Key Points
Patients with Hurler syndrome show significant residual disease burden despite HCT.
Early referral for HCT, using noncarrier donors and regimens designed to achieve full-donor chimerism, offers the best long-term prognosis.
Abstract
Mucopolysaccharidosis type I–Hurler syndrome (MPS-IH) is a lysosomal storage disease characterized by multisystem morbidity and death in early childhood. Although hematopoietic cell transplantation (HCT) has been performed in these patients for more than 30 years, large studies on the long-term outcome of patients with MPS-IH after HCT are lacking. The goal of this international study was to identify predictors of the long-term outcome of patients with MPS-IH after successful HCT. Two hundred seventeen patients with MPS-IH successfully engrafted with a median follow-up age of 9.2 years were included in this retrospective analysis. Primary endpoints were neurodevelopmental outcomes and growth. Secondary endpoints included neurologic, orthopedic, cardiac, respiratory, ophthalmologic, audiologic, and endocrinologic outcomes. Considerable residual disease burden was observed in the majority of the transplanted patients with MPS-IH, with high variability between patients. Preservation of cognitive function at HCT and a younger age at transplantation were major predictors for superior cognitive development posttransplant. A normal α-l-iduronidase enzyme level obtained post-HCT was another highly significant predictor for superior long-term outcome in most organ systems. The long-term prognosis of patients with MPS-IH receiving HCT can be improved by reducing the age at HCT through earlier diagnosis, as well as using exclusively noncarrier donors and achieving complete donor chimerism.
Introduction
Mucopolysaccharidosis type I–Hurler syndrome (MPS-IH) is a lysosomal storage disease caused by a deficiency of the lysosomal enzyme α-l-iduronidase (IDUA). Without treatment, this devastating disease is characterized by progressive multisystem morbidity including neurodevelopmental deterioration, severe orthopedic manifestations, and cardiopulmonary complications leading to death in early childhood.1 With more than 500 hematopoietic cell transplantation (HCT) procedures performed so far, MPS-IH is the most extensively transplanted inherited metabolic disorder, and therefore often serves as a paradigm disorder for HCT.2 Although enzyme replacement therapy (ERT) has become available for MPS-IH, HCT remains the standard of care because it is the only treatment that delivers the deficient enzyme to the central nervous system, HCT is associated with superior metabolic correction, and attenuating antibody formation accompanies ERT in MPS-IH.3,4
It has been known for many years that HCT dramatically alters the natural history of MPS-IH and allows affected individuals to achieve long-term survival.5 Historically, the success of HCT has been limited by low overall survival rates.6 Collaborative studies in the last decade have identified predictors for these poor graft outcomes. Because of adjusted international protocols and increased availability of well-matched donors, transplantation-related morbidity and mortality rates have been reduced, making HCT for MPS-IH a much safer procedure.6-10
There are, however, few series investigating the long-term clinical outcome of transplanted patients with MPS-IH.11-18 Moreover, limited data are available on clinically important long-term outcomes including neurodevelopmental and orthopedic parameters.19 We know residual disease burden is present in almost all patients with MPS-IH, although with a striking variability between patients. Various factors have been suggested to influence the prognosis, but the nature of these studies hampered the ability to draw firm conclusions.19
As MPS-IH is a relatively rare condition, only an international multicenter collaboration of experienced transplant centers committed to the care of these patients and extensive monitoring enables a meaningful analysis of patient, donor, and transplantation-related predictors of the long-term outcomes of patients with MPS-IH after HCT. This study is the largest study addressing MPS-IH outcomes post-HCT, including more than 70% of the patients with MPS-IH successfully transplanted worldwide.
Patients and methods
Data collection
One member of the study team (M.A.) visited all participating study centers. The medical records of all included patients were retrospectively evaluated according to a standardized set of potential patient, donor, and transplantation-related predictors (Table 1). On the basis of the medical records as well as the various involved specialists, endpoints were scored according to their presence and progression was compared with the pre-HCT status, as well as timing of interventions. The institutional review boards of all participating centers approved this study. Written informed consent was obtained from the parents or legal guardians of the patients.
Baseline patient, donor, and transplantation characteristics
| Characteristics . | N (%) . | Median (range) . |
|---|---|---|
| Patient characteristics | ||
| Overall | 217* | |
| Sex (male) | 122 (56) | |
| Ethnicity (Caucasian) | 198 (91) | |
| Genotype (nonsense-nonsense) | 76 (56)† | |
| Age at HCT, months | 16 (2-47) | |
| Age at diagnosis, months | 9 (0-42) | |
| Follow-up age, years | 9 (3-23) | |
| Donor characteristics | ||
| Source (CB/BM/PBSC) | 85/118/14 (39/54/7) | |
| Relation (related) | 73 (34) | |
| Carrier status (carrier) | 39 (19) | |
| Transplantation characteristics | ||
| Number of HCT (1/2/3) | 179/36/2 (83/16/1) | |
| Year of HCT | 2002 (1985-2011) | |
| ERT (yes) | 45 (21) | |
| TBI (yes) | 25 (12) | |
| Donor chimerism (<95%‡) | 49 (23) | |
| IDUA level¶ (<reference§) | 55 (26) | |
| IDUA level¶ (% of mean) | 82 (13-302) |
| Characteristics . | N (%) . | Median (range) . |
|---|---|---|
| Patient characteristics | ||
| Overall | 217* | |
| Sex (male) | 122 (56) | |
| Ethnicity (Caucasian) | 198 (91) | |
| Genotype (nonsense-nonsense) | 76 (56)† | |
| Age at HCT, months | 16 (2-47) | |
| Age at diagnosis, months | 9 (0-42) | |
| Follow-up age, years | 9 (3-23) | |
| Donor characteristics | ||
| Source (CB/BM/PBSC) | 85/118/14 (39/54/7) | |
| Relation (related) | 73 (34) | |
| Carrier status (carrier) | 39 (19) | |
| Transplantation characteristics | ||
| Number of HCT (1/2/3) | 179/36/2 (83/16/1) | |
| Year of HCT | 2002 (1985-2011) | |
| ERT (yes) | 45 (21) | |
| TBI (yes) | 25 (12) | |
| Donor chimerism (<95%‡) | 49 (23) | |
| IDUA level¶ (<reference§) | 55 (26) | |
| IDUA level¶ (% of mean) | 82 (13-302) |
All characteristics concern the last HCT. BM, bone marrow; CB, cord blood; PBSC, peripheral blood stem cells; TBI, total body irradiation.
Centers: University of Minnesota (n = 45), Duke University (n = 45), Royal Manchester Children’s Hospital (n = 30), Our Lady’s Children’s Hospital (n = 27), Hopital Universitaire Necker-Enfants Malades (n = 19), University Medical Center Utrecht (n = 14), Great Ormond Street Hospital (n = 13), San Gerardo University Hospital (n = 6), University Hospitals Leuven (n = 3), Ghent University Hospital (n = 3).
Of known mutations.
Median, 75% (range, 16%-94%).
Measured in leukocytes.
Lower limit of normal, as defined by the local reference laboratory testing IDUA activity.
Inclusion criteria
Patients with MPS-IH who received an allogeneic-HCT in 1 of the 10 participating centers within Europe and the United States between January 1985 and February 2011 were included in the study. Graft outcome data from some of the included patients have been reported previously.8,20 Assays of leukocyte IDUA activity at presentation in combination with the clinical phenotype confirmed the diagnosis in all patients. Patients with an attenuated phenotype (Hurler-Scheie) were excluded on the basis of the age of diagnosis, genotype, and neurodevelopmental presentation. All studied patients included in the study had at least 10% donor chimerism and a minimum follow-up of 3 years post-HCT.
Primary endpoints
Neurodevelopmental outcome.
The neurodevelopmental outcome was based on standardized and validated tests (supplemental Table 1, available on the Blood Web site). Age equivalents were used to permit comparisons across tests and to identify newly acquired skills. The results were compared with norms for typically developing children. Normal cognitive development was defined as a developmental quotient/intelligence quotient (DQ/IQ) of 85 or more, mild cognitive impairment as a DQ/IQ of 70 to 85, moderate cognitive impairment as a DQ/IQ of 55 to 70, and severe cognitive impairment as a DQ/IQ lower than 55.
Growth.
Growth data included weight, height, head circumference, and body mass index. Data obtained from patients who received growth hormone (GH) treatment were excluded from the start of treatment. Data distribution was depicted along with World Health Organization (WHO) reference curves.21 For analysis, height was expressed as a standard deviation (SD) score related to the WHO reference data. Where available, midparental target height was calculated according to the Tanner method,22 and sitting height, leg length, and arm span were compared with the reference curves of Fredriks et al.23
Secondary endpoints
Neurological endpoints included hydrocephalus and cerebral atrophy, according to radiologic imaging. Orthopedic endpoints included evidence of thoracolumbar kyphosis, cord compression, cervical instability, hip dysplasia with (sub)luxation, genu valgum, carpal tunnel syndrome, and trigger fingers and their surgical intervention, according to radiologic imaging, electrophysiological tests, and the involved orthopedic specialists. Cardiac endpoints included mitral and aortic valve insufficiency as well as cardiomyopathy (ejection fraction <55%) and the prescription of an angiotensin converting enzyme inhibitor, all based on cardiac ultrasounds and the involved cardiologists. Respiratory endpoints included overnight hypoxia and the need for respiratory support based on polysomnography and the involved pediatricians and ear, nose, and throat specialists. Ophthalmologic endpoints included corneal clouding, glaucoma, cataracts, and their intervention according to the involved eye specialists. Audiologic endpoints consisted of the presence of a defined hearing loss and the need for hearing aids based on audiologic tests. Endocrinologic endpoints included GH treatment as well as hypothyroidism requiring treatment.
Statistical analysis
The association between the various patient, donor, and transplantation-related predictors and the primary endpoints were analyzed using linear mixed models. For secondary endpoints, univariate and multivariate regression analysis were used: Cox proportional hazards regression analysis in case of clear event-time endpoints and logistic regression analysis in case of binary endpoints. Univariate predictors of outcome parameters that were statistically significant (P < .10) were selected for multivariate analysis. Results were expressed as estimate (β), hazard ratios (HRs), or odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs). P values <.05 were considered statistically significant. Linear and nonlinear regression models were used to depict the best-fit line through the longitudinal data. Cumulative incidence curves were used to depict event-time endpoints. The cutoff date for data analysis was April 2014. Statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL).
Results
Study population
Of the 222 patients included in the study, five were excluded based on an attenuated phenotype. The final 217 patients were transplanted at a median age of 16 months (range, 2-47 months) with a median age at last follow-up of 9.2 years (range, 3-23 years). Twenty-six percent of the patients obtained enzyme levels after transplant below the local lower reference limit. The baseline characteristics are shown in Table 1.
Primary endpoints
Neurodevelopmental outcome.
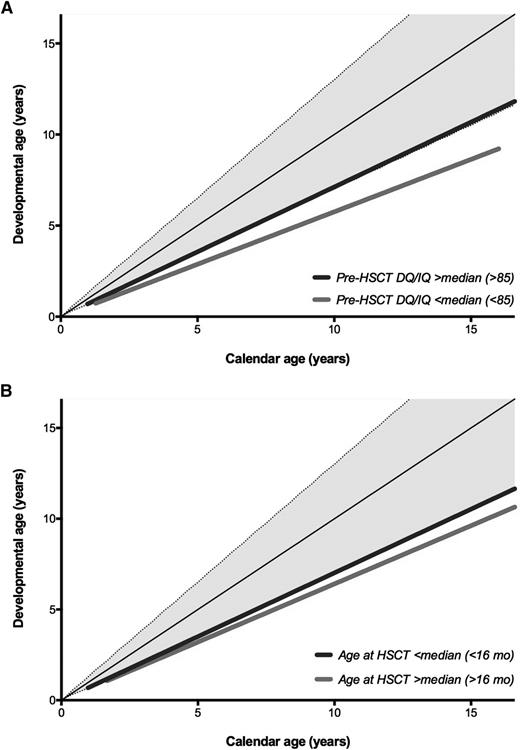
Pre-HCT, 56.9% and 26.6% of the patients showed a normal or only mildly impaired neurodevelopment, respectively. At last follow-up post-HCT, normal or only mildly impaired neurodevelopment was observed in 26.9% and 28.3% of the patients, respectively, and 44.9% suffered from moderate to severely impaired neurodevelopment. Male sex (β, −6.55; 95% CI, −12.86 to −0.24; P = .04), lower baseline DQ/IQ (β, −8.58; 95% CI, −14.95 to −2.21; P = .009), higher age at HCT (β, −8.40; 95% CI, −14.62 to −2.19; P = .009), the use of total body irradiation (TBI; β, −9.90; 95% CI, −18.82 to −0.98; P = .03), and higher age at evaluation (β, −0.09; 95% CI, −0.13 to −0.05; P < .001) were all statistically significant predictors of inferior neurodevelopmental outcome post-HCT (Table 2; Figure 1A-B). For example, at the age of 10 years, the average DQ/IQ was 81 if transplanted with a baseline DQ/IQ of 85 or higher, and 64 if the baseline DQ/IQ was lower than 85. Combining the predictors age at HCT and baseline DQ/IQ shows that 71.1% of the patients with an age at HCT younger than 12 months in combination with a baseline DQ/IQ lower than 70 develop severe cognitive impairment (DQ/IQ < 70) compared with 14.7% if the age at HCT is younger than 12 months combined with a baseline DQ/IQ higher than 70 (P < .001).
Linear mixed model analysis for longitudinal endpoints
| Endpoint, predictor, and cutoff . | β . | 95% CI . | P . |
|---|---|---|---|
| Neurodevelopment (DQ/IQ) | |||
| Sex | |||
| Male | 1 | ||
| Female | 6.55 | 0.24-12.86 | .04 |
| Baseline DQ/IQ | |||
| <85* | 1 | ||
| ≥85* | 8.58 | 2.21-14.95 | .009 |
| Age at HCT | |||
| <16 months† | 1 | ||
| ≥16 months† | −8.40 | −14.62 to −2.19 | .009 |
| TBI | |||
| No | 1 | ||
| Yes | −9.90 | −18.82 to −0.98 | .03 |
| Follow-up age, months | −0.09 | −0.13 to −0.05 | <.001 |
| Height (SD) | |||
| Baseline height (SD) | 0.49 | 0.39-0.60 | <.001 |
| IDUA level | |||
| <Reference‡ | 1 | ||
| ≥Reference‡ | 0.43 | 0.08-0.77 | .02 |
| TBI | |||
| No | 1 | ||
| Yes | −1.01 | −1.53 to −0.48 | <.001 |
| Follow-up age, months | −0.02 | −0.02 to −0.02 | <.001 |
| Endpoint, predictor, and cutoff . | β . | 95% CI . | P . |
|---|---|---|---|
| Neurodevelopment (DQ/IQ) | |||
| Sex | |||
| Male | 1 | ||
| Female | 6.55 | 0.24-12.86 | .04 |
| Baseline DQ/IQ | |||
| <85* | 1 | ||
| ≥85* | 8.58 | 2.21-14.95 | .009 |
| Age at HCT | |||
| <16 months† | 1 | ||
| ≥16 months† | −8.40 | −14.62 to −2.19 | .009 |
| TBI | |||
| No | 1 | ||
| Yes | −9.90 | −18.82 to −0.98 | .03 |
| Follow-up age, months | −0.09 | −0.13 to −0.05 | <.001 |
| Height (SD) | |||
| Baseline height (SD) | 0.49 | 0.39-0.60 | <.001 |
| IDUA level | |||
| <Reference‡ | 1 | ||
| ≥Reference‡ | 0.43 | 0.08-0.77 | .02 |
| TBI | |||
| No | 1 | ||
| Yes | −1.01 | −1.53 to −0.48 | <.001 |
| Follow-up age, months | −0.02 | −0.02 to −0.02 | <.001 |
Only statistically significant results are shown. β indicates estimate.
Median baseline DQ/IQ.
Median age at HCT.
Lower limit of local reference.
Cognitive development. Calendar age is depicted on the horizontal axis, with developmental age on the vertical axis. The continuous and dashed black lines represent the reference curves (+2 SD, 0 SD, and −2 SD). (A) Subdivided by the cognitive status (DQ/IQ) pre-HCT; median or higher (≥85) vs lower than median (<85). (B) Subdivided by the age at HCT; lower than median (<16 months) and median or higher (≥16 months).
Cognitive development. Calendar age is depicted on the horizontal axis, with developmental age on the vertical axis. The continuous and dashed black lines represent the reference curves (+2 SD, 0 SD, and −2 SD). (A) Subdivided by the cognitive status (DQ/IQ) pre-HCT; median or higher (≥85) vs lower than median (<85). (B) Subdivided by the age at HCT; lower than median (<16 months) and median or higher (≥16 months).
Growth.
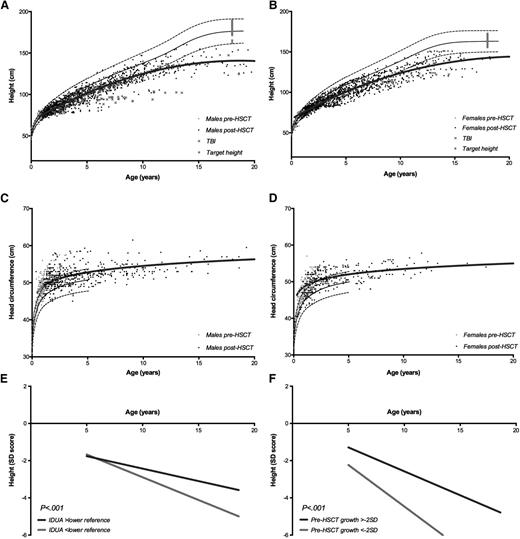
Longitudinal data on height and head circumference are shown in Figure 2A-D. Head circumference appears to normalize over time in the majority of the transplanted patients with MPS-IH. Longitudinal height is still significantly affected post-HCT, deviating from the reference curves, particularly after 10 years of age, in both sexes. The target height, known in 41% of the patients, represented the WHO reference population. Predominantly, sitting height appears to contribute to short stature, with relative sparing of the leg length and arm span (supplemental Figure 1). Although weight is appropriate for age, body mass index appeared to be increased (supplemental Figure 2). Lower baseline height SD score (β, −0.49; 95% CI, −0.60 to −0.39; P < .001), obtained IDUA enzyme level post-HCT below the local lower reference (β, −0.43; 95% CI, −0.77 to −0.08; P = .02), use of TBI (β, −1.01; 95% CI, −1.53 to −0.48; P < .001), and higher age at evaluation (β, −0.02; 95% CI, −0.02 to −0.02; P < .001) were shown to have a significant negative influence on the height of transplanted patients with MPS-IH (Table 2; Figure 2E-F).
Height and head circumference. Male and female height (A-B) and head circumference (C-D) for age before and after HCT. The continuous thick black lines represent the nonlinear regression models for the post-HCT data. The continuous and dashed black lines represent the reference curves (+2 SD, 0 SD, and −2 SD) according to the WHO.21 The red crosses represent data of patients receiving TBI, the gray squares represent the target height. (E) Height expressed as SD score for age subdivided by the IDUA level; normal IDUA level vs IDUA level below the local lower reference limit. (F) Height expressed as SD score for age subdivided by the baseline height at HCT; −2 SD or higher vs lower than −2 SD.
Height and head circumference. Male and female height (A-B) and head circumference (C-D) for age before and after HCT. The continuous thick black lines represent the nonlinear regression models for the post-HCT data. The continuous and dashed black lines represent the reference curves (+2 SD, 0 SD, and −2 SD) according to the WHO.21 The red crosses represent data of patients receiving TBI, the gray squares represent the target height. (E) Height expressed as SD score for age subdivided by the IDUA level; normal IDUA level vs IDUA level below the local lower reference limit. (F) Height expressed as SD score for age subdivided by the baseline height at HCT; −2 SD or higher vs lower than −2 SD.
Secondary endpoints
Neurological outcome.
Hydrocephalus was observed in 30.6% of the patients pre-HCT, requiring a ventriculoperitoneal shunt in 16.5% of the patients. Although signs of hydrocephalus were still present in 5.9% of the patients post-HCT, no new cases or progression of hydrocephalus were observed at long-term follow-up. All VP shunts were inserted either pre-HCT or within 2 months after HCT. A multivariate predictor of neurological outcome include age at HCT which appear to determine cerebral atrophy after HCT (Table 4).
Orthopedic outcome.
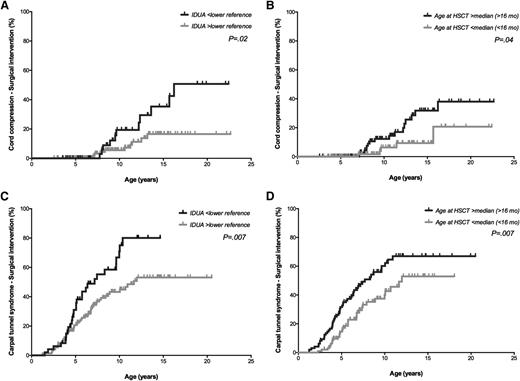
Although data were not available in all patients, the vast majority of the patients had evidence of orthopedic complications pre-HCT, including thoracolumbar kyphosis (97.2%), hip dysplasia (82.4%), and genu valgum (51.0%). Despite HCT, several orthopedic complications still progressed during follow-up, requiring surgical interventions in the majority of the patients. Intervention for severe complications affecting the spinal cord, including cervical instability and cord compression, occurred in only a minority of the patients (4.5% and 10.5%, respectively). Eighteen patients (9.2%) in the study cohort were using a wheelchair at latest follow-up. The leukocyte IDUA level obtained post-HCT was of importance in predicting the risk for most prevalent and severe orthopedic complications, as assessed by progression of the orthopedic complication and/or the need for surgical intervention (Tables 3 and 4; Figure 3). Other predictors that influenced the orthopedic outcome were age at HCT, follow-up age, and follow-up center.
Multivariate Cox regression analysis for event-time endpoints
| Endpoint, predictor, and cutoff . | N (%) . | % . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| Orthopedic endpoints | |||||
| Thoracolumbar kyphosis (surgical intervention) | 67 (34) | ||||
| Cervical instability (surgical intervention) | 9 (5) | ||||
| Cord compression (surgical intervention) | 21 (11) | ||||
| IDUA level | |||||
| <Reference* | 22 | 1 | |||
| ≥Reference* | 7 | 0.34 | 0.14-0.82 | .02 | |
| Age at HCT | |||||
| <16 months† | 5 | 1 | |||
| ≥16 months† | 16 | 2.84 | 1.02-1.41 | .04 | |
| Hip dysplasia (surgical intervention) | 71 (36) | ||||
| IDUA level | |||||
| <Reference* | 52 | 1 | |||
| ≥Reference* | 31 | 0.53 | 0.32-0.86 | .01 | |
| Genu valgum (surgical intervention) | 75 (38) | ||||
| IDUA level | |||||
| <Reference* | 54 | 1 | |||
| ≥Reference* | 33 | 0.50 | 0.31-0.81 | .005 | |
| Carpal tunnel syndrome (surgical intervention) | 89 (45) | ||||
| Age at HCT | |||||
| <16 months† | 33 | 1 | |||
| ≥16 months† | 56 | 1.72 | 1.11-2.68 | .02 | |
| IDUA level | |||||
| <Reference* | 61 | 1 | |||
| ≥Reference* | 39 | 0.58 | 0.37-0.92 | .02 | |
| Trigger fingers (surgical intervention) | 33 (18) | ||||
| Cardiac endpoints | |||||
| Cardiomyopathy (treatment) | 33 (18) | ||||
| Respiratory endpoint | |||||
| Overnight hypoxia (respiratory support) | 9 (4) | ||||
| IDUA level | |||||
| <Reference* | 12 | 1 | |||
| ≥Reference* | 2 | 0.15 | 0.03-0.78 | .02 | |
| Ophthalmologic endpoints | |||||
| Corneal clouding (surgical intervention) | 20 (10) | ||||
| Glaucoma (surgical intervention) | 11 (6) | ||||
| Cataract (surgical intervention) | 6 (3) | ||||
| TBI | |||||
| No | 0 | 1 | |||
| Yes | 22 | 40.13 | 4.66-345.72 | .001 | |
| Audiologic endpoint | |||||
| Hearing loss (hearing aids) | 59 (32) | ||||
| IDUA level | |||||
| <Reference* | 51 | 1 | |||
| ≥Reference* | 26 | 0.42 | 0.24-0.73 | .002 | |
| Endocrinologic endpoints | |||||
| Growth retardation (GH treatment) | 26 (13) | ||||
| TBI | |||||
| No | 11 | 1 | |||
| Yes | 32 | 4.82 | 1.84-12.65 | .001 | |
| Follow-up center | 1.20 | 1.02-1.41 | .03 | ||
| Hypothyroidism (treatment) | 12 (7) | ||||
| TBI | |||||
| No | 5 | 1 | |||
| Yes | 18 | 6.12 | 1.42-26.33 | .02 | |
| Follow-up center | 1.37 | 1.03-1.81 | .03 |
| Endpoint, predictor, and cutoff . | N (%) . | % . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| Orthopedic endpoints | |||||
| Thoracolumbar kyphosis (surgical intervention) | 67 (34) | ||||
| Cervical instability (surgical intervention) | 9 (5) | ||||
| Cord compression (surgical intervention) | 21 (11) | ||||
| IDUA level | |||||
| <Reference* | 22 | 1 | |||
| ≥Reference* | 7 | 0.34 | 0.14-0.82 | .02 | |
| Age at HCT | |||||
| <16 months† | 5 | 1 | |||
| ≥16 months† | 16 | 2.84 | 1.02-1.41 | .04 | |
| Hip dysplasia (surgical intervention) | 71 (36) | ||||
| IDUA level | |||||
| <Reference* | 52 | 1 | |||
| ≥Reference* | 31 | 0.53 | 0.32-0.86 | .01 | |
| Genu valgum (surgical intervention) | 75 (38) | ||||
| IDUA level | |||||
| <Reference* | 54 | 1 | |||
| ≥Reference* | 33 | 0.50 | 0.31-0.81 | .005 | |
| Carpal tunnel syndrome (surgical intervention) | 89 (45) | ||||
| Age at HCT | |||||
| <16 months† | 33 | 1 | |||
| ≥16 months† | 56 | 1.72 | 1.11-2.68 | .02 | |
| IDUA level | |||||
| <Reference* | 61 | 1 | |||
| ≥Reference* | 39 | 0.58 | 0.37-0.92 | .02 | |
| Trigger fingers (surgical intervention) | 33 (18) | ||||
| Cardiac endpoints | |||||
| Cardiomyopathy (treatment) | 33 (18) | ||||
| Respiratory endpoint | |||||
| Overnight hypoxia (respiratory support) | 9 (4) | ||||
| IDUA level | |||||
| <Reference* | 12 | 1 | |||
| ≥Reference* | 2 | 0.15 | 0.03-0.78 | .02 | |
| Ophthalmologic endpoints | |||||
| Corneal clouding (surgical intervention) | 20 (10) | ||||
| Glaucoma (surgical intervention) | 11 (6) | ||||
| Cataract (surgical intervention) | 6 (3) | ||||
| TBI | |||||
| No | 0 | 1 | |||
| Yes | 22 | 40.13 | 4.66-345.72 | .001 | |
| Audiologic endpoint | |||||
| Hearing loss (hearing aids) | 59 (32) | ||||
| IDUA level | |||||
| <Reference* | 51 | 1 | |||
| ≥Reference* | 26 | 0.42 | 0.24-0.73 | .002 | |
| Endocrinologic endpoints | |||||
| Growth retardation (GH treatment) | 26 (13) | ||||
| TBI | |||||
| No | 11 | 1 | |||
| Yes | 32 | 4.82 | 1.84-12.65 | .001 | |
| Follow-up center | 1.20 | 1.02-1.41 | .03 | ||
| Hypothyroidism (treatment) | 12 (7) | ||||
| TBI | |||||
| No | 5 | 1 | |||
| Yes | 18 | 6.12 | 1.42-26.33 | .02 | |
| Follow-up center | 1.37 | 1.03-1.81 | .03 |
Only statistically significant results are shown.
*Lower limit of local reference.
Median age at HCT.
Multivariate logistic regression analysis for binary endpoints
| Endpoint, predictor, and cutoff . | No (%) . | % . | OR . | 95% CI . | P . |
|---|---|---|---|---|---|
| Neurological endpoints | |||||
| Hydrocephalus (presence) | 10 (6) | ||||
| Cerebral atrophy (presence) | 56 (34) | ||||
| Age at HCT | |||||
| <16 months* | 23 | 1 | |||
| ≥16 months* | 46 | 3.22 | 1.60-6.50 | .001 | |
| Orthopedic endpoints | |||||
| Thoracolumbar kyphosis (progression) | 133 (75) | ||||
| IDUA level | |||||
| <Reference† | 93 | 1 | |||
| ≥Reference† | 69 | 0.19 | 0.05-0.64 | .008 | |
| Cervical instability (progression) | 18 (9) | ||||
| IDUA level | |||||
| <Reference† | 21 | 1 | |||
| ≥Reference† | 5 | 0.24 | 0.09-0.69 | .008 | |
| Follow-up age | |||||
| <9.2 years‡ | 5 | 1 | |||
| ≥9.2 years‡ | 14 | 3.23 | 1.04-10.01 | .04 | |
| Cord compression (progression) | 29 (15) | ||||
| IDUA level | |||||
| <Reference† | 34 | 1 | |||
| ≥Reference† | 9 | 0.19 | 0.08-0.45 | <.001 | |
| Age at HCT | |||||
| <16 months* | 8 | 1 | |||
| ≥16 months* | 21 | 3.02 | 1.17-7.76 | .02 | |
| Follow-up age | |||||
| <9.2 years‡ | 6 | 1 | |||
| ≥9.2 years‡ | 25 | 5.36 | 1.97-14.58 | .001 | |
| Hip dysplasia (progression) | 140 (79) | ||||
| IDUA level | 95 | 1 | |||
| <Reference† | 75 | 0.17 | 0.04-0.74 | .02 | |
| ≥Reference† | |||||
| Follow-up age | |||||
| <9.2 years‡ | 71 | 1 | |||
| ≥9.2 years‡ | 87 | 2.50 | 1.13-5.53 | .02 | |
| Genu valgum (progression) | 144 (84) | ||||
| Follow-up center | 1.20 | 1.05-1.38 | .008 | ||
| Orthopedic endpoints | |||||
| Carpal tunnel syndrome | 65 (71) | ||||
| IDUA level | |||||
| <Reference† | 85 | 1 | |||
| ≥Reference† | 64 | 0.29 | 0.09-0.94 | .04 | |
| Follow-up age | |||||
| <9.2 years‡ | 50 | 1 | |||
| ≥9.2 years‡ | 86 | 6.17 | 2.20-17.29 | .001 | |
| Trigger finger (progression) | 33 (18) | ||||
| Cardiac endpoints | |||||
| Cardiomyopathy (progression) | 8 (5) | ||||
| Mitral valve insufficiency (progression) | 71 (37) | ||||
| IDUA level | |||||
| <Reference† | 56 | 1 | |||
| ≥Reference† | 30 | 0.36 | 0.18-0.74 | .005 | |
| Age at HCT | |||||
| <16 months* | 26 | 1 | |||
| ≥16 months* | 47 | 2.46 | 1.30-4.65 | .006 | |
| Follow-up age | |||||
| <9.2 years‡ | 26 | 1 | |||
| ≥9.2 years‡ | 50 | 2.71 | 1.44-5.11 | .002 | |
| Aortic valve insufficiency (progression) | 53 (29) | ||||
| Age at HCT | |||||
| <16 months* | 19 | 1 | |||
| ≥16 months* | 37 | 2.40 | 1.19-4.82 | .01 | |
| Follow-up age | |||||
| <9.2 years‡ | 18 | 1 | |||
| ≥9.2 years‡ | 41 | 3.32 | 1.66-6.64 | .001 | |
| Respiratory endpoint | |||||
| Overnight hypoxia (progression) | 13 (8) | ||||
| IDUA level | |||||
| <Reference† | 27 | 1 | |||
| ≥Reference† | 3 | 0.10 | 0.03-0.35 | <.001 | |
| Respiratory endpoint | |||||
| Overnight hypoxia (progression) | |||||
| Follow-up age | |||||
| <9.2 years‡ | 3 | 1 | |||
| ≥9.2 years‡ | 15 | 4.42 | 1.09-17.83 | .04 | |
| Ophthalmologic endpoint | |||||
| Corneal clouding (progression) | 48 (26) | ||||
| IDUA level | |||||
| <Reference† | 46 | 1 | |||
| ≥Reference† | 20 | 0.28 | 0.11-0.67 | .005 | |
| Follow-up age | |||||
| <9.2 years‡ | 6 | 1 | |||
| ≥9.2 years‡ | 49 | 15.43 | 5.93-40.15 | <.001 | |
| Glaucoma | 12 (7) | ||||
| IDUA level | |||||
| <Reference† | 15 | 1 | |||
| ≥Reference† | 5 | 0.28 | 0.09-0.95 | .04 | |
| Cataract | 16 (8) | ||||
| TBI | |||||
| No | 4 | 1 | |||
| Yes | 39 | 12.53 | 3.73-42.08 | <.001 | |
| Follow-up age | |||||
| <9.2 years‡ | 1 | 1 | |||
| ≥9.2 years‡ | 16 | 16.51 | 2.05-133.26 | .008 | |
| Audiologic endpoint | |||||
| Hearing loss (progression) | 14 (9) | ||||
| Follow-up age | |||||
| <9.2 years‡ | 4 | 1 | |||
| ≥9.2 years‡ | 16 | 4.80 | 1.27-18.21 | .02 |
| Endpoint, predictor, and cutoff . | No (%) . | % . | OR . | 95% CI . | P . |
|---|---|---|---|---|---|
| Neurological endpoints | |||||
| Hydrocephalus (presence) | 10 (6) | ||||
| Cerebral atrophy (presence) | 56 (34) | ||||
| Age at HCT | |||||
| <16 months* | 23 | 1 | |||
| ≥16 months* | 46 | 3.22 | 1.60-6.50 | .001 | |
| Orthopedic endpoints | |||||
| Thoracolumbar kyphosis (progression) | 133 (75) | ||||
| IDUA level | |||||
| <Reference† | 93 | 1 | |||
| ≥Reference† | 69 | 0.19 | 0.05-0.64 | .008 | |
| Cervical instability (progression) | 18 (9) | ||||
| IDUA level | |||||
| <Reference† | 21 | 1 | |||
| ≥Reference† | 5 | 0.24 | 0.09-0.69 | .008 | |
| Follow-up age | |||||
| <9.2 years‡ | 5 | 1 | |||
| ≥9.2 years‡ | 14 | 3.23 | 1.04-10.01 | .04 | |
| Cord compression (progression) | 29 (15) | ||||
| IDUA level | |||||
| <Reference† | 34 | 1 | |||
| ≥Reference† | 9 | 0.19 | 0.08-0.45 | <.001 | |
| Age at HCT | |||||
| <16 months* | 8 | 1 | |||
| ≥16 months* | 21 | 3.02 | 1.17-7.76 | .02 | |
| Follow-up age | |||||
| <9.2 years‡ | 6 | 1 | |||
| ≥9.2 years‡ | 25 | 5.36 | 1.97-14.58 | .001 | |
| Hip dysplasia (progression) | 140 (79) | ||||
| IDUA level | 95 | 1 | |||
| <Reference† | 75 | 0.17 | 0.04-0.74 | .02 | |
| ≥Reference† | |||||
| Follow-up age | |||||
| <9.2 years‡ | 71 | 1 | |||
| ≥9.2 years‡ | 87 | 2.50 | 1.13-5.53 | .02 | |
| Genu valgum (progression) | 144 (84) | ||||
| Follow-up center | 1.20 | 1.05-1.38 | .008 | ||
| Orthopedic endpoints | |||||
| Carpal tunnel syndrome | 65 (71) | ||||
| IDUA level | |||||
| <Reference† | 85 | 1 | |||
| ≥Reference† | 64 | 0.29 | 0.09-0.94 | .04 | |
| Follow-up age | |||||
| <9.2 years‡ | 50 | 1 | |||
| ≥9.2 years‡ | 86 | 6.17 | 2.20-17.29 | .001 | |
| Trigger finger (progression) | 33 (18) | ||||
| Cardiac endpoints | |||||
| Cardiomyopathy (progression) | 8 (5) | ||||
| Mitral valve insufficiency (progression) | 71 (37) | ||||
| IDUA level | |||||
| <Reference† | 56 | 1 | |||
| ≥Reference† | 30 | 0.36 | 0.18-0.74 | .005 | |
| Age at HCT | |||||
| <16 months* | 26 | 1 | |||
| ≥16 months* | 47 | 2.46 | 1.30-4.65 | .006 | |
| Follow-up age | |||||
| <9.2 years‡ | 26 | 1 | |||
| ≥9.2 years‡ | 50 | 2.71 | 1.44-5.11 | .002 | |
| Aortic valve insufficiency (progression) | 53 (29) | ||||
| Age at HCT | |||||
| <16 months* | 19 | 1 | |||
| ≥16 months* | 37 | 2.40 | 1.19-4.82 | .01 | |
| Follow-up age | |||||
| <9.2 years‡ | 18 | 1 | |||
| ≥9.2 years‡ | 41 | 3.32 | 1.66-6.64 | .001 | |
| Respiratory endpoint | |||||
| Overnight hypoxia (progression) | 13 (8) | ||||
| IDUA level | |||||
| <Reference† | 27 | 1 | |||
| ≥Reference† | 3 | 0.10 | 0.03-0.35 | <.001 | |
| Respiratory endpoint | |||||
| Overnight hypoxia (progression) | |||||
| Follow-up age | |||||
| <9.2 years‡ | 3 | 1 | |||
| ≥9.2 years‡ | 15 | 4.42 | 1.09-17.83 | .04 | |
| Ophthalmologic endpoint | |||||
| Corneal clouding (progression) | 48 (26) | ||||
| IDUA level | |||||
| <Reference† | 46 | 1 | |||
| ≥Reference† | 20 | 0.28 | 0.11-0.67 | .005 | |
| Follow-up age | |||||
| <9.2 years‡ | 6 | 1 | |||
| ≥9.2 years‡ | 49 | 15.43 | 5.93-40.15 | <.001 | |
| Glaucoma | 12 (7) | ||||
| IDUA level | |||||
| <Reference† | 15 | 1 | |||
| ≥Reference† | 5 | 0.28 | 0.09-0.95 | .04 | |
| Cataract | 16 (8) | ||||
| TBI | |||||
| No | 4 | 1 | |||
| Yes | 39 | 12.53 | 3.73-42.08 | <.001 | |
| Follow-up age | |||||
| <9.2 years‡ | 1 | 1 | |||
| ≥9.2 years‡ | 16 | 16.51 | 2.05-133.26 | .008 | |
| Audiologic endpoint | |||||
| Hearing loss (progression) | 14 (9) | ||||
| Follow-up age | |||||
| <9.2 years‡ | 4 | 1 | |||
| ≥9.2 years‡ | 16 | 4.80 | 1.27-18.21 | .02 |
Only statistically significant results are shown.
Median age at HCT.
Lower limit of local reference.
Median age at follow-up.
Cumulative incidence curves. Age is depicted on the horizontal axis. The occurrence of surgical intervention regarding cord compression (A-B) and carpal tunnel syndrome (C-D) in percentages is depicted on the vertical axis, estimated by the IDUA enzyme level post-HCT (A,C) and age at HCT (B,D). The result of the log-rank tests for the comparison between the 2 depicted curves is shown.
Cumulative incidence curves. Age is depicted on the horizontal axis. The occurrence of surgical intervention regarding cord compression (A-B) and carpal tunnel syndrome (C-D) in percentages is depicted on the vertical axis, estimated by the IDUA enzyme level post-HCT (A,C) and age at HCT (B,D). The result of the log-rank tests for the comparison between the 2 depicted curves is shown.
Cardiac outcome.
Mitral and aortic valve insufficiency were observed pre-HCT in 46.5% and 10.1% of the patients, respectively. In 19.7% of the patients, cardiomyopathy was diagnosed pre-HCT. During follow-up post-HCT, a significant proportion of the patients showed progression of mitral (36.8%) and aortic valve (28.5%) insufficiency, and 18.4% were using an angiotensin converting enzyme inhibitor after HCT. In 2 patients, a coarctation of the aorta was described, requiring surgical intervention in 1 patient. Six patients suffered from a cardiac arrest at a median of 15.1 years post-HCT; in 5 cases, during surgical intervention (n = 2 kyphosis surgery, n = 2 cord compression surgery, n = 1 cardiac valve surgery). Four of 6 patients survived the cardiac arrest. Multivariate predictors of cardiac outcome include the IDUA enzyme level post-HCT and age at HCT, as well as age at follow-up (Tables 3 and 4).
Respiratory outcome.
Overnight hypoxia was still observed despite HCT, and 8 patients required overnight continuous positive airway pressure at a median of 7.8 years post-HCT. One patient received a tracheotomy for prolonged respiratory support 19.7 years post-HCT. The IDUA level obtained post-HCT was a significant multivariate predictor for respiratory support (Tables 3 and 4).
Ophthalmologic outcome.
Corneal clouding was observed pre-HCT in almost all (97.6%) patients and was stabilized or improved post-HCT in the majority (73.8%) of the patients. Progression of corneal clouding, resulting in corneal transplantation, occurred in 9.8% of the patients at a median follow-up of 11.1 years post-HCT. Permanent blindness resulting from hydrocephalus was already present pre-HCT in 3 patients. Glaucoma, requiring topical treatment, was observed in 11 patients. In 17 patients, cataracts were diagnosed, all in patients receiving TBI. Cataract surgery was performed in 6 patients. Multivariate predictors of ophthalmologic outcome include the obtained IDUA enzyme level post-HCT as well as the age at follow-up (Tables 3 and 4). The presence of cataracts was influenced by TBI only.
Audiologic outcome.
Hearing loss was encountered in 88.2% of the patients pre-HCT. Post-HCT, 62.8% of the patients still suffered from hearing loss; of these, 29.2% were sensorineural in nature, 31.0% were conductive, and 39.8% were of a mixed type. Hearing aids were required in 31.9% of the study population; in 14.6% of the patients, hearing aids were used before HCT. Both IDUA level post-HCT and follow-up age were significant predictors for hearing loss (Table 3).
Endocrinologic outcome.
GH treatment was prescribed in 13.1% of the patients at a median of 8.0 years post-HCT. In 12 patients, hypothyroidism was diagnosed, requiring oral intervention. Both TBI and center of follow-up were predictors for GH treatment and treatment of hypothyroidism (Table 3).
Discussion
After successful HCT, the clinical course of patients with MPS-IH is strikingly improved. Residual disease burden is, however, present in the majority of the patients. Because life expectancy is significantly increased after HCT, with survival up to 23 years in this study, several manifestations became apparent after longer follow-up. Despite the complex nature of the endpoints analyzed and the many centers caring for these patients, we were able to demonstrate that age at HCT, obtained IDUA level post-HCT, and baseline clinical status were all important predictors for the prognosis of patients with MPS-IH post-HCT. Previously, the identification of predictors that were associated with graft failure by this multicenter collaboration has led to markedly improved graft outcomes in this rare disease.6-10 Identification of the importance of age at HCT and delivered IDUA enzyme can therefore lead to improvements in the long-term clinical outcomes of transplanted patients with MPS-IH.
A normal leukocyte IDUA enzyme level obtained post-HCT was a predictor for better clinical outcome in most organ systems. This result supports the use of only noncarrier donors and striving to achieve full-donor chimerism, as both factors contribute to the post-HCT leukocyte IDUA level.4,8 The use of unrelated cord blood is of special interest, as it is associated with similar survival rates and higher rates of full-donor chimerism compared with other graft sources.8 In this study, fully engrafted patients with a noncarrier cord blood graft obtained higher IDUA levels compared with fully engrafted patients receiving noncarrier or carrier bone marrow/peripheral blood stem cell donors (supplemental Figure 3). This may be a result of the donor selection criteria of one of the larger centers, where cord blood units were also selected according to highest IDUA enzyme level. Within the normal range of IDUA, no differences in clinical outcome were found. Whether supranormal levels will improve the endpoints described remains an important question. Gene-transduced autologous-HCT protocols, using stronger promoters with resulting overexpression of IDUA, are of particular interest in this context. A recent gene therapy trial in patients with metachromatic leukodystrophy showed promising results.24 Clinical gene therapy studies including long-term follow-up are needed to prove efficacy in patients with MPS-IH.
Age at HCT is an important predictor for better outcomes, including neurodevelopment. Early diagnosis and timely HCT are therefore of utmost importance to minimize the risk for what seems to be irreversible tissue damage. The most effective strategy to identify MPS-IH early in the course of the disease is newborn screening, which has the potential to reduce the age at HCT to 3 to 4 months of age. This will very likely affect the baseline DQ/IQ, a second important predictor for post-HCT neurodevelopment. Identified patients with an obvious severe genotype (nonsense mutation on both alleles) should proceed to transplant as soon as possible. More challenging include cases in which it may be difficult to predict the phenotype. Such cases could be closely monitored and proceed to HCT as soon as a severe phenotype is suggested.25 An international expert consensus statement is important for further recommendations. Furthermore, considering the higher and more stable IDUA enzyme levels achieved after HCT, one might argue that patients with MPS type I with a more attenuated phenotype (MPS I Hurler-Scheie) are better off with HCT compared with ERT,4 especially as HCT has become much safer in recent years.9 Of interest, neither the genotype nor the transient use of ERT was found to be a predictor of any of the endpoints.
Determination of the baseline developmental level pre-HCT can be very helpful to predict the neurodevelopmental prognosis of patients with MPS-IH after transplant, as patients with lower baseline DQ/IQs show significantly inferior neurodevelopmental outcome post-HCT. The same holds true for age at transplantation: a higher age at HCT predicted inferior neurodevelopmental outcome. However, a clear cutoff baseline DQ/IQ or age at HCT that predicted moderate or severe cognitive impairment after transplant could not be found. In other words, there were patients with a severely impaired development pre-HCT, which showed only mild neurodevelopmental impairment after transplant. This might have been caused by, for instance, reversible hearing or vision impairment at time of transplant, which could have severely affected neurocognitive functioning at that time. Therefore, decisions on whether or not patients should be transplanted based on the baseline DQ/IQ or age at time of transplant alone must be made with caution.
The obtained IDUA enzyme level was not found as a predictor for the neurodevelopmental outcome after transplant. One might speculate that very low IDUA enzyme levels are already sufficient to prevent neurodegeneration in patients with MPS-IH. This is supported by the clinical presentation of patients with MPS type I–Scheie, who do not show neurodevelopmental deterioration despite the detection of only trace amounts of the IDUA enzyme. The neurodevelopmental prognosis of patients with MPS-IH after HCT is therefore predominantly determined by the degree of damage to the central nervous system that has already occurred at time of transplant. Therefore, early diagnosis and subsequent timely HCT, before the onset of irreversible pathology, is highly important.
In addition to the residual disease burden observed in the transplanted patients with MPS-IH, regimen-related toxicity might also have contributed to several of the observed manifestations. The negative effect of TBI concerning the endpoints neurodevelopment, growth, hypothyroidism, and cataracts was clearly shown. Fortunately, TBI as part of the conditioning regimen was abandoned and not used in this cohort since 2002. Although not specifically analyzed, other components of the regimen might have influenced the clinical prognosis of these patients as well. Furthermore, although not found in our study population, some manifestations might still arise in these patients as a result of regimen-related toxicity as patients are getting older, such as secondary malignancies.
The institution monitoring these patients was of importance with regard to several endpoints, suggesting the diagnosis or management of potential complications depended at least in part on the local follow-up protocol and/or involved specialist. One could imagine that some orthopedic surgeons are more eager to perform surgical intervention on the knees of patients with MPS-IH to correct the genu valgum than others, as there is still no clear consensus regarding the management of this frequently occurring manifestation. The same holds true for growth hormone therapy to treat growth retardation. Because it is still unclear whether this treatment will ameliorate the growth retardation observed in nearly all transplanted patients with MPS-IH, its use was only observed in 3 of the participating centers. Defining international consensus guidelines on the follow-up and management of residual disease burden in patients with MPS-IH is therefore of great importance. During the last years, 2 consensus meetings have already resulted in some clear guidelines.26,27 At present, specialists experienced in MPS disease are working on further guidelines on the follow-up and management of residual disease burden in patients with MPS-IH.
Our findings confirm that HCT results in a significantly improved clinical course for patients with MPS-IH, although a significant residual disease burden remains. Early referral for HCT, with the best available noncarrier donor, using a regimen designed to achieve full-donor engraftment, offers the best long-term prognosis. Unrelated cord blood units are particularly attractive, as these are readily available and have shown to result in high rates of full-donor chimerism and normal IDUA levels.7,8 In the near future, newborn screening programs enabling early HCT and strategies to provide supranormal levels post-HCT (eg, HCT in combination with alternative sources of enzyme or substrate reduction or using gene-transduced autologous cells) may further affect the long-term clinical outcome of patients with MPS-IH. Continuing international collaboration is of utmost importance to further optimize the therapies in patients with MPS-I and other lysosomal storage diseases.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We particularly acknowledge Professor J. Ed Wraith, one of the key contributors to this international collaboration, who sadly died last year. As a pioneer in therapies for lysosomal storage disorders, he hugely contributed to the increased knowledge concerning HCT for patients with Hurler syndrome and the subsequent improved patient outcomes. This work was supported by a research grant from the Netherlands Organisation for Scientific Research (92003535 to M.A.) and a fellowship grant from the European group for Blood and Marrow Transplantation (to M.A.).
Authorship
Contribution: M.A., J.J.B., and R.F.W. designed and supervised the study; M.A. visited the centers for data collection; M.A. and J.J.B. wrote the manuscript, performed statistical analysis, and analyzed and interpreted the data; R.F.W., P.J.O., A.O., P.V., S.A.J., T.J.d.K., and J.K. contributed to the critical revision of the manuscript; and R.F.W., P.J.O., A.O., P.V., A.F., V.V., B.N., A.R., V.K.P., J.T., H.A., S.A.J., R.P., M.R., V.B., N.M.W., T.J.d.K., E.G.S., J.K., and J.J.B. contributed to the acquisition of the data.
Conflict-of-interest disclosure: M.A. reports grants from Netherlands Organisation for Scientific Research (project 92003535) and European group for Blood and Marrow Transplantation during the conduct of the study. P.J.O. reports honoraria and grant support from Genzyme outside the submitted work. S.A.J. reports grants from Genzyme and BioMarin outside the submitted work. R.P. reports personal fees and nonfinancial support from Genzyme, Shire, and BioMarin outside the submitted work. E.G.S. reports grants from Shire and Genzyme outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Mieke Aldenhoven, University Medical Center Utrecht, Department of Pediatrics, Pediatric Blood and Marrow Transplantation Program, Office KE.04.133.1, Post box 85090, 3508 AB Utrecht, The Netherlands; e-mail: m.aldenhoven@umcutrecht.nl.