Key Points
VWFpp discriminates between type 3 VWD patients and severe type 1 VWD patients with very low VWF levels.
The pathophysiological mechanisms of all types of VWD can be defined by the combined ratios of VWFpp/VWF:Ag and FVIII:C/VWF:Ag.
Abstract
The ratios between von Willebrand factor propeptide (VWFpp) or factor VIII activity (FVIII:C) and VWF antigen (VWF:Ag) reflect synthesis, secretion, and clearance of VWF. We aimed to define the pathophysiology of 658 patients with type 1, 2, or 3 von Willebrand disease (VWD) with VWF levels ≤30 U/dL from the Willebrand in The Netherlands (WiN) study using the VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios. We evaluated the use of VWFpp in the classification and diagnosis of VWD. On the basis of the ratios, reduced VWF synthesis was observed in 18% of type 1 and only 2% of type 2 patients. A significant proportion of type 3 patients had detectable VWFpp (41%). These patients had a lower bleeding score than type 3 patients who had a complete absence of VWF:Ag and VWFpp (14.0 vs 19.5; P = .025). The majority of these patients had missense mutations with rapid VWF clearance, whereas type 3 patients with no VWFpp were homozygous for null alleles. In conclusion, VWFpp identified severe type 1 VWD with very low VWF levels in patients who had previously been classified as type 3 VWD. This study underlines the clinical significance of the VWFpp assay in the diagnosis and classification of VWD.
Introduction
Von Willebrand factor (VWF) is a large multimeric glycoprotein that mediates platelet adhesion and aggregation at sites of vascular injury and serves as the carrier of factor VIII (FVIII) to prevent its premature clearance.1 Synthesis of VWF is restricted to endothelial cells and megakaryocytes,2 where it is formed as a precursor protein, pre-proVWF, with a signal peptide, a propeptide, and a mature subunit.3 After translocation to the endoplasmic reticulum, the signal peptide is cleaved, and the pro-VWF undergoes extensive posttranslational modifications, including dimerization in the endoplasmic reticulum and multimerization in the Golgi system.4 The VWF propeptide (VWFpp) is subsequently removed from the mature VWF; however, it stays noncovalently bound.5 Part of VWF is thought to be secreted constitutively into the plasma, and the remaining part is stored in Weibel-Palade bodies in the endothelium or α-granules in megakaryocytes.6 After release into the circulation, the VWFpp and the mature VWF completely dissociate.6
Plasma VWF concentrations represent a balance between synthesis, secretion, and clearance from the circulation. VWF and VWFpp are secreted equimolarly but are cleared independently with estimated half-lives of 8 to 12 hours for VWF and 2 hours for VWFpp.7 The ratio between VWFpp and VWF antigen (VWF:Ag) can therefore be used to assess the rates of synthesis, secretion, and clearance of VWF.7,8 In addition to VWFpp, the ratio between FVIII coagulant activity (FVIII:C) and VWF:Ag can also be used to assess VWF synthesis and clearance.9 Because FVIII and VWF circulate in blood as a complex and are cleared together, their half-lives are strongly related.10
VWF is deficient or defective in von Willebrand disease (VWD), the most common inherited bleeding disorder.11 Low VWF levels are caused by reduced VWF synthesis or secretion, increased VWF clearance, or a combination thereof. Recently, Eikenboom et al12 have shown that the VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios represent the pathophysiology of VWD and correspond to different VWF gene mutations in type 1 VWD. However, it is still unknown whether these ratios can also provide insight into the pathophysiological mechanism in type 2 and 3 VWD.
VWD patients are classified as type 1, 2, or 3 VWD according to their remaining level of functional VWF.11 Type 1 VWD is characterized by partially reduced VWF levels, whereas in type 3 VWD, plasma is completely devoid of VWF. In type 2 VWD, functionally abnormal variants of VWF are synthesized. Type 2 VWD is subclassified as 2A, 2B, 2M, or 2N on the basis of the type of functional defect in VWF.11 In some patients, it is difficult to classify VWD correctly because the available diagnostic laboratory tests do not provide adequate information to distinguish between VWD (sub)types. For instance, type 2M may be caused by a binding defect to collagen, which will be missed by the regular VWF assays. In addition, these laboratory assays are less sensitive at measuring extremely low VWF and FVIII levels and are therefore less reliable.
In this large nationwide cross-sectional Willebrand in The Netherlands (WiN) study, we aimed to define the pathophysiology of type 1, 2, and 3 VWD by using the VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios irrespective of the VWF gene mutation. In addition, we evaluated whether the use of VWFpp can improve the diagnosis and classification of VWD.
Methods
Participants
The WiN study included 804 VWD patients who had previously been diagnosed with type 1, 2, or 3 VWD.13,14 The inclusion criteria for the WiN study were hemorrhagic diathesis or a family history of VWD and historically low VWF levels ≤30 U/dL (VWF:Ag and/or VWF ristocetin cofactor [VWF:RCo]) and/or FVIII:C levels ≤40 U/dL. Patients were excluded if they were known to have other hemostatic disorders resulting in hemorrhagic diathesis. The medical ethical committees at all participating centers approved this study, and all participants gave informed consent.
For this study, only patients from whom plasma was obtained were selected (n = 681). Exclusion criteria were pregnancy (n = 7) and the recent use of desmopressin or replacement therapy at the time of blood sampling (n = 16). Therefore, a total of 658 VWD patients were included in this study.
Assessment of bleeding symptoms
All patients completed a questionnaire regarding bleeding episodes, treatment of VWD, comorbidity, and quality of life.14,15 To calculate a bleeding score (BS), as previously described by Tosetto,16 we used information on the number and severity of clinically relevant bleeding of 12 specific bleeding symptoms. The Tosetto BS assigns a high score when desmopressin or replacement therapy is needed to control bleeding; however, when this replacement therapy was used prophylactically before a surgical intervention, dental extraction, or delivery to prevent bleeding, this bleeding symptom was not scored as a bleeding that required replacement therapy because that would overestimate the score.14,17 However, if bleeding did occur despite prophylactic treatment, this bleeding was scored according to the Tosetto BS.
Laboratory measurements
Patients’ plasma was obtained at inclusion in the study. Venous whole blood was collected in 0.105 M sodium citrate tubes and centrifuged twice at 2200g for 10 minutes at room temperature and stored at −80°C. VWF:Ag, VWF activity (VWF:Act), FVIII:C, VWF binding to FVIII (VWF:FVIIIB), and VWF multimers were determined centrally at the Erasmus University Medical Center (Rotterdam, The Netherlands) as described.14
VWF:Act was assessed with a latex immune assay on an automated coagulometer. The latex immune assay uses monoclonal antibodies directed against the GpIbα binding site of VWF and thereby reflects the binding activity of VWF to GpIbα (HemosIL von Willebrand Factor Activity; Instrumentation Laboratory B.V., Breda, The Netherlands).18 We were not able to use the laborious VWF:RCo test in this study for logistical reasons, although it is the most widely used and most preferable test. Because of the known variability of VWF testing in 13 different laboratories in The Netherlands, we preferred to perform all VWF tests centrally in a laboratory with expertise on VWF. We have previously found a very strong correlation (Spearman correlation coefficient 0.942; P < .0001) between the VWF:RCo and the VWF:Act tests in a random set of patients (n = 122). The diagnostic accuracy of this test was also confirmed by others.19
VWFpp was measured centrally at the Leiden University Medical Center (Leiden, The Netherlands). This assay was also used in the previous report by Eikenboom et al12 and was measured at the same center. VWFpp antigen was determined with an enzyme-linked immunoassay by using antibodies from Sanquin (Amsterdam, The Netherlands) as described.7,12 First, microtiter plates were coated with antibody CLB-Pro 35 overnight at 4°C and then blocked with 1% bovine serum albumin at room temperature for 2 hours. Next, the diluted samples were incubated for 2 hours at 37°C. VWFpp was detected with peroxidase-conjugated antibody CLB-Pro 14.3. Pooled normal plasma was used to create a standard curve. At the time of the study, no international standard was yet available for VWFpp, and therefore the plasma pool was arbitrarily set at 100 U/dL. Details on the blood-sampling procedure and laboratory measurements at inclusion in the WiN study have been described in more detail by de Wee et al.14
Definitions
Determination of VWD type was based on the current International Society on Thrombosis and Haemostasis (ISTH) guidelines11 by using centrally measured plasma concentrations of VWF:Ag, VWF:Act, and FVIII:C; VWF multimer patterns; VWF:FVIIIB assay; and locally performed ristocetin-induced platelet aggregation tests.14
Type 1 VWD patients had a VWF:Act/VWF:Ag ratio ≥0.70, whereas type 2 patients had a ratio <0.70. Considering the strong correlation between VWF:RCo and VWF:Act, we used the VWF:Act/VWF:Ag ratio instead of VWF:RCo/VWF:Ag. If type 2 patients had normal multimers, they were classified as 2M. Type 2A or 2B VWD patients showed abnormal multimers. If the locally performed ristocetin-induced platelet aggregation test showed increased VWF affinity for platelets, patients were classified as type 2B. Type 2N patients had an FVIII:C/VWF:Ag ratio of <0.70 and an VWF:FVIIIB ratio of <60%.20 Type 3 VWD was defined as having both VWF:Ag and VWF:Act levels <5 U/dL, irrespective of FVIII:C level.
The normal range (2.5th to 97.5th percentile) used for VWFpp was 82 to 173 U/dL, for the VWFpp/VWF:Ag ratio 0.8 to 2.2, and for the FVIII:C/VWF:Ag ratio 0.6 to 1.9, as previously reported by Eikenboom et al.12
Statistical methods
Descriptive statistics for categorical data are presented as numbers with percentages. Because data were nonnormally distributed, continuous variables are presented as median with 25% to 75% interquartile ranges [IQRs]. For comparison of proportions, χ2 tests were used. The Kruskal-Wallis test was used to test differences in VWFpp, VWFpp/VWF:Ag ratio, and FVIII:C/VWF:Ag ratio between types 1, 2A, 2B, 2M, 2N, and 3 VWD. Mann-Whitney U tests were used to detect differences between types and to compare BS between groups. Statistical analyses were performed with SPSS for Windows, version 21.0 (SPSS, Chicago, IL). A P value < .05 was considered statistically significant.
Results
A total of 658 VWD patients were included in these analyses, 381 of whom had type 1, 240 type 2, and 37 type 3 VWD, according to the current ISTH guidelines.11 Baseline characteristics are presented in Table 1.
Baseline characteristics
| Characteristic . | VWD patients (n = 658) . | |
|---|---|---|
| No. . | % . | |
| Age, y | ||
| Median | 43.5 | |
| Range | 1-83 | |
| Male sex | 251 | 38 |
| VWD type | ||
| 1 | 380 | 58 |
| 2 | 241 | 37 |
| 2A | 158 | |
| 2B | 42 | |
| 2M | 27 | |
| 2N | 14 | |
| 3 | 37 | 6 |
| Blood group O* | 398 | 61 |
| VWF:Ag (IU/dL) | ||
| Median | 29 | |
| 25%-75% IQR | 18-45 | |
| VWF:Act (U/dL) | ||
| Median | 22 | |
| 25%-75% IQR | 8-53 | |
| FVIII:C (IU/dL) | ||
| Median | 51 | |
| 25%-75% IQR | 32-73 | |
| BS† | ||
| Median | 11 | |
| 25%-75% IQR | 6-16 | |
| Characteristic . | VWD patients (n = 658) . | |
|---|---|---|
| No. . | % . | |
| Age, y | ||
| Median | 43.5 | |
| Range | 1-83 | |
| Male sex | 251 | 38 |
| VWD type | ||
| 1 | 380 | 58 |
| 2 | 241 | 37 |
| 2A | 158 | |
| 2B | 42 | |
| 2M | 27 | |
| 2N | 14 | |
| 3 | 37 | 6 |
| Blood group O* | 398 | 61 |
| VWF:Ag (IU/dL) | ||
| Median | 29 | |
| 25%-75% IQR | 18-45 | |
| VWF:Act (U/dL) | ||
| Median | 22 | |
| 25%-75% IQR | 8-53 | |
| FVIII:C (IU/dL) | ||
| Median | 51 | |
| 25%-75% IQR | 32-73 | |
| BS† | ||
| Median | 11 | |
| 25%-75% IQR | 6-16 | |
VWF:Ag, VWF:Act, and FVIII:C levels were measured centrally at time of inclusion in the study.
Total of 4 missing.
Total of 32 missing.
VWF parameters and ratios per type of VWD
In type 1 patients, median VWFpp was 91 U/dL (IQR, 68 to 116 U/dL), median VWFpp/VWF:Ag ratio was 2.2 (IQR, 1.7 to 3.1), and median FVIII:C/VWF:Ag ratio was 1.8 (IQR, 1.4 to 2.3) (Table 2 and Figure 1A). This is in accordance with previously published data by Eikenboom et al.12 In type 2 patients, median VWFpp was 104 U/dL ( IQR, 81 to 136 U/dL), which was higher than that in type 1 patients (P < .001). Median VWFpp/VWF:Ag ratio was 4.5 (IQR, 3.2 to 6.0), and median FVIII:C/VWF:Ag ratio was 1.6 (IQR, 1.2 to 2.0) in the type 2 patients. The VWFpp/VWF:Ag ratio was higher in type 2 VWD than in type 1 VWD (P < .001), and the FVIII:C/VWF:Ag ratio was lower in type 2 VWD compared with type 1 VWD (P < .001) (Table 2 and Figure 1A). This difference in VWFpp, VWFpp/VWF:Ag, and FVIII:C/VWF:Ag between type 1 and type 2 VWD was also observed after exclusion of type 2N patients (all P < .001). Median VWFpp in type 2A, 2B, and 2M VWD was 103 U/dL (IQR, 81 to 135 U/dL), median VWFpp/VWF:Ag was 4.6 (IQR, 3.4 to 6.2), and median FVIII:C/VWF:Ag was 1.6 (IQR, 1.3 to 2.1).
VWF parameters and ratios per type of VWD
| VWD type . | No. of Patients . | VWF:Ag (IU/dL) . | VWF:Act (U/dL) . | FVIII:C (IU/dL) . | VWFpp (U/dL) . | VWF:Act/VWF:Ag ratio . | VWFpp/VWF:Ag ratio . | FVIII:C/VWF:Ag ratio . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | ||
| 1* | 381 | 38 | 23-53 | 46 | 24-72 | 67 | 49-88 | 91 | 68-116 | 1.2 | 1.0-1.4 | 2.2 | 1.7-3.1 | 1.8 | 1.4-2.3 |
| 2 | 240 | 24 | 16-34 | 8 | 4-16 | 37 | 27-48 | 104 | 81-136 | 0.4 | 0.2-0.5 | 4.5 | 3.2-6.0 | 1.6 | 1.2-2.0 |
| 2A | 157 | 22 | 14-32 | 8 | 3-15 | 37 | 27-47 | 99 | 79-123 | 0.4 | 0.2-0.5 | 4.5 | 3.3-6.2 | 1.7 | 1.3-2.2 |
| 2B | 42 | 28 | 23-40 | 8 | 6-14 | 39 | 28-48 | 137 | 106-157 | 0.3 | 0.2-0.4 | 4.6 | 3.7-5.8 | 1.3 | 1.1-1.5 |
| 2M | 27 | 24 | 16-30 | 5 | 3-13 | 42 | 31-52 | 93 | 75-119 | 0.3 | 0.1-0.4 | 4.8 | 3.1-6.2 | 1.9 | 1.6-2.1 |
| 2N | 14 | 34 | 28-44 | 45 | 29-54 | 19 | 13-28 | 111 | 71-157 | 1.2 | 1.0-1.5 | 2.7 | 2.0-4.4 | 0.6 | 0.4-0.7 |
| 3 | 37 | 1 | 0-4 | 0 | 0-1 | 3 | 1-9 | 0 | 0-60† | NA | NA | NA | |||
| Normal range‡ | 47-169 | 45-213 | 57-185 | 82-173 | 0.6-1.8 | 0.8-2.2 | 0.6-1.9 | ||||||||
| VWD type . | No. of Patients . | VWF:Ag (IU/dL) . | VWF:Act (U/dL) . | FVIII:C (IU/dL) . | VWFpp (U/dL) . | VWF:Act/VWF:Ag ratio . | VWFpp/VWF:Ag ratio . | FVIII:C/VWF:Ag ratio . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | Median . | IQR . | ||
| 1* | 381 | 38 | 23-53 | 46 | 24-72 | 67 | 49-88 | 91 | 68-116 | 1.2 | 1.0-1.4 | 2.2 | 1.7-3.1 | 1.8 | 1.4-2.3 |
| 2 | 240 | 24 | 16-34 | 8 | 4-16 | 37 | 27-48 | 104 | 81-136 | 0.4 | 0.2-0.5 | 4.5 | 3.2-6.0 | 1.6 | 1.2-2.0 |
| 2A | 157 | 22 | 14-32 | 8 | 3-15 | 37 | 27-47 | 99 | 79-123 | 0.4 | 0.2-0.5 | 4.5 | 3.3-6.2 | 1.7 | 1.3-2.2 |
| 2B | 42 | 28 | 23-40 | 8 | 6-14 | 39 | 28-48 | 137 | 106-157 | 0.3 | 0.2-0.4 | 4.6 | 3.7-5.8 | 1.3 | 1.1-1.5 |
| 2M | 27 | 24 | 16-30 | 5 | 3-13 | 42 | 31-52 | 93 | 75-119 | 0.3 | 0.1-0.4 | 4.8 | 3.1-6.2 | 1.9 | 1.6-2.1 |
| 2N | 14 | 34 | 28-44 | 45 | 29-54 | 19 | 13-28 | 111 | 71-157 | 1.2 | 1.0-1.5 | 2.7 | 2.0-4.4 | 0.6 | 0.4-0.7 |
| 3 | 37 | 1 | 0-4 | 0 | 0-1 | 3 | 1-9 | 0 | 0-60† | NA | NA | NA | |||
| Normal range‡ | 47-169 | 45-213 | 57-185 | 82-173 | 0.6-1.8 | 0.8-2.2 | 0.6-1.9 | ||||||||
IQR, 25% to 75% interquartile range; NA, not applicable.
Median and 25% to 75% IQRs of VWFpp level and ratios in type 1 VWD patients are in accordance with the results from Eikenboom et al.12
VWFpp levels below the assay detection limit (<4 U/dL) were considered 0 (n = 25).
The normal reference ranges (2.5th to 97.5th percentile) are based on 387 healthy controls and were taken from our previous publication Eikenboom et al12 ; however, VWF activity in that study was based on VWF:RCo. VWFpp in the WiN study was measured in this same laboratory.

VWFpp levels and VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios per type of VWD. (A) VWFpp levels and VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios are shown for type 1 and type 2 VWD patients. For a better presentation of the graphic VWFpp/VWF:Ag ratio, one type 1 VWD patient with both a high VWFpp/Ag ratio (33.7) and a FVIII:C/VWF:Ag ratio of 3.4 was omitted. (B) For all patients with type 2A, 2B, 2M, and 2N VWD, the VWFpp levels and the VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios are shown. The central horizontal line represents the median. The upper and lower horizontal lines show the 25% to 75% interquartile ranges. The dashed lines show the normal ranges (2.5th to 97.5th percentile) for VWFpp (range, 82 to 173 U/dL), for VWFpp/VWF:Ag ratio (range, 0.8 to 2.2), and for FVIII:C/VWF:Ag ratio (range, 0.6 to1.9).

VWFpp levels and VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios per type of VWD. (A) VWFpp levels and VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios are shown for type 1 and type 2 VWD patients. For a better presentation of the graphic VWFpp/VWF:Ag ratio, one type 1 VWD patient with both a high VWFpp/Ag ratio (33.7) and a FVIII:C/VWF:Ag ratio of 3.4 was omitted. (B) For all patients with type 2A, 2B, 2M, and 2N VWD, the VWFpp levels and the VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios are shown. The central horizontal line represents the median. The upper and lower horizontal lines show the 25% to 75% interquartile ranges. The dashed lines show the normal ranges (2.5th to 97.5th percentile) for VWFpp (range, 82 to 173 U/dL), for VWFpp/VWF:Ag ratio (range, 0.8 to 2.2), and for FVIII:C/VWF:Ag ratio (range, 0.6 to1.9).
VWF parameters and ratios for different type 2 VWD subtypes
Median VWFpp was significantly higher in VWD 2B patients than in 2A patients or 2M patients: 137 U/dL (IQR, 106 to 157 U/dL) vs 99 U/dL (IQR, 79 to 123 U/dL] or 93 U/dL (IQR, 75 to 119 U/dL] with P < .001 for both (Figure 1B and Table 2). VWFpp/VWF:Ag ratio was increased in 2A, 2B, and 2M patients based on the normal range (range, 0.8 to 2.2)12 ; 4.5 (IQR, 3.3 to 6.2) in 2A, 4.6 (IQR, 3.7 to 5.8) in 2B, and 4.8 (IQR, 3.1 to 6.2) in 2M VWD. Type 2B patients had significantly lower FVIII:C/VWF:Ag ratios than 2A or 2M patients (1.3 [IQR, 1.1 to 1.5] vs 1.7 [IQR, 1.3 to 2.2] or 1.9 [IQR, 1.6 to 2.1]; P < .001 for both). Median VWFpp and median VWFpp/VWF:Ag ratio were similar between type 2N and type 1 VWD. Type 2N patients had a significantly decreased FVIII:C/VWF:Ag ratio compared with type 1, 2A, 2B, and 2M VWD (all P < .001), as expected by the decreased FVIII binding affinity in type 2N.
Pathophysiological mechanisms in type 1 VWD
An increased VWFpp/VWF:Ag ratio (>2.2) defines accelerated clearance of VWF, and an increased FVIII:C/VWF:Ag ratio (>1.9) correlates with reduced VWF synthesis (Figure 2A). The pathophysiological mechanisms of type 1 VWD were variable, with increased clearance of VWF in 23% of type 1 patients (89 of 380), reduced synthesis of VWF in 18% (67 of 380), and a combination of these in 23% (86 of 380). In 36% of type 1 patients (138 of 380), other pathophysiological mechanisms may play a role, because VWFpp/VWF:Ag and FVIII:C/VWF:Ag were within normal ranges (Figure 2).
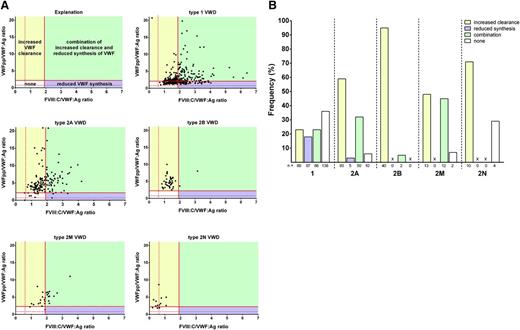
Pathophysiological mechanisms per type of VWD. (A) Scatter plots of FVIII:C/VWF:Ag (x-axis) vs VWFpp/VWF:Ag (y-axis) for type 1, 2A, 2B, 2M, and 2N VWD. Shown are patients with increased clearance (yellow), with reduced synthesis (purple), with a combination of both mechanisms (green), and absence of these pathophysiological mechanisms (white). Because of the FVIII binding defect in type 2N VWD, the FVIII:C/VWF:Ag ratio is low by definition and cannot be assessed reliably with respect to aspects of synthesis in type 2N. For a better presentation of the graphics, one type 1 VWD patient with a very high VWFpp/Ag ratio (33.7) and two type 1 VWD patients with high FVIII:C/VWF:Ag ratios (8.4 and 9.7) were omitted. (B) Proportion of VWD patients with increased clearance (yellow), reduced synthesis (purple), combination of increased clearance and reduced synthesis (green), and none of these mechanisms (white). X, not present.

Pathophysiological mechanisms per type of VWD. (A) Scatter plots of FVIII:C/VWF:Ag (x-axis) vs VWFpp/VWF:Ag (y-axis) for type 1, 2A, 2B, 2M, and 2N VWD. Shown are patients with increased clearance (yellow), with reduced synthesis (purple), with a combination of both mechanisms (green), and absence of these pathophysiological mechanisms (white). Because of the FVIII binding defect in type 2N VWD, the FVIII:C/VWF:Ag ratio is low by definition and cannot be assessed reliably with respect to aspects of synthesis in type 2N. For a better presentation of the graphics, one type 1 VWD patient with a very high VWFpp/Ag ratio (33.7) and two type 1 VWD patients with high FVIII:C/VWF:Ag ratios (8.4 and 9.7) were omitted. (B) Proportion of VWD patients with increased clearance (yellow), reduced synthesis (purple), combination of increased clearance and reduced synthesis (green), and none of these mechanisms (white). X, not present.
Pathophysiological mechanisms in type 2 VWD variants
On the basis of increased VWFpp/VWF:Ag or FVIII:C/VWF:Ag ratios, the majority of patients with type 2A VWD (59%; 93 of 158) were characterized by an increased clearance of VWF, followed by a combination of increased clearance and reduced synthesis (32%; 50 of 158). In only five patients with 2A VWD (3%; 5 of 158), reduced synthesis of VWF predominated. In ten 2A patients (6%; 10 of 158), the VWD was characterized by neither increased clearance nor reduced synthesis. Ninety-five percent of type 2B patients (40 of 42) showed an increased VWFpp/VWF:Ag ratio with a normal FVIII:C/VWF:Ag ratio, suggesting that 2B VWD is completely characterized by an increased clearance of normally synthesized and secreted VWF. The pathophysiological mechanisms of type 2M VWD were an increased clearance in almost half the patients (48%; 13 of 27) and a combination of increased clearance and reduced synthesis in the other half (44%; 12 of 27). None of the 2M patients had only an increased FVIII:C/VWF:Ag ratio, indicating that 2M VWD is not solely characterized by reduced synthesis. Ten 2N patients (71%; 10 of 14) had an increased VWFpp/VWF:Ag ratio, indicating that apart from reduced FVIII binding, type 2N VWD is also characterized by increased clearance (Figure 2).
Type 3 VWD patients
Thirty-seven patients with VWF:Ag and VWF:Act <5 U/dL were classified as type 3 VWD, according to the current ISTH guidelines.11 VWFpp was undetectable in 59% (22 of 37) of those patients, which fits with type 3 VWD having complete absence of VWF production. In all other patients classified as type 3 (41%; 15 of 37), high levels of VWFpp were measured with a median of 72 U/dL (Table 3). Compared with the type 3 VWD patients with virtually complete absence of VWFpp, the patients classified as type 3 but with ample VWFpp had higher VWF:Ag levels (0 vs 4 IU/dL; P < .001), higher VWF:Act levels (0 vs 1 U/dL; P = .036), and higher FVIII:C levels (2 vs 9 IU/dL; P < .001). The frequency of blood group O was significantly higher in patients with detectable VWFpp than in the type 3 patients without detectable VWFpp (12 of 15 vs 8 of 22; P = .009). In 12 of 22 type 3 patients without detectable VWFpp, historical data on VWF mutations were available; 10 of those patients were homozygous or compound heterozygous for a null allele (p.R2535X, p.N2546Y, p.Y1570X, p.S330KfsX4, p.D1333EfsX43, and deletion of exons 17 to 25). Of the 15 patients with detectable VWFpp, the VWF gene mutation was known in 14, 10 of whom had missense mutations in regions associated with rapid clearance of VWF (p.R1205H and p.S1285P) or mutations that are considered to rapidly clear VWF from the circulation (p.Y1584C and p.Y1146C).8,21-23 Additionally, 3 patients were compound heterozygous for a null allele and an unknown defect on the other allele.
Characteristics of type 3 VWD patients with and without VWFpp
| Characteristic . | Type 3 VWD patients . | P . | |||
|---|---|---|---|---|---|
| Without VWFpp (n = 22) . | With VWFpp (n = 15) . | ||||
| No. . | % . | No. . | % . | ||
| Age, y | .636 | ||||
| Median | 22 | 35 | |||
| Range | 2-60 | 4-65 | |||
| Male sex | 9 | 41 | 7 | 47 | .729 |
| Blood group O | 8 | 36 | 12 | 80 | .009 |
| VWFpp, U/dL | <.001 | ||||
| Median | 0 | 72 | |||
| 25%-75% IQR | 0-0 | 37-94 | |||
| VWF:Ag, IU/dL | <.001 | ||||
| Median | 0 | 4 | |||
| 25%-75% IQR | 0-1 | 2-4 | |||
| VWF:Act, U/dL | .036 | ||||
| Median | 0 | 1 | |||
| 25%-75% IQR | 0-0 | 0-3 | |||
| FVIII:C, IU/dL | <.001 | ||||
| Median | 2 | 9 | |||
| 25%-75% IQR | 1-3 | 8-13 | |||
| Multimers | |||||
| Absent | 19 | 86 | 3 | 20 | <.001 |
| Abnormal | 3 | 14 | 12 | 80 | |
| BS | .025 | ||||
| Median | 19.5 | 14.0 | |||
| 25%-75% IQR | 11.3-23.8* | 7.0-17.0 | |||
| Characteristic . | Type 3 VWD patients . | P . | |||
|---|---|---|---|---|---|
| Without VWFpp (n = 22) . | With VWFpp (n = 15) . | ||||
| No. . | % . | No. . | % . | ||
| Age, y | .636 | ||||
| Median | 22 | 35 | |||
| Range | 2-60 | 4-65 | |||
| Male sex | 9 | 41 | 7 | 47 | .729 |
| Blood group O | 8 | 36 | 12 | 80 | .009 |
| VWFpp, U/dL | <.001 | ||||
| Median | 0 | 72 | |||
| 25%-75% IQR | 0-0 | 37-94 | |||
| VWF:Ag, IU/dL | <.001 | ||||
| Median | 0 | 4 | |||
| 25%-75% IQR | 0-1 | 2-4 | |||
| VWF:Act, U/dL | .036 | ||||
| Median | 0 | 1 | |||
| 25%-75% IQR | 0-0 | 0-3 | |||
| FVIII:C, IU/dL | <.001 | ||||
| Median | 2 | 9 | |||
| 25%-75% IQR | 1-3 | 8-13 | |||
| Multimers | |||||
| Absent | 19 | 86 | 3 | 20 | <.001 |
| Abnormal | 3 | 14 | 12 | 80 | |
| BS | .025 | ||||
| Median | 19.5 | 14.0 | |||
| 25%-75% IQR | 11.3-23.8* | 7.0-17.0 | |||
Historical data on mutations (total of 11 missing): for those without VWFpp, 10 of 12 were homozygous or compound heterozygous for null alleles; for those with VWFpp, 14 patients were genotyped, 10 of whom had a mutation associated with increased clearance. VWFpp, VWF:Ag, VWF:Act, and FVIII:C levels were measured centrally at time of inclusion in the study.
Total of 2 missing.
Bleeding phenotype, VWFpp, and pathophysiological mechanism
BS was significantly higher in type 3 patients with undetectable VWFpp than in those with detectable VWFpp levels: 19.5 (IQR, 11.3 to 23.8) vs 14.0 (IQR, 7.0 to 17.0) (P = .025) (Table 3). In patients with type 1 VWD, the BS was not associated with the pathophysiological mechanisms of VWD (ie, increased clearance, reduced synthesis, combination of increased clearance and reduced synthesis, or none of these mechanisms: median BS 10.0 vs 9.0 vs 8.5 vs 9.0, respectively) (Figure 3A). However, all type 2 patients with increased clearance, reduced synthesis, or combination had significantly higher BS than type 2 patients with none of these pathophysiologic mechanisms (P = .010). Median BS in type 2 patients with increased clearance or a combination of increased clearance and reduced synthesis was significantly higher than in those with normal ratios: 13.0 (IQR, 9.0 to 17.5) or 13.0 (IQR, 8.0 to16.3) vs 7.5 (IQR, 5.5 to 10.8); P = .001 or P = .007. Median BS in type 2 VWD characterized by reduced synthesis was 17.0 (IQR, 8.0 to 26.0) vs 7.5 (IQR, 5.5 to 10.8) in those with normal ratios (P = .051) (Figure 3B and supplemental Figure 1, available on the Blood Web site).
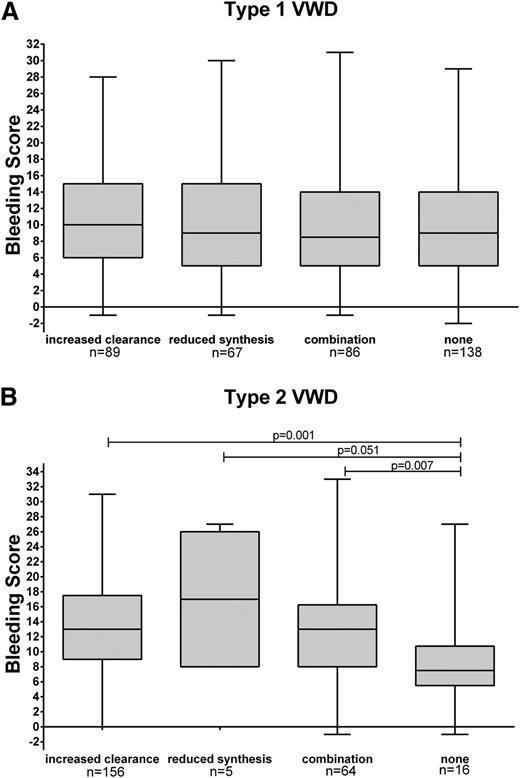
BS per pathophysiological mechanisms for type 1 and 2 VWD. BS according to different pathophysiological mechanisms of VWD for (A) type 1 VWD and (B) type 2 VWD. Boxplots show median, 25% to 75% interquartile range, and minimum and maximum score.
Discussion
Using data from our WiN study cohort, we are the first to report that VWFpp may help improve the discrimination between type 3 VWD patients who have a virtually complete absence of VWF:Ag and VWFpp and severe type 1 VWD patients who have extremely rapid VWF clearance leading to VWF:Ag plasma levels below 5 IU/dL and relatively high VWFpp levels. Moreover, the ratios of VWF:Ag with VWFpp or FVIII:C differ between type 1 and 2 VWD. Type 2 VWD was mainly characterized by an increased clearance of VWF, and type 1 VWD had a more heterogeneous pathophysiology. Finally, low VWFpp was associated with higher BS in type 3 and type 2 VWD.
VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios have been shown to identify type 1 VWD patients by giving insight into the pathophysiological mechanisms behind the low VWF levels (ie, increased clearance and reduced synthesis of VWF).8,12,24 We confirmed this in our study and showed that the pathophysiology of type 1 VWD is diverse because our type 1 patients showed a mixture of VWF synthesis and clearance defects when FVIII:C/VWF:Ag and VWFpp/VWF:Ag ratios were used. Almost two fifths of patients did not have increased ratios. VWD in these patients probably resulted from other as yet unidentified mechanisms. Although VWFpp/VWF:Ag and FVIII:C/VWF:Ag ratios are correlated or consistent with increased clearance or reduced secretion of VWF, definitively demonstrating one mechanism or the other would require expression or clearance studies of the various VWD mutants.
We hypothesized that as a result of our inclusion criteria (historically lowest VWF levels of <30 U/dL), we selected more type 1 patients with accelerated clearance of VWF or reduced synthesis, and therefore patients with higher ratios. However, the VWFpp levels and ratios thereof in our type 1 patients are in agreement with a previous study in type 1 VWD patients.12 This is remarkable because in the MCMDM-1VWD study (Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD), milder cases were also included. Regression to the mean or the effect of aging on VWF and FVIII levels may have been observed.
We observed a higher VWFpp/VWF:Ag ratio in type 2 VWD patients compared with type 1 VWD patients, suggesting a bigger contribution of increased clearance in type 2 patients, whereas type 1 patients had a significantly higher FVIII:C/VWF:Ag ratio than type 2 patients, indicating that reduced synthesis is more important as a pathophysiological mechanism in type 1 than type 2 VWD. Previous studies of type 2A mutations demonstrated reduced synthesis as a mechanism causing type 2A VWD; however, those in vitro expression studies could not analyze the component of clearance. In a recent expression study, it was shown that type 2A mutations are often characterized by a combination of intracellular retention, defective multimerization, loss of regulated secretion, and increased proteolysis by ADAMTS13.25 Our data support that this increased clearance is an important component. This also suggests that these ratios may discriminate between type 1 and type 2 VWD, which has been stated before.26
The main pathophysiological mechanism of type 2 VWD is increased VWF clearance: the majority of type 2A, 2B, and 2M patients showed an increased VWFpp/VWF:Ag ratio. In addition, a small proportion of type 2 patients showed a combination of reduced VWF survival and reduced synthesis, but this was seen only in 2A and 2M VWD. The effect of proteolysis of VWF on clearance has been argued.22,27 Proteolysis itself probably does not play a major role in VWF clearance. If increased proteolysis leads to loss of high molecular weight multimers only, with no shortening of the VWF half-life, then the ratio of VWFpp/VWF:Ag is not influenced by the proteolysis and this will not be picked up by the VWFpp/VWF:Ag ratio. The VWF type 2A mutations will often lead to a combination of pathophysiological mechanisms such as those reported by Jacobi et al.25 In addition to increased susceptibility for ADAMTS13 proteolysis, the mutant VWF may also have a shortened half-life resulting in an increased VWFpp/VWF:Ag ratio. We found no differences between 2A VWD with defects in multimerization (and possibly enhanced ADAMTS13-mediated proteolysis) and 2M VWD with normal multimer patterns. Type 2M patients characterized by defects in collagen binding may have normal ratios and would require VWF collagen binding assay to be identified. Because of small sample size, these findings should be interpreted with care. Our data indicate that 2B VWD is completely characterized by a normal synthesis and an increased clearance of VWF, which has been shown before in 18 type 2B VWD patients.28 It has been suggested that the mutant VWF is cleared faster in these patients because of the spontaneous binding of VWF to platelets.29 Our results also showed that the mutant type 2N VWF is cleared faster than normal VWF.
To the best of our knowledge, this is the first study to assess VWFpp in VWD patients with VWF:Ag and VWF:Act <5 U/dL, the diagnostic criteria used in the current ISTH classification for type 3 VWD.11 We showed that VWFpp is detectable in almost half of our type 3 VWD patients, indicating that VWF is actually produced but very rapidly cleared from the circulation in these patients. Our findings suggest that these type 3 VWD patients should actually be reclassified as severe type 1 VWD patients. We therefore conclude that VWFpp can discriminate between type 3 VWD with a complete absence of both VWF:Ag and VWFpp and severe type 1 VWD with extremely low VWF levels. A significant proportion of these severely affected type 1 VWD patients had blood group O, which may have contributed to the low VWF levels in these patients. Distinguishing between type 3 VWD and severe type 1 VWD is of clinical importance because VWD patients with lack of VWF but with detectable VWFpp have clinical characteristics different from those of patients with a complete absence of both VWF and VWFpp.
Because VWF:Ag and FVIII:C levels are associated with bleeding phenotype in VWD,14,16 it may be expected that VWFpp also associates with bleeding phenotype in VWD patients, either indirectly through its association with VWF:Ag or by binding to mature VWF in the circulation.30 Interestingly, we found that the presence of VWFpp in patients with VWF:Ag levels <5 IU/dL correlates with a milder bleeding phenotype. We also observed that increased clearance, reduced synthesis, and a combination thereof associates with higher BSs in type 2 patients.
To assess the functionality of VWF we used the HemosIL VWF Activity assay. We were not able to use the VWF:RCo test in this study for logistical reasons, although it is still the gold standard for assessing VWF functionality. To avoid the inevitable variability of VWF testing in the 13 different laboratories in The Netherlands, we preferred to perform all VWF tests centrally. The VWF:Act assay should be used with caution because it is not standard and does not completely replace the VWF:RCo test.
Mutation analysis of the VWF gene has been advised in type 2N, type 2B, and type 3 VWD.31 However, sequencing of the VWF gene is expensive and not widely available. Looking at historical data on the molecular background of the type 3 VWD patients, we observed homozygosity and compound heterozygosity for null alleles in the type 3 VWD patients with complete absence of VWF:Ag and VWFpp. In the type 3 VWD patients with detectable VWFpp, missense mutations in the D3 and D4 domain of the VWF gene (p.R1205H, p.S1285P) were identified that are associated with accelerated clearance of VWF.8,21,22 Therefore assessing VWFpp may substitute for VWF gene analyses because it identifies carriers of two null alleles and thus type 3 VWD without detectable VWFpp. In addition, the MCMDM-1VWD study has recently shown that a high VWFpp/VWF:Ag ratio predicts the detection of VWF gene mutations in type 1 VWD patients.12 Furthermore, patients with mutations in regions of the VWF gene associated with reduced VWF survival can be identified with the VWFpp/VWF:Ag ratio.8,21,22 On the basis of our results and the current literature, we believe that measurement of VWFpp should be implemented as a standard diagnostic in VWD for patients with low VWF levels and may render mutation analysis of the VWF gene unnecessary.
VWFpp measurement is of clinical importance because its ratio with VWF:Ag predicts reduced VWF survival and may therefore predict the VWF clearance after desmopressin treatment. In patients with increased VWF clearance, VWF disappears rapidly after desmopressin administration. The desmopressin response in these patients is probably sufficient for hemostasis in case of minor bleeding, but it is inadequate in case of major bleeding or surgery because these patients lack a sustained response.21,32 The FVIII:C/VWF:Ag ratio can also help in the classification of VWD patients, because those patients with an increased FVIII:C/VWF:Ag mainly classify as type 1 VWD. With the current novel insights into the pathophysiology and molecular biology of VWD, the classification of VWD should be reviewed in the near future.
In conclusion, VWFpp discriminates between type 3 VWD patients with complete lack of VWF and severe type 1 VWD patients with very low VWF levels. An increased FVIII:C/VWF:Ag ratio may help identify type 1 VWD patients. This study shows the clinical significance of the VWFpp assay; it is of added value for the classification of VWD, for the assessment of the bleeding phenotype, and for genetic counseling. It also has therapeutic consequences.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr E. M. de Wee and all hemophilia nurses for their work on including patients; and Sanquin (Amsterdam, The Netherlands) for providing the antibodies CLB-Pro 35 and CLB-Pro 14.3 for measurement of von Willebrand factor propeptide (VWFpp) and Richard Dirven for determining VWFpp.
This work (the Willebrand in The Netherlands [WiN] study) was supported by research funding from the Dutch Hemophilia Foundation (Stichting Hemophilia) and CSL Behring (unrestricted grant).
Authorship
Contribution: F.W.G.L. and J.E. designed the research, analyzed and interpreted data, and wrote the manuscript; Y.V.S. and D.G. performed research, analyzed and interpreted data, and wrote the manuscript; K.M., K.F., M.H.C., J.G.v.d.B., B.A.P.L.-v.G., and E.P.M.-B. designed research, analyzed and interpreted data, and critically reviewed the manuscript; M.C. and J.d.M. analyzed and interpreted data and critically reviewed the manuscript; and all authors gave their consent to the final version of the manuscript.
The membership of the Willebrand in The Netherlands (WiN) study group is included in the Appendix.
Conflict-of-interest disclosure: F.W.G.L. received research support from CSL Behring for performing the Willebrand in The Netherlands (WiN) study and has served on advisory boards of CSL Behring and Baxter. J.E. received research support from CSL Behring and has been a teacher for Roche educational activities. E.P.M.-B. received research support from CSL Behring, Bayer, Baxter, Novo Nordisk, Pfizer, and Sanquin. J.G.v.d.B. has received unrestricted research/educational funding from Bayer Schering Pharma, Baxter, CSL Behring, Novo Nordisk, and Pfizer, has been a consultant to Baxter and Pfizer, and has been a teacher for Bayer Schering Pharma educational activities. M.H.C. has received unrestricted research/educational funding from Bayer Schering Pharma, Baxter, Novo Nordisk, and Pfizer. K.M. received research support from Bayer and Baxter, served on an advisory board for CSL Behring, and received speaker fees from Sanquin. B.A.P.L.-v.G. has received unrestricted educational grants from Baxter and CSL Behring and speaker fees from Sanquin. The remaining authors declare no competing financial interests.
Correspondence: Jeroen Eikenboom, Leiden University Medical Center, Department of Thrombosis and Haemostasis, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: h.c.j.eikenboom@lumc.nl.
Appendix: WiN study group members
Academic Medical Center, Amsterdam: K. Fijnvandraat, M. Coppens; VU University Medical Center, Amsterdam: A. Kors, S. Zweegman; Netherlands Hemophilia Society, Nijkerk: J. de Meris; Amphia Hospital, Breda: G. J. Goverde, M. H. Jonkers; Catharina Hospital, Eindhoven: N. Dors; Maxima Medical Center, Eindhoven: M. R. Nijziel; University Medical Center Groningen, Groningen: K. Meijer, R. Y. J. Tamminga; Kennemer Gasthuis, Haarlem: P. W. van der Linden; HagaZiekenhuis, The Hague: P. F. Ypma; Leiden University Medical Center, Leiden: J. G. van der Bom, J. Eikenboom, F. J. W. Smiers; Maastricht University Medical Center: B. Granzen, K. Hamulyák; Radboud university medical center, Nijmegen: P. Brons, B. A. P. Laros-van Gorkom; Erasmus University Medical Center, Rotterdam: M. H. Cnossen, F. W. G. Leebeek (principal investigator), Y. V. Sanders; Van Creveld, University Medical Center, Utrecht: E. P. Mauser-Bunschoten (chairman steering committee).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal