Key Points
G-CSF activates autophagy in HSCs and neutrophils.
The response and survival of HSCs mobilized by G-CSF in vivo require autophagy.
Abstract
Granulocyte colony-stimulating factor (G-CSF) is widely used clinically to prevent neutropenia after cytotoxic chemotherapy and to mobilize hematopoietic stem cells (HSCs) for transplantation. Autophagy, a process of cytoplasmic component recycling, maintains cellular homeostasis and protects the cell during periods of metabolic stress or nutrient deprivation. We have observed that G-CSF activates autophagy in neutrophils and HSCs from both mouse and human donors. Furthermore, G-CSF–induced neutrophil and HSC mobilization is impaired in the absence of autophagy. In contrast, autophagy is dispensable for direct HSC mobilization in response to the CXCR4 antagonist AMD3100. Altogether, these data demonstrate an important role for G-CSF in invoking autophagy within hematopoietic and myeloid cells and suggest that this pathway is critical for ensuring cell survival in response to clinically relevant cytokine-induced stress. These findings have direct relevance to HSC transplantation and the increasing clinical use of agents that modulate autophagy.
Introduction
Hematopoietic stem cells (HSCs) maintain life-long blood production.1 During stress, HSCs must be able to rapidly proliferate and differentiate to regenerate mature blood cells. Granulocyte colony-stimulating factor (G-CSF) is used to mobilize HSCs before transplantation and prevent neutropenia in patients treated with chemotherapy. G-CSF treatment has both direct effects on HSCs, leading to proliferation, and indirect effects on the interactions between HSCs and the bone marrow (BM) stroma.2,3
Autophagy encompasses the self-degradation of intracytoplasmic components, including damaged proteins and organelles,4,5 and is induced by nutrition deprivation, drugs such as rapamycin, and cytokine-induced cellular stress.6-9 Recent studies have demonstrated that autophagy is required for HSC survival at homeostasis and during aging.10-12 G-CSF and other inflammatory cytokines induce HSC proliferation and the accumulation of genotoxic stress.13 We therefore examined the requirement for autophagy in G-CSF responses.
Study design
Mice
Wild-type (WT) or Atg5−/− fetal liver cells were transplanted into lethally irradiated Ptprca primary recipients. Animal studies were performed in accordance with QIMR-Berghofer Ethics Protocols A01047M (G.R.H.), A08608M (K.P.A.M.), and A11605M (S.W.L.). LysMCre:Atg7fl/fl mice have been previously described.14
Cell count, flow cytometry, and sorting
Blood counts were determined with the Hemavet 950FS (Drew Scientific). Antibodies were purchased from BD Biosciences or Biolegend. HSC flow cytometry was performed as previously described.15
Colony-forming unit (CFU) assay
A total of 100 µL of blood or 1 × 104 BM cells were plated in M3234 methylcellulose (StemCell Technologies) with stem cell factor, erythropoietin, interleukin-3, and interleukin-6 and counted after 1 week.
Imaging flow cytometry
In vivo assay, LC3-GFP mice were injected with chloroquine (CQ) (80 mg/kg per day, Sigma-Aldrich) for 3 days before analysis. Cells were flow-sorted and analyzed with ImageStreamX (Amnis). In vitro, cells were cultured in CQ (80 µM) for 6 hours. Images (×60) and statistics were processed by using IDEAS software (version 6.0, Amnis).
Western blotting
Cells were sorted, treated for 6 hours with or without CQ (80 µM) or G-CSF (100 ng/mL, Amgen), and prepared for immunoblotting as described.16 Antibodies were from Cell Signaling or Sigma-Aldrich.
G-CSF or AMD3100 mobilization
Human G-CSF mobilization
Primary clinical samples were collected from healthy stem cell donors before and after 5 days of G-CSF treatment (10 mcg/kg per day) in accordance with human ethics protocol (Protocol identifier LR 09/10) and the Declaration of Helsinki. Following red cell lysis, neutrophils were stained and underwent fluorescence-activated cell sorting (forward-scatter/side-scatter characteristics and CD14neg).
Real-time quantitative reverse transcription polymerase chain reaction
RNA extraction and reverse transcription were performed as per manufacturer’s instructions (QIAGEN and Thermo Scientific). Human ATG5 (5′-AGCAACTCTGGATGGGATTG-3′, 5′-AGGTCTTTCAGTCGTTGTCTG-3′) and ATG7 (5′-CTTGGATGTTGGGTTTTGGC-3′, 5′-GAACTCCAATGTTAAGCGAGC-3′) messenger RNA levels were determined from sorted neutrophils using SYBR Green (Invitrogen) and normalized to β2 microglobulin.
Statistical analysis
Data are shown as mean ± standard error of the mean (SEM). An unpaired 2-tailed Mann-Whitney U test or a paired Student t test was used (GraphPad Prism, version 6.01, software).
Results and discussion
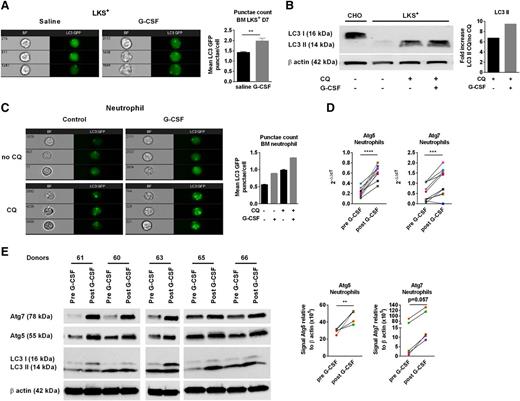
To examine the effect of G-CSF administration on autophagy, we treated LC3-GFP mice with G-CSF (10 µg/day subcutaneously, 6 days) and CQ (80 mg/kg per day intraperitoneally, 3 days) to block lysosomal degradation of LC3. LKS+ cells were sort-purified from the BM and LC3 punctae quantified by imaging flow cytometry. G-CSF treatment increased LC3-GFP punctae in LKS+ cells, demonstrating that G-CSF enhances autophagic activity (Figure 1A). This was a direct effect of G-CSF on LKS+ cells because LC3-II protein accumulation was greater after ex vivo G-CSF treatment of purified LKS+ cells (Figure 1B). G-CSF administration also increased LC3 punctae in BM neutrophils (Figure 1C). To confirm these findings, we examined paired samples from clinical stem cell donors before and after G-CSF mobilization. Sorted neutrophils from the peripheral blood (PB) of these donors were analyzed for the expression of autophagy-associated genes and autophagic flux (stabilization of LC3 II).7 G-CSF mobilization increased transcriptional expression of ATG5 and ATG7 (Figure 1D), which was confirmed at the protein level (Figure 1E). LC3 I-II was variable between samples, possibly explained by variation in the lysosomal degradation of LC3, which confounds the accurate measurement of autophagic flux. Unfortunately, CQ administration to test this was not feasible in healthy donors. Altogether, these data demonstrate increased autophagy in both mouse and human cells after G-CSF mobilization.
G-CSF induces autophagy in HSCs and neutrophils. (A) Representative images of LC3-GFP punctae formation in sorted BM LKS+ cells from LC3-GFP mice treated with saline or G-CSF. Mean LC3-GFP punctae/cell ± SEM (n = 6-7, 2 experiments). (B) Total cell lysates from LKS+ were immunoblotted with anti-LC3B or anti-β actin. CHO cell lines were used as a control expressing LC3 I. The fold increase of LC3 II band intensities, normalized to β actin, was determined by the ratio of CQ-treated and CQ-untreated cells (representative experiment). (C) LC3-GFP punctae in purified BM neutrophils (CD11b+Gr1hi) culture in duplicate for 6 hours ±G-CSF and ±CQ. LC3-GFP punctae quantification in neutrophils (representative experiment). (D) Blood from human donors were collected before and after 5 days of G-CSF treatment (10 mcg/kg per day). Sorted neutrophils were collected for quantitative real-time polymerase chain reaction analyses and western blot. Relative gene expression of ATG5 and ATG7 from sorted human blood neutrophils before and after G-CSF treatment (n = 10). (E) Total cell lysates were immunoblotted with anti-ATG5, ATG7, LC3, or anti-β actin antibodies. A paired Student t test was performed for statistical analyses (**P < .01, ***P < .001, ****P < .0001). The mean ± SEM is presented. Each dot corresponds to an individual donor. The data before and after G-CSF are color-paired.
G-CSF induces autophagy in HSCs and neutrophils. (A) Representative images of LC3-GFP punctae formation in sorted BM LKS+ cells from LC3-GFP mice treated with saline or G-CSF. Mean LC3-GFP punctae/cell ± SEM (n = 6-7, 2 experiments). (B) Total cell lysates from LKS+ were immunoblotted with anti-LC3B or anti-β actin. CHO cell lines were used as a control expressing LC3 I. The fold increase of LC3 II band intensities, normalized to β actin, was determined by the ratio of CQ-treated and CQ-untreated cells (representative experiment). (C) LC3-GFP punctae in purified BM neutrophils (CD11b+Gr1hi) culture in duplicate for 6 hours ±G-CSF and ±CQ. LC3-GFP punctae quantification in neutrophils (representative experiment). (D) Blood from human donors were collected before and after 5 days of G-CSF treatment (10 mcg/kg per day). Sorted neutrophils were collected for quantitative real-time polymerase chain reaction analyses and western blot. Relative gene expression of ATG5 and ATG7 from sorted human blood neutrophils before and after G-CSF treatment (n = 10). (E) Total cell lysates were immunoblotted with anti-ATG5, ATG7, LC3, or anti-β actin antibodies. A paired Student t test was performed for statistical analyses (**P < .01, ***P < .001, ****P < .0001). The mean ± SEM is presented. Each dot corresponds to an individual donor. The data before and after G-CSF are color-paired.
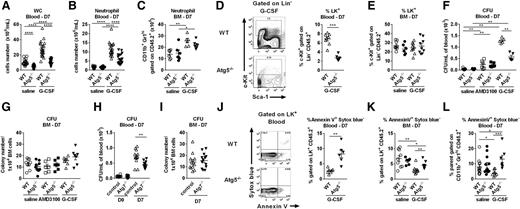
We next examined HSC mobilization in mice deficient for autophagy in hematopoietic cells. In Atg5−/− chimeric mice, G-CSF had a reduced capacity to increase blood white cell and neutrophil counts compared with WT mice (Figure 2A-B); however, there was no difference in BM neutrophils (Figure 2C). Similarly, following G-CSF administration, only the circulating progenitor cells were reduced in Atg5−/− chimeras compared with WT (Figure 2D-E). Confirming these results, mobilized PB CFUs were reduced in Atg5−/− chimeras compared with controls; however, no differences were seen in nonmobilized CFUs or BM CFUs between Atg5−/− and WT (Figure 2F-G). Therefore, although G-CSF induces autophagy in vivo, autophagy is only functionally required upon cellular activation and mobilization to the peripheral circulation.
Autophagy is required for G-CSF responsiveness in vivo. (A) Total white cell (WC) and (B) neutrophil counts in the PB and (C) BM neutrophil counts after 6 days’ G-CSF or saline. (D) Representative flow cytometry plots from the PB of WT or Atg5−/− FLC after G-CSF. Progenitors are enriched in linnegc-Kit+ (LK+) populations. Quantification of mobilized LK+ after G-CSF in the PB and (E) BM. (F) CFU in the PB (CFU/mL of blood) and (G) BM (CFU/1 × 104 BM cells) of Atg5−/− or WT chimeras after 6 days of G-CSF or 1 dose of AMD3100 analyzed 1 hour after injection (n = 5-11, 2-3 experiments). (H) CFU in the PB (CFU/mL of blood) and (I) BM (CFU/1 × 104 BM cells) of LysMCre:Atg7fl/fl or controls after 6 days of G-CSF (n = 11-12, 3 experiments). (J) Representative plots of Annexin V and Sytox blue gated on LK+ cells from blood of WT and Atg5−/− chimeras at D7 after G-CSF treatment. Percentage of Annexin V+ Sytox blue− of blood LK+ cells (n = 6-8, 2 experiments). (K) Percentage of Annexin V+ Sytox blue− cells within BM LK+ population after G-CSF treatment (n = 6-9, 3 experiments). (L) Percentage of Annexin V+ Sytox blue− of blood neutrophil (CD11b+Gr1hiCD45.2+) at D7 after G-CSF treatment (n = 7-12, 3 experiments). A Mann-Whitney U test was performed for statistical analyses (*P < .05, **P < .01, ***P < .001, ****P < .0001). For all experiments, data are expressed as mean ± SEM. (Each dot corresponds to an individual mouse.) D7, day 7.
Autophagy is required for G-CSF responsiveness in vivo. (A) Total white cell (WC) and (B) neutrophil counts in the PB and (C) BM neutrophil counts after 6 days’ G-CSF or saline. (D) Representative flow cytometry plots from the PB of WT or Atg5−/− FLC after G-CSF. Progenitors are enriched in linnegc-Kit+ (LK+) populations. Quantification of mobilized LK+ after G-CSF in the PB and (E) BM. (F) CFU in the PB (CFU/mL of blood) and (G) BM (CFU/1 × 104 BM cells) of Atg5−/− or WT chimeras after 6 days of G-CSF or 1 dose of AMD3100 analyzed 1 hour after injection (n = 5-11, 2-3 experiments). (H) CFU in the PB (CFU/mL of blood) and (I) BM (CFU/1 × 104 BM cells) of LysMCre:Atg7fl/fl or controls after 6 days of G-CSF (n = 11-12, 3 experiments). (J) Representative plots of Annexin V and Sytox blue gated on LK+ cells from blood of WT and Atg5−/− chimeras at D7 after G-CSF treatment. Percentage of Annexin V+ Sytox blue− of blood LK+ cells (n = 6-8, 2 experiments). (K) Percentage of Annexin V+ Sytox blue− cells within BM LK+ population after G-CSF treatment (n = 6-9, 3 experiments). (L) Percentage of Annexin V+ Sytox blue− of blood neutrophil (CD11b+Gr1hiCD45.2+) at D7 after G-CSF treatment (n = 7-12, 3 experiments). A Mann-Whitney U test was performed for statistical analyses (*P < .05, **P < .01, ***P < .001, ****P < .0001). For all experiments, data are expressed as mean ± SEM. (Each dot corresponds to an individual mouse.) D7, day 7.
We therefore examined the effects of AMD3100, a direct antagonist of the CXCR4–CXCL12 interaction that, in contrast to G-CSF, does not directly signal HSCs or induce cell proliferation. Accordingly, there were no differences in mobilized CFUs between WT and Atg5−/− chimeras, either 1 or 3 hours after AMD3100 (Figure 2F-G; supplemental Figure 1A). Although combined G-CSF and AMD3100 resulted in greater mobilized CFUs in both groups than either agent alone, Atg5−/− chimeras had fewer mobilized CFUs after combined G-CSF/AMD3100 treatment (supplemental Figure 1A). These findings were confirmed using LysMCre:Atg7fl/fl mice, which lack autophagy in myelomonocytic cells and committed myeloid progenitors.19 G-CSF–mobilized CFUs were reduced in LysMCre:Atg7fl/fl mice compared with Cre negative controls; however, there was no difference in BM cells or PB from nonmobilized mice (Figure 2H-I). We observed fewer monocytes and a trend to decreased white cells and neutrophils after G-CSF treatment (supplemental Figure 1B) and significantly fewer mobilized progenitors in LysMCre:Atg7fl/fl (supplemental Figure 1C). These data confirm that autophagy is required for efficient G-CSF–induced mobilization.
Finally, to investigate how autophagy regulates G-CSF–induced HSC/progenitor cell mobilization, we examined the cellular responses induced by G-CSF in Atg5−/− and WT cells. There were no differences observed between WT and Atg5−/− chimeras in regard to protease expression, degradation of CXCL12, depletion of phagocytic macrophages, or in the expression of cell adhesion molecules (supplemental Figure 1D-G). We therefore tested whether autophagy activation was essential for the survival of mobilized HSC/progenitor cells and neutrophils rather than BM egress per se. We observed increased apoptotic progenitors from Atg5−/− compared with WT chimeras in the blood after G-CSF treatment; however, only minor differences were seen in BM (Figure 2J-K). Similarly, PB Atg5−/− neutrophils exhibited a striking increase in apoptosis after G-CSF, but not in WT controls (Figure 2L). These findings demonstrate that autophagy is required for optimal G-CSF–induced mobilization and cell-intrinsic survival after G-CSF therapy.
We have found that the clinically relevant stress mediated by exogenous cytokines such as G-CSF acts directly to increase autophagy in HSCs and neutrophils and that this induction of autophagy is essential for their survival in the periphery after G-CSF mobilization. This role of autophagy in G-CSF responses has clinical relevance in transplantation and in the use of immunomodulatory drugs that modulate autophagy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark Smyth, Christian Engwerda, Stephen Sykes, and the members of the MacDonald, Lane, and Hill laboratory for helpful discussion and suggestions; the staff at the QIMR-Berghofer including the animal facility, Grace Chojnowski, Paula Hall, and Melinda Christensen (flow cytometry), Siok Tey for consenting donors, and Antiopi Varelias and Stuart Olver for assisting with blood processing; Noboru Mizushima for providing Atg5+/− mice; James Harris for providing LysMCre:Atg7fl/fl mice and for helpful comments; and nurses Elise Sturgeon, Judy Avery, and Justine Leach from the Royal Brisbane and Women’s Hospital for the collection of human samples.
These studies were supported by the University of Queensland International Research Tuition Award and University of Queensland Research Scholarship (L.L.-E.M.), the Novo Nordisk Foundation (F.O.B.), and the National Health and Medical Research Council (NHMRC) (grants 1011787, 1059286, and 1089138). J.D.M. and S.W.L. are Australia Fellows and Queensland Career Development Fellows, G.R.H. is an NHMRC and Queensland Health Senior Clinical Research Fellow, K.P.A.M. is a Cancer Council Queensland Senior Research Fellow, and S.W.L. has received fellowship funding from the Leukaemia Foundation.
Authorship
Contribution: L.L.-E.M. designed and performed experiments and wrote the manuscript; T.V., K.E.L., R.D.K., B.E.T., and M.L. performed experiments; F.O.B., G.M.B., C.B., and J.D.M. contributed to experimental design and interpretation of data; G.R.H., K.P.A.M., and S.W.L. contributed to experimental design and interpretation of data and cowrote the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: G.R.H. has received a consultancy payment from Amgen within the past 2 years. The remaining authors declare no competing financial interests.
Correspondence: Kelli P. A. MacDonald, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, Brisbane 4006, Australia; e-mail: kelli.macdonald@qimrberghofer.edu.au; and Steven W. Lane, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Herston, Brisbane 4006, Australia; e-mail: steven.lane@qimrberghofer.edu.au.
References
Author notes
K.P.A.M. and S.W.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal