Key Points
FDG-PET–assessed response to ST according to Deauville criteria predicts outcome post-ASCT for rel/ref DLBCL.
Abstract
High-dose chemotherapy (HDT) plus autologous stem cell transplantation (ASCT) is the standard of care for chemosensitive relapsed and refractory diffuse large B-cell lymphoma (rel/ref DLBCL). Interim restaging with functional imaging by positron emission tomography using 18F-deoxyglucose (FDG-PET) has not been established after salvage chemotherapy (ST) and before HDT-ASCT by modern criteria. Herein, we evaluated 129 patients with rel/ref DLBCL proceeding to HDT-ASCT, with ST response assessment by FDG-PET according to the contemporary Deauville 5-point scale. At 3 years, patients achieving a Deauville response of 1 to 3 to ST experienced superior progression-free survival (PFS) and overall survival (OS) rates of 77% and 86%, respectively, compared with patients achieving Deauville 4 (49% and 54%, respectively) (P < .001). No other pre-HDT-ASCT risk factors significantly impacted PFS or OS. Despite achieving remission to ST, patients with Deauville 4 should be the focus of risk-adapted investigational therapies.
Introduction
High-dose therapy (HDT) plus autologous stem cell transplantation (ASCT) is standard-of-care as consolidation for patients with relapsed and refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL).1 HDT-ASCT is indicated in other rel/ref aggressive B-cell non-Hodgkin lymphomas (B-NHL), including transformed histology.2 Two recent large prospective randomized phase III studies with interventions of conditioning modification3 and maintenance therapy4 failed to improve long-term disease-free survival rates above 50% to 60%.
Eligibility for HDT-ASCT is based on the presence of chemosensitive disease to salvage therapy (ST) as defined by complete or partial remission assessed by computed tomography (CT) imaging.5 The controversy surrounding the prognostic significance of interim positron emission tomography (PET) using 18F-deoxyglucose (FDG) restaging during induction therapy for DLBCL has prompted the generation of revised criteria for more accurate determination of chemosensitive response.6 The prognostic significance of FDG-PET pre-HDT-ASCT for DLBCL has been demonstrated previously7-10 ; however, not with modern criteria. We aimed to determine the prognostic impact of FDG-PET-assessed response to ST before HDT-ASCT for rel/ref DLBCL/transformed B-NHL by modern criteria established at the first international workshop on FDG-PET reporting in lymphoma held in Deauville, France 11 and recently recommended at the 12th International Conference of Malignant Lymphoma.12
Study design
Patients
We retrospectively reviewed a database of 129 adult patients with rel/ref DLBCL and transformed B-NHL previously exposed to at least1 prior line of induction chemotherapy and who were chemosensitive to ST and proceeding to HDT-ASCT after a restaging FDG-PET scan at Memorial Sloan-Kettering Cancer Center (MSKCC) from 2002 to December 2012. Rel/ref disease was confirmed by biopsy in 123 of 129 (95%) of patients. Eligibility for HDT-ASCT was based on ST response per above, as well as adequate organ function, performance status, and CD34+ stem cells for re-infusion per institutional standard. A waiver of authorization to carry out this analysis was approved by the MSKCC Institutional Review Board.
Pre-HDT-ASCT FDG-PET scans and risk factors
Chemosensitivity was assessed per standard CT criteria for B-NHL.5 All PET/CT scans were obtained 60 minutes after radiotracer injection, extending from skull base to upper thigh, on Discovery STE or LS cameras (GE Medical Systems, Milwaukee, WI). A low-dose CT scan (120-140 kV, ∼80 mA) was followed by emission images (3 minutes per bed position). Deauville criteria11 for FDG-PET response was applied for purposes of analysis on the 5-point scale: 1, no uptake; 2, uptake ≤mediastinal blood pool; 3, uptake > mediastinal blood pool but ≤ liver; 4, uptake > liver at any site; and 5, uptake > liver and/or new sites of disease. No patients had Deauville 5 response, because this represented progression of disease to ST, thus determining ineligibility to proceeding to HDT-ASCT consolidation. Two staff nuclear medicine radiologists (H.S. and G.A.U.) reviewed and scored all images. Traditional pre-ST risk factors4 were applied for purposes of analysis, including histologic phenotype at relapse (including cell of origin in DLBCL as determined by Hans criteria13 ), secondary age-adjusted International Prognostic Index (sAA-IPI), and previous remission duration to initial induction chemotherapy.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were defined as the time from HDT-ASCT until death from any cause and time from HDT-ASCT to disease progression or death, respectively. Univariate probabilities and 95% confidence intervals (CIs) of OS and PFS were estimated using Kaplan-Meier methodology, and survival distributions were compared across patient and treatment characteristics using a log-rank test, with P values <.05 considered significant.
Results and discussion
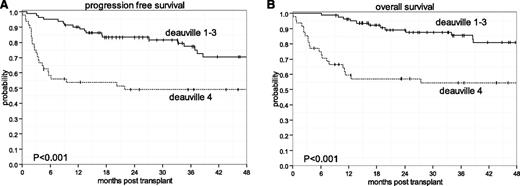
The characteristics of the 129 patients appear in Table 1. The majority of patients (66%) were de novo rel/ref DLBCL. All patients were previously exposed to rituximab and were chemosensitive by CT criteria.5 FDG-PET scans after ST occurred at a median of 34 days (range: 5-173 days) before HDT-ASCT and at a median of 14 days after ST. Of the 6 patients with pre-HDT-ASCT FDG-PET occurring >3 months from HDT-ASCT, all received pre-HDT-ASCT IFRT. The median follow-up among survivors was 43.6 months (range: 4.6-135.6 months). PFS and OS rates for the entire cohort at 3 years were 67% (95% CI: 58-75) and 74% (95% CI: 65-81), respectively (Figure 1A). No significant differences in PFS and OS were observed according to standard pre-ST risk factors including sAA-IPI, relapse <12 months or primary refractory disease vs relapse ≥12 months, and type of ST. In addition, transformed or de novo DLBCL and cell-of-origin factors lacked prognostic significance. The only pre-HDT-ASCT factor associated with PFS and OS was response to ST by FDG-PET Deauville criteria. At 3 years, patients with Deauville 1 to 3 experienced superior PFS (Figure 1A) and OS rates (Figure 1B) of 77% (95% CI: 65- 86) and 86% (95% CI: 75-92), respectively, compared with patients with Deauville 4 who had respective PFS and OS rates of 49% (95% CI: 34-62) and 54% (95% CI: 39-68) (P < .001).
Patient characteristics (N = 129)
| Median age, y (range) | 56.5 (21-73) |
| Gender | |
| Male | 77 (59.7) |
| Female | 52 (40.3) |
| Deauville response to ST | |
| 1 | 64 (49.6) |
| 2 | 10 (7.8) |
| 3 | 7 (5.4) |
| 4 | 48 (37.2) |
| IFRT pre-HDT-ASCT | |
| No | 75 (58.1) |
| Yes | 54 (41.9) |
| Remission duration | |
| <12 mo* | 81 (62.8) |
| ≥12 mo | 48 (37.2) |
| Histology | |
| Activated B-cell phenotype | 47 (41.2) |
| Germinal center phenotype | 39 (34.2) |
| Primary mediastinal large B-cell lymphoma | 9 (7.9) |
| T-cell histiocyte-rich large B-cell lymphoma | 4 (3.5) |
| Transformed | 15 (13.2) |
| Missing | 15 |
| ST | |
| DHAP | 17 (13.2) |
| EPOCH | 11 (8.5) |
| ICE | 87 (67.4) |
| Other | 6 (4.7) |
| ST × 2 | 8 (6.2) |
| sAA-IPI | |
| High | 43 (35.0) |
| Low | 80 (65.0) |
| Missing | 6 |
| Median age, y (range) | 56.5 (21-73) |
| Gender | |
| Male | 77 (59.7) |
| Female | 52 (40.3) |
| Deauville response to ST | |
| 1 | 64 (49.6) |
| 2 | 10 (7.8) |
| 3 | 7 (5.4) |
| 4 | 48 (37.2) |
| IFRT pre-HDT-ASCT | |
| No | 75 (58.1) |
| Yes | 54 (41.9) |
| Remission duration | |
| <12 mo* | 81 (62.8) |
| ≥12 mo | 48 (37.2) |
| Histology | |
| Activated B-cell phenotype | 47 (41.2) |
| Germinal center phenotype | 39 (34.2) |
| Primary mediastinal large B-cell lymphoma | 9 (7.9) |
| T-cell histiocyte-rich large B-cell lymphoma | 4 (3.5) |
| Transformed | 15 (13.2) |
| Missing | 15 |
| ST | |
| DHAP | 17 (13.2) |
| EPOCH | 11 (8.5) |
| ICE | 87 (67.4) |
| Other | 6 (4.7) |
| ST × 2 | 8 (6.2) |
| sAA-IPI | |
| High | 43 (35.0) |
| Low | 80 (65.0) |
| Missing | 6 |
Values are n (%) unless otherwise indicated.
DHAP, dexamethasone/cytarabine/cisplatin; EPOCH, etoposide, doxorubicin, vincristine, prednisone, cyclophosphamide; ICE: ifosfamide/etoposide/carboplatin; IFRT, involved-field radiotherapy.
Includes primary refractory disease.
Kaplan-Meier survival estimates based on Deauville responses to ST. (A) PFS. (B) OS.
Kaplan-Meier survival estimates based on Deauville responses to ST. (A) PFS. (B) OS.
This analysis represents the only series of chemosensitive rel/ref DLBCL and transformed B-NHL patients, predominately of de novo DLBCL histologic phenotype, proceeding to HDT-ASCT with responses to ST assessed by FDG-PET according to modern Deauville criteria on the 5-point scale. Our retrospective data demonstrate significant prognostic impact of FDG-PET response to ST (Deauville 1-3) with significant PFS and OS benefit (P < .001). Whereas other series’ have demonstrated prognostic significance with FDG-PET response before HDT-ASCT,7-9,14 none has used the contemporary Deauville 5-point scale.11 This 5-point scale of response criteria was recommended by consensus of the Imaging Working Group at the 12th International Conference of Malignant Lymphoma in Lugano, Switzerland.12 Additional key differences of previous publications assessing risk by FDG-PET pre-HDT-ASCT, as compared to our study, include patients undergoing HDT-ASCT in first remission,9 varying lymphoma histologies,7,14 and smaller numbers of patients.7-9 In the other larger, and most contemporary, study of 143 patients with rel/ref DLBCL or transformed lymphoma from the Dana-Farber Cancer Institute/Massachusetts General Hospital, a negative FDG-PET scan conferred a rate of OS benefit at 4 years of 75% vs 56% in FDG-PET-positive patients (P = .025), results notably similar to those obtained in our study.10 Response criteria by FDG-PET were not specified in the Dana-Farber Cancer Institute/Massachusetts General Hospital study.
Limitations of our study should be noted. First, this is not a prospective study and, thus, it is subject to the inherent limitations of retrospective data. Patient selection within this single-center study may account for the improved PFS and OS over historic controls of 2 recent prospective multicenter studies of HDT-ASCT for de novo rel/ref DLBCL.3,4 Also, our study contained 15 patients (12% of the cohort) with transformed histology DLBCL and 8 patients (6%) who received 2 lines of ST, which was different from the recent prospective HDT-ASCT studies for de novo DLBCL. Lastly, 54 of the 129 patients with limited-stage disease received pre-HDT-ASCT IFRT after post-ST restaging as part of the HDT-ASCT15,16 at the discretion of the treating physician. It is important to note that the PFS and OS rates between pre-HDT-ASCT IFRT and no pre-HDT-ASCT IFRT were similar (P = .20 and P = .73, respectively), even when restricted to the 48 patients who achieved Deauville 4 response to ST (PFS: P = .32; OS: P = .40).
In conclusion, our study represents the first report of the prognostic impact of pre-HDT-ASCT FDG-PET response to ST according to Deauville criteria in chemosensitive rel/ref DLBCL. Clearly, given the inferiority of patients achieving a Deauville 4 response to ST, this group of patients should be the subject of risk-adapted investigation. Such investigational interventions could include, but are not limited to, immunotherapeutic approaches to overcome relative chemotherapy insensitivity such as consolidation with chimeric antigen receptor–modified autologous T-cell therapy directed against CD19 post-HDT-ASCT (NCT01840566), immune-checkpoint blockade post-HDT-ASCT,17 or allogeneic hematopoietic cell transplantation. With regard to the latter treatment modality, we have recently demonstrated that the allogeneic graft-versus-lymphoma effect may overcome relative chemotherapy insensitivity, achieving similar long-term outcomes with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning in chemosensitive patients to ST according to CT criteria across all Deauville responses by FDG-PET.18
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Lymphoma and Adult Bone Marrow Transplant Service at MSKCC for their enduring dedication to patient care.
Authorship
Contribution: C.S.S. and C.H.M. designed the study; C.S.S., M.J.M., A.D.Z., and C.H.M. interpreted the data; C.S.S., J.M, J.C.M, S.M.D, P.H, G.A.U., and H.S. analyzed the data; all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig S. Sauter, Memorial Sloan Kettering Cancer Center, Box 276, 1275 York Ave, New York, NY 10065; e-mail: sauterc@mskcc.org.