medical Center. Frozen blood peripheral blood mononuclear cells (PBMCs) from healthy donors were used as controls. The local ethics committees approved the sampling, and all patients gave informed consent in accordance with the Declaration of Helsinki (approving board: 159/03 [Freiburg], HEM/004/SH/shVBM2013.12 [Leiden]).
Cells and cell-culture conditions
Phoenix and triple-knockout (TKO) cells were cultured in Iscove medium (Biochrom AG) containing 10% fetal calf serum (FCS) (PAN-Biotech), 10 mM l-glutamine, 100 U/mL penicillin/streptomycin, and 50 μM 2-mercaptoethanol (all Gibco). For culture of TKO samples, the medium was supplemented with supernatant of interleukin-7–producing J558L mouse plasmacytoma cells. The TKO cell line was established from the bone marrow of mice deficient for λ5, RAG2, and SLP65. Owing to the absence of RAG2 and λ5, the cells cannot express endogenous BCR or pre-BCR and thus can be reconstituted with an HC and light chain (LC) of interest. In order to study signaling events downstream of the BCR, TKO cells were reconstituted with a tamoxifen-sensitive ERT2-SLP65 fusion protein that allows inducible activation of SLP65 function by the addition of 4-hydro-tamoxifen (OHT).24-26
Human cell lines were cultured in RPMI containing 10% FCS, 10 mM l-glutamine, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Gibco), 100 U/mL penicillin/streptomycin, and 50 μM 2-mercaptoethanol. Primary B cells were thawed and cultured in RPMI containing 10% FCS, 10 mM l-glutamine, and 100 U/mL penicillin/streptomycin for 1 hour prior to fluorescence-activated cell sorter (FACS) experiments.
Plasmids and retroviral transduction
ERT2-SLP6526 was expressed using a vector containing tdTomato as expression control (a kind gift from R. Y. Tsien). Lymphoma-derived BCRs were amplified from sequencing vectors (CellGenix) using sequence-specific primers (sequences available upon request). HC and LC were expressed using the BiFC vector system.24,25 Phoenix cells were transfected using Gene Juice (Novagen), and virus-containing supernatants were harvested 36 hours posttransfection and used for transduction of TKO cells as described previously.27
Antigen biotinylation
One milligram protein was incubated with 0.1 mg biotinamidohexanoic acid N-hydroxysuccinimide ester (Sigma-Aldrich) dissolved in dimethylformamide (Sigma-Aldrich) for 1 hour at room temperature in the dark on a shaker followed by dialysis in phosphate-buffered saline.
Bacterial lectin biotinylation
The bacterial lectins Bc2L-A and LecB were a kind gift from Anne Imberty’s group (CERMAV-Grenoble). The recombinant lectins were produced in Escherichia coli as previously described.28,29 Biotinylation was performed using the EZ-Link NHS-PEG4 biotinylation kit (Thermo Scientific) according to the manufacturer’s instructions.
Flow cytometry
TKO cells were stained with goat anti-mouse immunoglobulin M (IgM) Cy5 or Alexa Fluor 647 (Jackson ImmunoResearch), goat anti-human/mouse κ/λ-biotin (Southern Biotech), and streptavidin-PerCP (BD Biosciences). Antigen binding was performed with 1 μg/mL NIP7 bovine serum albumin (BSA) biotin (Biosearch Technologies) and 5 μg/mL sHEL-biotin (Sigma). Concanavalin A (ConA), Galanthus nivalis agglutinin (GNA), peanut agglutinin–biotin (all Vector Labs), and DC-SIGN/Fc (20 μg/mL, R&D Systems) binding was performed in lectin buffer23 and detected using streptavidin–Alexa Fluor 647 (Jackson ImmunoResearch) or polyclonal anti-human immunoglobulin G (IgG) antibody (Southern Biotech, allophycocyanin labeled using AbD Serotech LNK032APC labeling kit), respectively. Binding of Bc2L-A-biotin and LecB-biotin (20 μg/mL) was performed in Iscove medium and detected using streptavidin–Alexa Fluor 647.
Human samples were stained using anti-human CD19-phycoerythrin (BD Biosciences), CD43–fluorescein isothiocyanate (eBioscience), κ/λLC-biotin (Southern Biotech), and streptavidin–Pacific Blue (Invitrogen) or streptavidin–Alexa Fluor 647. FACS analysis was performed with FACSCalibur, LSR II, or Fortessa (BD). Data were analyzed using FlowJo software (TreeStar).
Calcium-flux analysis
Calcium-influx measurements were performed as described previously.30 Briefly, 1 × 106 cells were loaded with Indo-1 (Invitrogen) using Pluronic (Invitrogen) for 45 minutes at 37°C. A total of 2 μM 4-OHT (Sigma-Aldrich) was used for ERT2-SLP65 induction in TKO cells. Primary human samples were prestained using anti-human CD43–fluorescein isothiocyanate (eBioscience); CD43−-gated lymphocytes are declared as B cells. Measurement was performed in medium supplemented with 1% FCS or lectin buffer as stated. Stimulation was carried out using 1 μg/mL NIP7-BSA and sHEL, 1 to 20 μg/mL Bc2L-A and LecB, or 10 μg/mL DC-SIGN/Fc (preincubated with mouse anti-human IgG at a 1:1 ratio). Goat anti-mouse/human IgM/κ/λLC (10 μg/mL; Southern Biotech) was used as control. Calcium-flux measurements were performed using a BD LSRII and analyzed using FlowJo software.
Statistical analysis
For statistical analyses, GraphPad Prism software was used. A Mann-Whitney U test was performed to assess statistical differences of antigen binding and Ca2+ kinetics. A paired t test was used for forward scatter and R1 gate changes.
Additional materials and methods are described in supplemental Methods (available on the Blood Web site).
Results
V-region mannosylation interferes with antigen binding of model BCRs
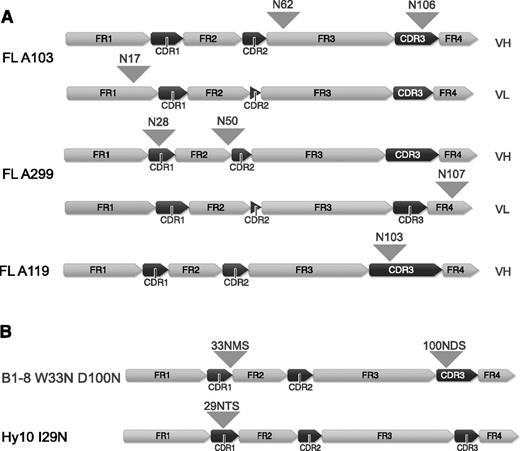
FL arises in GCs and FL B cells undergo SHM and show intraclonal variability due to ongoing SHM. Herein, the majority (79%-100%) of FLs acquire novel N-linked glycosylation sites, whereas the V-region glycosylation frequency is rather low in the healthy B-cell population (9%).8-11 Attachment of bulky oligosaccharides to V-region glycosylation sites may affect antigen binding. For this study, we first investigated the consequence of FL-type glycosylation for the interaction with antigen of the model BCRs B1-8, specific for hapten 4-hydroxy-3-nitrophenylacetic acid or 4-hydroxy-3-iodo-5-nitrophenylacetyl (NIP)31-33 and for the HyHEL10 (Hy10) BCR specific for hen egg lysozyme (HEL).34 We therefore introduced N-glycosylation sequons into the model BCRs based on alignments to our own and published sequences. A glycosylation sequon was inserted at the border of complementarity-determining region (CDR) 1/framework region 2 at position 33 of the B1-8 receptor (W33N) similar to FL-A139 in our set35 and to the previously reported FL receptors.10 We also inserted a glycosylation sequon at position 100 (D100N) similar to FL-BCR A11935 (Figure 1B and supplemental Figure 1A). Insertion of a FL-characteristic glycosylation sequon (similar to FL-A29935 ) in the Hy10 BCR was achieved by the replacement of 1 nucleotide (T→A) to obtain the glycosylation sequon NTS at position 29 to 31 (Figure 1B and supplemental Figure 1B). We expressed the HC and LC of the receptors using bimolecular fluorescence-complementation expression vectors in a murine TKO B-cell line described previously.24-26
V-region sequences of FL receptors and B1-8 and Hy10 variants containing N-glycosylation sites. (A) V-region sequences of FL receptors are shown. The distinct framework region and CDR regions are indicated and positions of the acquired N-glycosylation sites are highlighted with arrowheads. (B) V-region sequences of B1-8 and Hy10 receptor variants containing FL-characteristic glycosylation sequons. N-glycosylation sites are indicated with arrowheads. All peptide sequence numbering without leader sequence. Figure generated using Geneious version 6 created by Biomatters. FR, framework region; VH, variable heavy, VL, variable light.
V-region sequences of FL receptors and B1-8 and Hy10 variants containing N-glycosylation sites. (A) V-region sequences of FL receptors are shown. The distinct framework region and CDR regions are indicated and positions of the acquired N-glycosylation sites are highlighted with arrowheads. (B) V-region sequences of B1-8 and Hy10 receptor variants containing FL-characteristic glycosylation sequons. N-glycosylation sites are indicated with arrowheads. All peptide sequence numbering without leader sequence. Figure generated using Geneious version 6 created by Biomatters. FR, framework region; VH, variable heavy, VL, variable light.
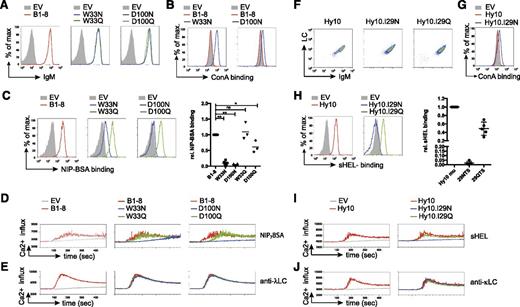
We detected similar BCR surface expression for B1-8 and all related variants (Table 1 and Figure 2A). Increased binding of the plant-derived lectin ConA, which binds to oligomannose,36 to the B1-8 variants W33N and D100N indicated that the N-glycosylation sites harbor oligomannose in the V region similar to human FL-BCRs (Figure 2B). Glycosylation of the VH reduced or even abrogated binding to the multivalent antigen NIP7- BSA (Figure 2C). To test whether this was a consequence of additional glycosylation rather than loss of antigen affinity due to changes in the amino acid sequence itself, we used control variants with glutamine replacing the asparagine residue required for N-glycosylation (Table 1). B1-8 control variants showed comparable surface expression and normal, or only slightly reduced, antigen binding as unmodified B1-8 (Figure 2A,C). Additional controls containing incomplete glycosylation sites (33NMH and 100NYY, Table 1) supported these results. Consistent with reduced antigen binding, BCR-signal induction was impaired in B1-8 variants W33N and D100N, but not (or only slightly) in the respective controls (Figure 2D). To rule out differences in the stimulation conditions, we performed the calcium-flux measurements for each receptor alone or in combination with cells expressing B1-8 unmodified, glycosylated, and control-receptor–expressing cells in the same tube after labeling the different receptor-expressing cells with a distinct amount of eFluor670 (supplemental Figure 2). Stimulation with anti-λ LC antibody verified the functionality of the glycosylated variants (Figure 2E).
Position of FL receptor N-linked glycosylation sequons and sequence of B1-8 and Hy10 variants
| FL receptor . | B1-8 receptor variants . | Hy10 receptor variants . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Glycosylation . | Position . | Position . | Variant . | Sequence . | Position . | Variant . | Sequence . |
| A103HC | 62 NSS | FR3 | 33, CDR1/FR2 | B1-8 WT | WMH | 29, CDR1 | Hy10 WT | ITS |
| 106 NYS | CDR3 | W33N | NMS | I29N | NTS | |||
| A103 LC | 17 NVS | FR1 | W33Q | QMS | I29Q | QTS | ||
| A299 HC | 28NFT | CDR1 | W33N (control) | NMH | ||||
| 50 NIS | CDR2 | 100, CDR3 | B1-8 WT | DYY | ||||
| A299 LC | 107 NLT | FR4 | D100N | NDS | ||||
| A119 HC | 103 NDS | CDR3 | D100Q | QDS | ||||
| D100N (control) | NYY | |||||||
| FL receptor . | B1-8 receptor variants . | Hy10 receptor variants . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Glycosylation . | Position . | Position . | Variant . | Sequence . | Position . | Variant . | Sequence . |
| A103HC | 62 NSS | FR3 | 33, CDR1/FR2 | B1-8 WT | WMH | 29, CDR1 | Hy10 WT | ITS |
| 106 NYS | CDR3 | W33N | NMS | I29N | NTS | |||
| A103 LC | 17 NVS | FR1 | W33Q | QMS | I29Q | QTS | ||
| A299 HC | 28NFT | CDR1 | W33N (control) | NMH | ||||
| 50 NIS | CDR2 | 100, CDR3 | B1-8 WT | DYY | ||||
| A299 LC | 107 NLT | FR4 | D100N | NDS | ||||
| A119 HC | 103 NDS | CDR3 | D100Q | QDS | ||||
| D100N (control) | NYY | |||||||
FR, framework region; WT, wild-type.
Mannose-terminating N-linked glycosylation of high-affinity receptor VH decreases antigen-affinity and antigen-induced BCR stimulation. (A) Flow-cytometric measurement of surface expression of TKO cells expressing the indicated B1-8 receptor variants in comparison with empty-vector–transduced cells. (B-C) Surface binding profile of ConA (B) and NIP7-BSA (C) to B1-8 receptor and the indicated receptor variants compared with empty-vector–transduced TKO cells. Statistical analysis by nonparametric Mann-Whitney U test of NIP7-BSA binding is shown (mean; n = 5: B1-8 and N variants; n = 3: Q variants; **P < .01, *P < .05). (D-E) Kinetics of Ca2+ mobilization upon addition of OHT and the indicated stimuli after 1-minute baseline measurement in TKO cells expressing ERT2-SLP65 and the indicated B1-8 receptor variants measured in 1 tube after distinct eFluor670 labeling. (F) Flow-cytometric measurement of surface expression of TKO cells expressing the indicated Hy10 receptor variants. (G-H) Surface binding profile of ConA (G) and sHEL (H) to the indicated receptor variants compared with empty-vector–transduced TKO cells. Statistical analysis by nonparametric Mann-Whitney U test of sHEL binding is shown (mean; n = 5). (I-J) Kinetics of Ca2+ mobilization upon addition of OHT and the indicated stimuli after 1-minute baseline measurement in TKO cells expressing ERT2-SLP65 and the indicated Hy10 receptor variants or empty-vector–transduced cells measured in 1 tube after distinct eFluor670 labeling. Data are representative of >3 independent experiments. EV, empty vector.
Mannose-terminating N-linked glycosylation of high-affinity receptor VH decreases antigen-affinity and antigen-induced BCR stimulation. (A) Flow-cytometric measurement of surface expression of TKO cells expressing the indicated B1-8 receptor variants in comparison with empty-vector–transduced cells. (B-C) Surface binding profile of ConA (B) and NIP7-BSA (C) to B1-8 receptor and the indicated receptor variants compared with empty-vector–transduced TKO cells. Statistical analysis by nonparametric Mann-Whitney U test of NIP7-BSA binding is shown (mean; n = 5: B1-8 and N variants; n = 3: Q variants; **P < .01, *P < .05). (D-E) Kinetics of Ca2+ mobilization upon addition of OHT and the indicated stimuli after 1-minute baseline measurement in TKO cells expressing ERT2-SLP65 and the indicated B1-8 receptor variants measured in 1 tube after distinct eFluor670 labeling. (F) Flow-cytometric measurement of surface expression of TKO cells expressing the indicated Hy10 receptor variants. (G-H) Surface binding profile of ConA (G) and sHEL (H) to the indicated receptor variants compared with empty-vector–transduced TKO cells. Statistical analysis by nonparametric Mann-Whitney U test of sHEL binding is shown (mean; n = 5). (I-J) Kinetics of Ca2+ mobilization upon addition of OHT and the indicated stimuli after 1-minute baseline measurement in TKO cells expressing ERT2-SLP65 and the indicated Hy10 receptor variants or empty-vector–transduced cells measured in 1 tube after distinct eFluor670 labeling. Data are representative of >3 independent experiments. EV, empty vector.
We performed similar experiments with the Hy10 wild-type, I29N, and I29Q Hy10 BCR variants34 (Figure 1B and Table 1). Accordingly, the glycosylation abolished soluble HEL (sHEL) binding and subsequent receptor stimulation (Figure 2F-J).
Together, the data demonstrate that the addition of sugar moieties to the BCR V region impairs antigen binding and subsequent antigen-induced BCR stimulation. The observation that BCR expression is maintained on FL cells, together with our findings that V-region mannosylation impairs responsiveness to model antigen stimulation, raised the question whether mannose-binding lectins might lead to the activation of FL-BCRs.
FL-specific BCR glycosylation pattern is reconstituted in the TKO cell line
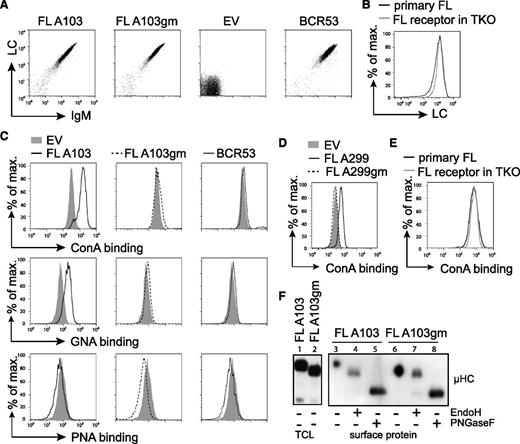
To study the stimulation of FL-BCRs with mannose-specific lectins, we expressed FL-BCRs (Table 1) in TKO cells. The V regions containing the glycosylation sites were cloned from patient lymphoma cells, and glycosylation-defective variants of respective receptors were generated by replacing asparagine with glutamine (Figure 1A and Table 1). Receptor surface expression levels of fully glycosylated FL receptors, the glycosylation mutant (gm) variants, and single glycosylation-site mutants were comparable to a control receptor (BCR53) isolated from a normal peripheral human B cell25,37 (Figure 3A and data not shown). Furthermore, surface expression of FL-BCRs in TKO cells was comparable to BCR expression on primary FL B cells, as demonstrated by LC staining (Figure 3B).
FL receptors expressed in TKO cells are mannosylated on V-region glycosylation sites. (A) TKO cells were transduced with the respective HCs and LCs. Receptor surface expression of cYFP-positive cells expressing receptor FL A103, its V-region glycosylation-defective mutant FL A103gm, with glutamine replacing asparagine in the glycosylation sequon, the empty vector control, and control receptor BCR53 that was isolated from a healthy human mature B cell are shown. FACS analysis was performed using anti-human LC and anti-murine IgM antibodies. (B) FL receptor surface expression in TKO cells was compared with receptor surface expression on a primary FL sample. λ LC expression on IgM+-gated cells is shown. (C-D) Surface binding profile of indicated glycan-specific lectins to the designated receptors expressed on TKO cells. (E) ConA binding to FL receptor expressed on TKO cells in comparison with a primary FL sample. (F) Western blot analysis of total cell lysate and isolated surface proteins of TKO cells transduced with receptor FL A103 and FL A103gm subjected to glycosidase treatment as indicated. Size shift was analyzed by immunoblotting against anti-μHC. Data are representative of ≥3 independent experiments. EV, empty vector; TCL, total cell lysate.
FL receptors expressed in TKO cells are mannosylated on V-region glycosylation sites. (A) TKO cells were transduced with the respective HCs and LCs. Receptor surface expression of cYFP-positive cells expressing receptor FL A103, its V-region glycosylation-defective mutant FL A103gm, with glutamine replacing asparagine in the glycosylation sequon, the empty vector control, and control receptor BCR53 that was isolated from a healthy human mature B cell are shown. FACS analysis was performed using anti-human LC and anti-murine IgM antibodies. (B) FL receptor surface expression in TKO cells was compared with receptor surface expression on a primary FL sample. λ LC expression on IgM+-gated cells is shown. (C-D) Surface binding profile of indicated glycan-specific lectins to the designated receptors expressed on TKO cells. (E) ConA binding to FL receptor expressed on TKO cells in comparison with a primary FL sample. (F) Western blot analysis of total cell lysate and isolated surface proteins of TKO cells transduced with receptor FL A103 and FL A103gm subjected to glycosidase treatment as indicated. Size shift was analyzed by immunoblotting against anti-μHC. Data are representative of ≥3 independent experiments. EV, empty vector; TCL, total cell lysate.
To prove that FL-BCRs expressed in TKO cells harbor oligomannose in the V region similar to BCRs in human FL, we used different plant-derived lectins with distinct carbohydrate-binding specificities.36 Increased binding of the oligomannose-specific ConA and GNA, which specifically binds to terminal α-linked mannose, was observed when glycosylation-proficient FL-BCRs were expressed and compared with controls or gm variants (Figure 3C-D). ConA binding to TKO expressing FL-BCRs was similar to ConA binding to primary FL B cells (Figure 3E). Peanut agglutinin, which binds to galactosyl-β1-3-N-acetylgalactosamine, served as negative control.38 This suggests that V-region N-glycosylation sequons carry mannose-terminating oligosaccharides. We further validated these results by isolating surface proteins and subjecting them to the specific glycosidases, EndoH (digestion of high-mannose oligosaccharides) and PNGaseF (digestion of all N-linked glycans), as described previously.23,39 Oligomannose was attached to VH glycosylation sequons as indicated by the shift of EndoH-digested FL-BCRs, but not of glycosylation-defective mutants (Figure 3E). Mature PNGaseF-sensitive glycans were attached to the constant regions of surface IgM, revealing that mannosylation is specific to V-region glycosylation sites. This picture resembles the glycosidase sensitivity of surface IgM isolated from primary FL cells (supplemental Figure 3) described previously.23 Thus, TKO cells reproduce the N-glycosylation pattern of FL receptors seen in primary FL cells.
Binding of endogenous lectins to V-region glycans is not sufficient for BCR activation in TKO cells
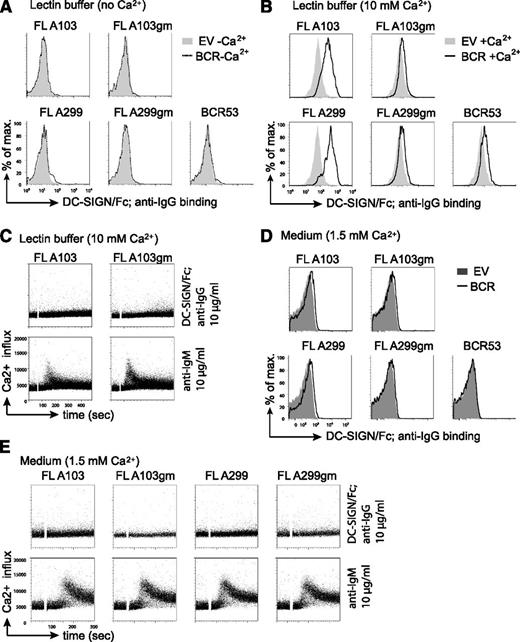
Increasing evidence points to a role of the microenvironment in stimulating BCR signaling and lymphomagenesis. The binding of mannose-specific lectins expressed on cells of the innate immune system to mannosylated BCRs on FL cells represents a potential mechanism for FL-BCR stimulation.23 One of the lectins tested in our previous study23 was DC-SIGN, which is able to interact with high-mannose–type oligosaccharides.40,41 DC-SIGN is a member of the C-type lectin family that requires coordination of Ca2+ ions for carbohydrate binding.42 Thus, in absence of Ca2+, no binding of a solubilized chimeric DC-SIGN fused to the constant part of IgG1 (DC-SIGN/Fc) to FL-BCRs was detected (Figure 4A). Binding of DC-SIGN/Fc to FL-BCRs expressed in TKO cells was observed when the binding was carried out in lectin buffer containing an elevated Ca2+ concentration (10 mM) (Figure 4B). This binding was reduced to background binding when V-region glycosylation sites were removed. However, DC-SIGN/Fc binding to FL-BCRs at 10mM Ca2+ was not sufficient to induce calcium mobilization in TKO cells (Figure 4C). Interestingly, the anti-BCR-induced signal was markedly reduced in lectin buffer as compared with normal medium. Moreover, lectin buffer induced morphologic changes in TKO cells and primary human B cells as evident by the decreased values of forward scatter and percentage of cells in the live-cell gate (supplemental Figure 4). This might interfere or augment signal induction depending on the experimental system. When DC-SIGN/Fc binding was performed in medium in the presence of 1.5 mM Ca2+, a weak binding to mannosylated FL-BCRs was detected (Figure 4D). However, this binding was not sufficient to induce BCR-downstream signaling, although DC-SIGN/Fc was multimerized by preincubation with anti-human IgG antibody (Figure 4E). Nonetheless, due to the additional effect of the lectin buffer on cell morphology and probably cytoskeleton that may influence BCR responsiveness, we performed all following Ca2+ measurements in medium with 1% FCS containing a final concentration of ∼1.5 mM Ca2+ as used for previous studies.24-26
DC-SIGN/Fc binding to FL receptor V-region mannosylation is not sufficient to induce Ca2+ influx. (A-B) Lectin binding to transduced TKO cells in lectin buffer either in the absence (A) or in the presence (B) of Ca2+ was measured by FACS. (A) Gray shaded area represents binding to empty-vector–transduced cells in absence of Ca2+; dotted line represents binding to TKO cells expressing the indicated receptors in absence of Ca2+. (B) Gray shaded area shows binding to empty-vector–transduced cells in the presence of Ca2+; black line represents binding to receptor-positive cells in the presence of Ca2+. (C) FACS analyses of Ca2+ flux of TKO cells transduced with ERT2-SLP65 and FL receptors in lectin buffer containing 10 mM Ca2+. After 1-minute baseline measurement, cells were stimulated with OHT and DC-SIGN/Fc that was preincubated with anti-IgG for further multimerization. Anti-μHC stimulation served as control. (D-E) Lectin binding and Ca2+-flux measurements were carried out in medium supplemented with 1% FCS and physiological Ca2+-ion concentration. (D) Gray shaded area represents binding to empty-vector–transduced cells in medium; black line represents binding to TKO cells expressing the indicated receptors in medium (D) FACS analyses of Ca2+ flux of TKO cells transduced with ERT2-SLP65 and FL receptors in medium containing 1% FCS as described in (C). Results are representative of >3 independent experiments. EV, empty vector.
DC-SIGN/Fc binding to FL receptor V-region mannosylation is not sufficient to induce Ca2+ influx. (A-B) Lectin binding to transduced TKO cells in lectin buffer either in the absence (A) or in the presence (B) of Ca2+ was measured by FACS. (A) Gray shaded area represents binding to empty-vector–transduced cells in absence of Ca2+; dotted line represents binding to TKO cells expressing the indicated receptors in absence of Ca2+. (B) Gray shaded area shows binding to empty-vector–transduced cells in the presence of Ca2+; black line represents binding to receptor-positive cells in the presence of Ca2+. (C) FACS analyses of Ca2+ flux of TKO cells transduced with ERT2-SLP65 and FL receptors in lectin buffer containing 10 mM Ca2+. After 1-minute baseline measurement, cells were stimulated with OHT and DC-SIGN/Fc that was preincubated with anti-IgG for further multimerization. Anti-μHC stimulation served as control. (D-E) Lectin binding and Ca2+-flux measurements were carried out in medium supplemented with 1% FCS and physiological Ca2+-ion concentration. (D) Gray shaded area represents binding to empty-vector–transduced cells in medium; black line represents binding to TKO cells expressing the indicated receptors in medium (D) FACS analyses of Ca2+ flux of TKO cells transduced with ERT2-SLP65 and FL receptors in medium containing 1% FCS as described in (C). Results are representative of >3 independent experiments. EV, empty vector.
Together, DC-SIGN/Fc binding to BCR V-region mannosides did not induce detectable Ca2+ influx in the TKO cells, but binding and stimulation by other endogenous lectins cannot be ruled out.
Bacterial lectins bind to FL-receptor glycans and induce Ca2+ influx
Proteins capable of binding specific carbohydrates are present in various species. In bacteria, surface lectins serve as adhesion molecules that mediate attachment to the host cell and thereby initiate infection.43 Some soluble bacterial lectins were described to possess affinity to high-mannose oligosaccharides.43,44 We tested binding to FL-BCRs and concomitant BCR-signal induction by 2 bacterial lectins from common environmental bacteria: P aeruginosa second lectin (LecB), which is attached to the bacterial surface by binding to ligands in the outer membrane,45 and the related soluble B cenocepacia lectin A (Bc2L-A). Both lectins require Ca2+ cooperation for glycan binding.28,29,46,47 The tetrameric LecB has outstandingly high affinity to fucose and also binds mannose with an affinity comparable to ConA.48 The homodimeric protein Bc2L-A exhibits strict specificity to mannose-terminating N-glycans.28
Bc2L-A bound strongly to the glycosylated V-region of both tested FL-BCRs. Likewise, stronger LecB binding to FL-A103 and FL-A299 as compared with gm variants was detected (Figure 5A). Importantly, binding was performed under physiological 1.5mM Ca2+ and, in contrast to DC-SIGN/Fc, the bacterial lectins triggered B-cell activation (Figure 5B). Signal induction enabled by FL-characteristic V-region mannosylation was measurable at lectin concentrations from 1 μg/mL to 20 μg/mL. The kinetics of signaling at higher lectin concentrations resembled that of anti-BCR stimulation. No lectin-induced signal induction was observed for the BCR53 control receptor or for the gm variants. Importantly, all receptors were functional as demonstrated by anti-BCR treatment (Figure 5B). Together, these results demonstrate selective binding of mannose-specific soluble bacterial lectins to glycosylated FL-BCRs in medium and BCR-signal induction at low lectin concentrations.
Binding of mannose-specific bacterial lectins stimulates FL receptors via V-region N-glycosylation. (A) Binding profile of bacterial lectins to FL-BCRs and the glycosylation-defective mutants expressed on TKO cells by FACS analyses. Empty-vector–transduced cells and BCR53+ cells served as controls. (B) Ca2+ mobilization of ERT2-SLP65+ TKO cells reconstituted with the indicated receptor variants upon stimulation with bacterial lectins or anti-BCR stimulation at the indicated concentrations in medium containing 1% FCS. (C) BCR surface expression on primary FL and HD PBMCs was acquired using anti-human CD19 and anti-human LC antibodies. Binding profile of Bc2L-A to CD19+- compared with CD19−-gated cells in FL sample from lymph node biopsy specimen or healthy donor control measured by FACS. (D) Ca2+-influx measurement of primary FL sample and healthy control PBMCs pregated on CD43− cells upon addition of the indicated stimuli after 1-minute baseline measurement. Experiments were performed in medium containing 1.5 mM Ca2+. (E) Mean fluorescence intensity values of Bc2L-A binding in CD19+ referred to CD19− cells revealed 2 FL subsets: binders and nonbinders (left). Statistical analysis of Ca2+-influx measurements of primary samples displayed as percentage of cells over threshold after Bc2L-A stimulation (FL binder, n = 4; FL nonbinder, n = 4; HD, n = 7; box and whiskers). *P = .0286 (binder vs nonbinder), P = .242 (binder vs HD), P = .5237 (nonbinder vs HD). Data are representative of >3 independent experiments.
Binding of mannose-specific bacterial lectins stimulates FL receptors via V-region N-glycosylation. (A) Binding profile of bacterial lectins to FL-BCRs and the glycosylation-defective mutants expressed on TKO cells by FACS analyses. Empty-vector–transduced cells and BCR53+ cells served as controls. (B) Ca2+ mobilization of ERT2-SLP65+ TKO cells reconstituted with the indicated receptor variants upon stimulation with bacterial lectins or anti-BCR stimulation at the indicated concentrations in medium containing 1% FCS. (C) BCR surface expression on primary FL and HD PBMCs was acquired using anti-human CD19 and anti-human LC antibodies. Binding profile of Bc2L-A to CD19+- compared with CD19−-gated cells in FL sample from lymph node biopsy specimen or healthy donor control measured by FACS. (D) Ca2+-influx measurement of primary FL sample and healthy control PBMCs pregated on CD43− cells upon addition of the indicated stimuli after 1-minute baseline measurement. Experiments were performed in medium containing 1.5 mM Ca2+. (E) Mean fluorescence intensity values of Bc2L-A binding in CD19+ referred to CD19− cells revealed 2 FL subsets: binders and nonbinders (left). Statistical analysis of Ca2+-influx measurements of primary samples displayed as percentage of cells over threshold after Bc2L-A stimulation (FL binder, n = 4; FL nonbinder, n = 4; HD, n = 7; box and whiskers). *P = .0286 (binder vs nonbinder), P = .242 (binder vs HD), P = .5237 (nonbinder vs HD). Data are representative of >3 independent experiments.
To test that bacterial lectins bind not only to FL-BCRs in TKO cells but also to primary FL B cells, we stained primary FL samples and healthy-donor (HD) samples with the strictly mannose-specific Bc2L-A. The results showed binding of Bc2L-A to CD19+ B cells in 4 out of 8 FL samples, whereas no Bc2L-A binding was observed for HD B cells (Figure 5C,E). Binding of bacterial lectin induced Ca2+ influx in these 4 primary FL samples (Figure 5D-E). No significant difference was observed for the anti-IgM-induced calcium response for all FL samples as compared with HD (Figure 5D and supplemental Figure 5).
To further confirm the specific activation of FL-BCRs by bacterial lectins, we sought a human FL B-cell lymphoma line that contains FL-characteristic BCR glycosylation. We investigated the human B-cell line WSU-FSCCL, which was established from the blood of a patient with low-grade follicular small cleaved-cell lymphoma and harbors the FL-characteristic t(14;18) in addition to a t(8;11) chromosomal translocation.49 As a control human cell line, we used the Ramos Burkitt B-cell lymphoma line.50 Both cell lines express VH4-34 gene containing a natural N-glycosylation site in the CDR2, whereas WSU-FSCCL cells expressed κLC and Ramos cells expressed λLC (Figure 6A). Interestingly, sequence analysis revealed preservation of the VH4-34 glycosylation sequon in Ramos-cell BCR, whereas the WSU-FSCCL cells lost this site through 2 nucleotide changes (supplemental Figure 6A). However, 2 additional glycosylation sites have been introduced by SHM in the VH of the WSU-FSCCL BCR (supplemental Figure 6A). Importantly, when using VH4-34 receptors in a previous study, we did not observe attachment of oligosaccharide to this germline-encoded V-region glycosylation site.10 Increased ConA binding to WSU-FSCCL cells compared with Ramos cells suggested the presence of oligomannose on the WSU-FSCCL BCR (Figure 6B). When subjecting surface proteins to glycosidases EndoH and PNGaseF, EndoH digestion resulted in a mobility shift of WSU-FSCCL surface IgM, but not Ramos surface IgM. Treatment with PNGaseF resulted in increased mobility in both cell lines, confirming that oligomannose is on the surface IgM of WSU-FSCCL cells (Figure 6C). In accordance, we detected binding of bacterial lectins Bc2L-A and LecB to WSU-FSCCL cells (Figure 6D). Consistently, treatment with bacterial lectins triggered BCR signaling in WSU-FSCCL cells, but not in Ramos cells (Figure 6E). However, anti-BCR stimulation using anti-LC antibodies resulted in signal induction in both cell lines (Figure 6E). Expression of the WSU-FSCCL-derived receptor and glycosylation mutant thereof in TKO cells suggested that the V-region glycosylation was important for lectin binding and lectin-induced calcium mobilization (supplemental Figure 6B). In summary, the data obtained from the human FL B-cell line WSU-FSCCL confirmed that BCRs containing FL-characteristic V-region mannosylation can specifically be stimulated by bacterial lectins under physiological Ca2+ conditions.
Bacterial lectin binding induced Ca2+ flux in the human cell line WSU-FSCCL under physiological Ca2+ conditions. (A) Flow-cytometric measurement of BCR surface expression on WSU-FSCCL and Ramos cell lines using anti-human IgM and anti-human κLC or λLC antibodies, respectively. (B) Surface binding profile of ConA to the indicated cells. (C) Western blot analysis of isolated surface proteins of the human cell lines subjected to glycosidase treatment as indicated. Size shift was analyzed by immunoblotting against anti-human μHC. (D) Binding profile of bacterial lectins to WSU-FSCCL and Ramos cell line cells measured by FACS. (E) Ca2+ mobilization of the indicated cell lines upon stimulation with bacterial lectins or anti-LC antibodies at the indicated concentrations. Data are representative of >3 independent experiments.
Bacterial lectin binding induced Ca2+ flux in the human cell line WSU-FSCCL under physiological Ca2+ conditions. (A) Flow-cytometric measurement of BCR surface expression on WSU-FSCCL and Ramos cell lines using anti-human IgM and anti-human κLC or λLC antibodies, respectively. (B) Surface binding profile of ConA to the indicated cells. (C) Western blot analysis of isolated surface proteins of the human cell lines subjected to glycosidase treatment as indicated. Size shift was analyzed by immunoblotting against anti-human μHC. (D) Binding profile of bacterial lectins to WSU-FSCCL and Ramos cell line cells measured by FACS. (E) Ca2+ mobilization of the indicated cell lines upon stimulation with bacterial lectins or anti-LC antibodies at the indicated concentrations. Data are representative of >3 independent experiments.
Discussion
The high abundance (79%-100%)8-11 of non–germline-encoded potential N-glycosylation sites in the VH and VL of FL-BCRs compared with normal B cells (9%)8 suggests a role of the attached oligosaccharide for lymphoma generation and proliferation or survival. Some FL cases may acquire N-glycosylation sites during VDJ recombination (Scherer Florian et al, manuscript in preparation). Most N-glycosylation sequons, however, arise during SHM, and other GC-B-cell malignancies also harbor higher amounts of glycosylation sites in the V regions.8 Carbohydrate-linking motifs are probably commonly acquired during SHM.51 However, the occurrence of V-region N-glycosylation sites in normal B cells is rather low.8,14 This raises the question whether B cells carrying N-glycosylation sites in the V regions are removed from the repertoire during normal GC reaction or whether FL cells are positively selected for the acquisition of N-glycans. Using the Hy10 antibody transgenic mouse model with HEL as foreign and self-antigen, N-glycosylation was identified as a mechanism to reduce self-reactivity. Interestingly, a single somatic mutation was sufficient to diminish self-reactivity.14 Likewise, our work shows that the attachment of 1 oligomannose-glycan to the antigen-binding site of a high-affinity BCR, but not just a change in the protein sequence itself, abrogates antigen binding. Thus, V-region glycosylation hinders binding of conventional antigens, suggesting that cells expressing such BCRs are not selected during normal antigen-driven affinity maturation. Importantly, N-glycosylation sites in FL are most likely not just unselected sequences, as high replacement-to-silent mutation ratio in the CDRs of IgM FL receptors has been reported,22,52 suggesting that the FL receptors underwent positive selection. However, a recent study described negative selection against replacement mutations in the framework regions but no positive selection of replacement mutations in the CDRs, indicating a selection to maintain the structural integrity of the FL BCRs rather than a positive selection for antigen recognition.53 Our unpublished data suggest that novel N-glycosylation sites appeared as one of the first events within the genealogical BCR trees and that SHM eradicated potential N-glycosylation sites in only around 1% of the FL cases (Scherer Florian et al, manuscript in preparation). Together, these observations suggest a positive selection for the acquisition of N-glycosylation sites in FL.
As our data demonstrate that BCR mannosylation interferes with antigen binding, it seems that in antigen recognition, that initially stimulated SHM in the respective t(14;18)+ B cell is lost or reduced by the acquired glycosylation. However, mannosylation-mediated lectin binding depicts an alternative way for BCR stimulation in FL, eg, through endogenous C-type lectins expressed on the surface of dendritic cells or macrophages. In agreement with previous data,23 we found selective, Ca2+-dependent binding of DC-SIGN/Fc to FL BCR-mannosylation. DC-SIGN binding to mannosylated FL receptors expressed in TKO cells or human WSU-FSCCL cells did not induce BCR signaling in medium. Nonetheless, primary FL cells seem to induce some BCR signaling in response to soluble DC-SIGN (F.K.S., unpublished data), which might be due to differences in the BCR downstream signaling cascade as compared with the cell lines.
Given the already mentioned natural occurrence of N-glycosylation sequons in the V region of normal B cells, eg, ConA binding was observed for 12% of IgG F(ab’)2 in healthy humans,54 the expression of DC-SIGN on dendritic cells that are present during a normal GC reaction makes DC-SIGN a potential autoantigen for B cells carrying mannosylated receptors in normal individuals. The exact role of this potential autoantigen in the activation of 12% of normal B cells and in FL pathogenesis remains to be investigated.
Lectins from opportunistic pathogens such as B cenocepacia or P aeruginosa represent potent stimulants for B cells expressing N-linked glycan-containing BCRs. Although Bc2L-A and LecB require 2 Ca2+ ions in their carbohydrate binding sites,28,29,41,46 treatment with the soluble bacterial lectins induced stimulation of FL-BCR expressed on TKO cells, the human WSU-FSCCL cell line, and 4 out of 8 primary FL cells under physiological Ca2+ concentrations. The fact that not all respond to the same lectin suggests that binding might be influenced by the number and positions of the introduced N-glycans in the V region. This observation and the possibility that different lectins may exist and interact with distinct FL cells remain to be elucidated.
Importantly, regular human exposure to such bacteria is likely, as both are found in soil and water and are involved in decomposition processes.55,56 For immunocompromised humans, they act as aggressive opportunistic pathogens.57 B cenocepacia can cause epidemic infections in cystic fibrosis patients, although infections in healthy individuals remain unnoticed.
An interesting conclusion of our experiments is that treatment with antibiotics to clear the opportunistic bacteria might represent a potential therapy for FL. Thus, characterizing these lectins and the respective bacteria might well be key for understanding the pathogenesis of FL and establishing novel therapeutic approaches.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Imberty for providing the bacterial lectins and critically reading the manuscript; A. Würch, A. Hobitz, and M. Knoblauch for help with FACS experiments; and P. J. Nielsen and E. Hug for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (JU463/2-1 and TRR130), the Baden-Württemberg Stiftung (P-BWS-Glyko/14), and the Deutsche Krebshilfe (project 108935). F.K. acknowledges the support of Worldwide Cancer Research. W.R. acknowledges the support of the European Research Council (ERC-2011-StG 282105). This work was supported in part by the Excellence Initiative of the Deutsche Forschungsgemeinschaft (GSC-4 [Spemann Graduate School] and EXC 294 [Centre for Biological Signaling Studies]).
Authorship
Contribution: D.S. designed and performed experiments, created the figure layout, and wrote the manuscript; M.D.-v.M., A.A., and C.S. performed experiments with human cell lines; W.R., I.W., and S.V. provided native and labeled bacterial lectins; F.K.S., S.K., and G.P. helped with DC-SIGN experiments and cell-surface protein isolation; C.A.M.v.B., M.B.-P., K.Z., and H.V. characterized follicular lymphoma patients and provided primary human samples; C.B. provided cell lines and discussed data; and H.J. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hassan Jumaa, Institute of Immunology, Ulm University Medical Center, Albert-Einstein-Allee 11, 89081 Ulm, Germany; e-mail: hassan.jumaa@uni-ulm.de.