Key Points
Antigenic modulation significantly impacts natural killer cell and macrophage ability to mediate Fc γ receptor-dependent killing.
hIgG1 mAbs are unable to elicit natural killer–mediated ADCC in the mouse, supporting ADCP as the dominant effector mechanism.
Abstract
Following the success of rituximab, 2 other anti-CD20 monoclonal antibodies (mAbs), ofatumumab and obinutuzumab, have entered clinical use. Ofatumumab has enhanced capacity for complement-dependent cytotoxicity, whereas obinutuzumab, a type II mAb, lacks the ability to redistribute into lipid rafts and is glycoengineered for augmented antibody-dependent cellular cytotoxicity (ADCC). We previously showed that type I mAbs such as rituximab have a propensity to undergo enhanced antigenic modulation compared with type II. Here we assessed the key effector mechanisms affected, comparing type I and II antibodies of various isotypes in ADCC and antibody-dependent cellular-phagocytosis (ADCP) assays. Rituximab and ofatumumab depleted both normal and leukemic human CD20-expressing B cells in the mouse less effectively than glycoengineered and wild-type forms of obinutuzumab, particularly when human immunoglobulin G1 (hIgG1) mAbs were compared. In contrast to mouse IgG2a, hIgG1 mAbs were ineffective in ADCC assays with murine natural killer cells as effectors, whereas ADCP was equivalent for mouse IgG2a and hIgG1. However, rituximab’s ability to elicit both ADCC and ADCP was reduced by antigenic modulation, whereas type II antibodies remained unaffected. These data demonstrate that ADCP and ADCC are impaired by antigenic modulation and that ADCP is the main effector function employed in vivo.
Introduction
Rituximab is the archetypal anti-CD20 monoclonal antibody (mAb), licensed in 1997 and now used alongside chemotherapy to treat non-Hodgkin lymphoma.1 It is proposed to operate through 4 effector functions: programmed cell death, complement-dependent cytotoxicity (CDC), and the 2 Fc γ receptor (FcγR)-dependent mechanisms antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).1-3
Following on from the success of rituximab are the next-generation anti-CD20 mAbs ofatumumab and obinutuzumab. Ofatumumab was approved for chronic lymphocytic leukemia (CLL) treatment in 2009 and shows enhanced CDC, likely through its low off-rate and cell-surface proximal epitope.4,5 Obinutuzumab, licensed in 2013 for first-line CLL treatment (in combination with chlorambucil), has a glycoengineered Fc region, resulting in higher affinity binding to human FcγRIIIa (hFcγRIIIa) and b, thus enhancing hFcγRIII-dependent effector functions.6-9
Despite the success of rituximab, patients often become resistant to therapy and relapse. Acute resistance can be associated with loss of CD20 from the cell surface, particularly in the case of CLL.10 We have previously demonstrated that antigenic modulation, whereby CD20 antibody:antigen complexes are internalized after type I mAb binding, will contribute to CD20 loss and that this process is accelerated by hFcγRIIb.11-13
Anti-CD20 mAbs are categorized as type I (rituximab, ofatumumab) or II (tositumomab, obinutuzumab) depending upon their propensity to elicit CD20 redistribution into lipid rafts and trigger efficient CDC (type I) or homotypic adhesion and lysosomal nonapoptotic programmed cell death (type II).14-16 Type II antibodies are also resistant to antigenic modulation and display an enhanced B-cell depletion in hCD20 mice.11,17
Using a combination of mouse FcγR−/− (mFcγR−/−) mice18 and intravital imaging,19,20 it has been clearly shown that anti-CD20 mAbs require FcγR-mediated effector mechanisms for successful therapy. Further evidence for FcγR effector mechanisms came from the observations that hFcγRIIIa and hFcγRIIa polymorphisms correlate with clinical efficacy,21,22 although roles for complement activation and apoptosis induction have also been proposed.1,23
Multiple cell types express FcγR with variable expression patterns: With the exception of a minor proportion of the human population who express an open reading frame for hFcγRIIc,24 B cells express only the inhibitory hFcγRIIb (mFcγRII), natural killer (NK) cells express only hFcγRIIIa (mFcγRIII), and macrophages variably express the full repertoire of FcγR.25
Here we assessed the potential effector functions employed by type I and II mAbs, how they are affected by the internalization process, and, through the use of isotype variants, identified the key effector mechanisms involved in B-cell depletion. We demonstrate that antigenic modulation affects ADCP and ADCC in both the mouse and human systems in vitro and that ADCP is the dominant effector mechanism of B-cell depletion in the mouse. These data explain the greater efficacy of type II antibodies in vivo in mice and have implications for future antibody selection and development in humans.
Methods
Animals, clinical samples, and antibodies
Details relating to animal experiments, clinical sample preparation, and antibody generation/preparation can be found in the supplemental Methods, available on the Blood Web site.
Flow cytometry
Flow cytometry was as described previously26 with samples assessed on a FACScan, FACSCalibur, or FACSCanto II (BD Biosciences) and data analyzed with FCS Express V3 (De Novo Software). To determine cell-surface expression of CD20, cells were incubated with anti-CD20 mAbs, washed twice, and then stained using phycoerythrin-F(ab′)2 goat-anti-mouse or anti-human Fcγ-specific reagents (Jackson ImmunoResearch). The mean number of antibodies/cell was determined using BD QuantiBRITE beads (BD Biosciences).
ADCP
ADCC
Target cells were loaded with calcein-AM (10 µg/mL), opsonized with anti-CD20 antibody (10 µg/mL), and cultured with murine NK (mNK) cells for 2 hours at an ratio effector to target (E:T) ratio of 10:1 or peripheral blood mononuclear cells for 4 hours at an E:T of 50:1. Subsequently, 75 µL of supernatant was collected and analyzed using a Varioskan Flashplate reader at 495 nm (Thermo Scientific). Results are reported as percentage maximum lysis obtained when incubating targets alone with 4% triton X-100. For human targets, ADCC was also measured using the ADCC Reporter Bioassay (Promega) according to the manufacturer’s instructions.
Detection of hIgG
The level of human immunoglobulin G (hIgG) in mouse serum was assessed by standard enzyme-linked immunosorbent assay (supplemental Methods).
SPR
Surface plasmon resonance (SPR) analysis of FcγR:mAb binding was performed as described previously.27 Further details are in the supplemental Methods.
In vivo B-cell depletions
Systemic B-cell depletion assays were performed as previously described.11 Mice were given a single intravenous dose of anti-CD20 mAb (250 µg) and the proportion of B cells remaining in the blood assessed by flow cytometry over time using dual staining with anti-B220-PerCP and anti-CD19-APC (BD Biosciences).
Eμ-Tcl-1 × hCD20 Tg leukemia studies
Eμ-Tcl-1 × hCD20 transgenic (Tg) tumors were generated by intercrossing Eμ-Tcl-1 and hCD20 Tg mice and harvesting the resulting tumors. Splenic tumors were confirmed as expressing hCD20 by flow cytometry and stored in liquid nitrogen. For therapy experiments, cells were thawed and 1 × 107 given intraperitoneally to congenic female hCD20 Tg C57BL/6 mice. Leukemic burden was assessed by monitoring the percentage of CD5+B220lo cells by flow cytometry. When tumor cells could be clearly observed in the blood (after approximately 35-42 days), mice were randomized to receive different mAbs (250 µg/mouse) intravenously and the effect on circulating tumor and normal B cells was monitored.
Statistical analysis
Statistical analysis comparing treatment groups was performed using 1- or 2-way analysis of variance (ANOVA) where appropriate. Statistical analysis was performed using GraphPad Prism, version 6, for Windows (GraphPad Software).
Results
hIgG1 type II but not type I mAbs effectively deplete B cells in hCD20 transgenic mice
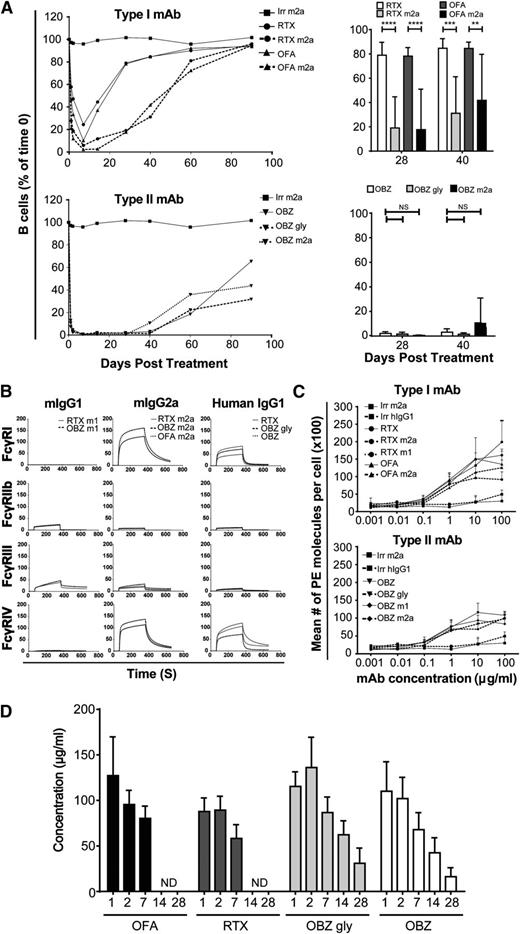
We previously showed that type II anti-CD20 mAbs are superior to type I in depleting hCD20 transgenic B cells in vivo and that this superior efficacy correlates with the propensity of type I mAbs to undergo antigenic modulation.11,17 Previous work has also demonstrated a hierarchical role for IgG isotype in FcγR interaction, effector cell function, and target cell deletion capacity in vivo.28 Therefore, to extend our previous observations, we investigated the impact that alternate antibody isotypes had on B-cell depletion with type I and II mAbs. We treated hCD20 Tg mice with either type I or type II anti-CD20 antibodies engineered with mIgG2a or hIgG1 isotypes.
For type I antibodies, the mIgG2a isotype provided significantly prolonged B-cell depletion compared with hIgG1 (Figure 1A). However, there was no difference in B-cell depletion when comparing hIgG1 (glycoengineered obinutuzumab and wild-type non-glycoengineered obinutuzumab [OBZ gly]) and wild-type non-glycoengineered mIgG2a versions of the type II reagents. As anticipated from previous work with mCD20 as a target,28 the mIgG2a isotype was superior to mouse IgG1 (mIgG1), independent of whether type I or II anti-hCD20 reagents were examined (supplemental Figure 1).
In vivo depletion using human and mouse type I and II antibodies. (A) Left panels show that transgenic hCD20 mice were administered 250 µg anti-CD20 by tail-vein injection and the percentage of circulating B220, CD19+ B cells measured over 90 days (n = 4). Right panels show statistical comparison of human vs mouse isotype mAbs at days 28 and 40. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at ****P < .0001, ***P < .001, and **P < .01. (B) SPR analysis of anti-human CD20 mAbs (hIgG1, mIgG1, and mIgG2a) binding to mouse FcγRI, IIb, III, and IV. Recombinant, soluble FcγR proteins were passed over mAbs immobilized at 2000 RU. Sensorgrams are shown. (C) Binding comparison of type I and II anti-CD20 mAbs to transgenic hCD20 B cells. Cells were opsonized with 0.001 to 100 µg/mL anti-CD20 mAbs and analyzed by flow cytometry. The mean number of phycoerythrin (PE) molecules per cell was quantified by indirect staining with anti-mouse or anti-human Fc PE-conjugated F(ab′)2 and comparison of the geometric mean fluorescent index with BD QuantiBRITE beads. (D) The concentration of anti-CD20 mAbs in the sera of mice administered 250 µg of human IgG1 mAbs was determined by enzyme-linked immunosorbent assay; n = 4 mice per group. ND, not detectable. Bars represent mean ± standard deviation. Irr, WR17; OFA, ofatumumab; RTX, rituximab.
In vivo depletion using human and mouse type I and II antibodies. (A) Left panels show that transgenic hCD20 mice were administered 250 µg anti-CD20 by tail-vein injection and the percentage of circulating B220, CD19+ B cells measured over 90 days (n = 4). Right panels show statistical comparison of human vs mouse isotype mAbs at days 28 and 40. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at ****P < .0001, ***P < .001, and **P < .01. (B) SPR analysis of anti-human CD20 mAbs (hIgG1, mIgG1, and mIgG2a) binding to mouse FcγRI, IIb, III, and IV. Recombinant, soluble FcγR proteins were passed over mAbs immobilized at 2000 RU. Sensorgrams are shown. (C) Binding comparison of type I and II anti-CD20 mAbs to transgenic hCD20 B cells. Cells were opsonized with 0.001 to 100 µg/mL anti-CD20 mAbs and analyzed by flow cytometry. The mean number of phycoerythrin (PE) molecules per cell was quantified by indirect staining with anti-mouse or anti-human Fc PE-conjugated F(ab′)2 and comparison of the geometric mean fluorescent index with BD QuantiBRITE beads. (D) The concentration of anti-CD20 mAbs in the sera of mice administered 250 µg of human IgG1 mAbs was determined by enzyme-linked immunosorbent assay; n = 4 mice per group. ND, not detectable. Bars represent mean ± standard deviation. Irr, WR17; OFA, ofatumumab; RTX, rituximab.
To better understand why type II reagents are superior and why mIgG2a and hIgG1 isotypes are equally efficacious, we assessed the ability of these mAbs to engage mFcγR using SPR. As seen in Figure 1B and supplemental Table 1, mIgG2a isotypes possess similar mFcγR binding profiles in accordance with those previously reported,27 with strong affinity for mFcγRI and IV, and we report similar binding profiles for hIgG1. Although the mIgG2a had an intermediate affinity for mFcγRIII, hIgG1 had a lower affinity. In contrast, mIgG1 mAbs had no measurable binding to mFcγRI or IV, but did have an intermediate affinity for mFcγRIII. Importantly, these binding patterns were independent of antibody type and equivalent for both types I and II and therefore do not explain the propensity of type II reagents to provide prolonged B-cell depletion in vivo.
Antibody-binding levels of type I and II reagents may provide a trivial explanation for their differing efficacy in vivo; therefore, we stained hCD20 Tg B cells with type I or type II reagents and looked for the amount of cell surface–bound antibody. Figure 1C shows that 10 µg/mL is sufficient to saturate hCD20 Tg B cells with both types of mAbs and that, as previously reported,17 at 10 µg/mL, type I antibodies bind ∼2-fold compared with type II antibodies, although some variation in binding was seen, presumably from differences in affinity.
After ruling out differences in Fc:FcγR binding affinities and antibody binding levels as explanations for our earlier observations, we next determined the serum half-life of hIgG1 mAbs during our in vivo depletions (Figure 1A). Figure 1D demonstrates that type I antibodies have a shorter serum half-life compared with type II reagents, likely because of B cell–dependent internalization of antibody.11-13 The reduced quantity of type I antibody available may therefore affect effector cell mechanisms.
Previously, we showed that complement and apoptosis were redundant effector mechanisms in depleting hCD20+ B cells with mIgG2a anti-CD20 mAbs, whereas depletion of macrophages with clodronate or treatment of γ chain−/− mice arrested B-cell depletion11 ; we carried out similar experiments here confirming these observations for hIgG1 mAbs (supplemental Figures 2 and 3). Given these confirmatory findings, we decided to investigate the differential effects of FcγR-mediated ADCC and ADCP on B-cell depletion using clinical CLL samples and in the hCD20 mouse.
Type II reagents show superior ADCC activity, but ADCC is not the dominant mechanism of action in vivo
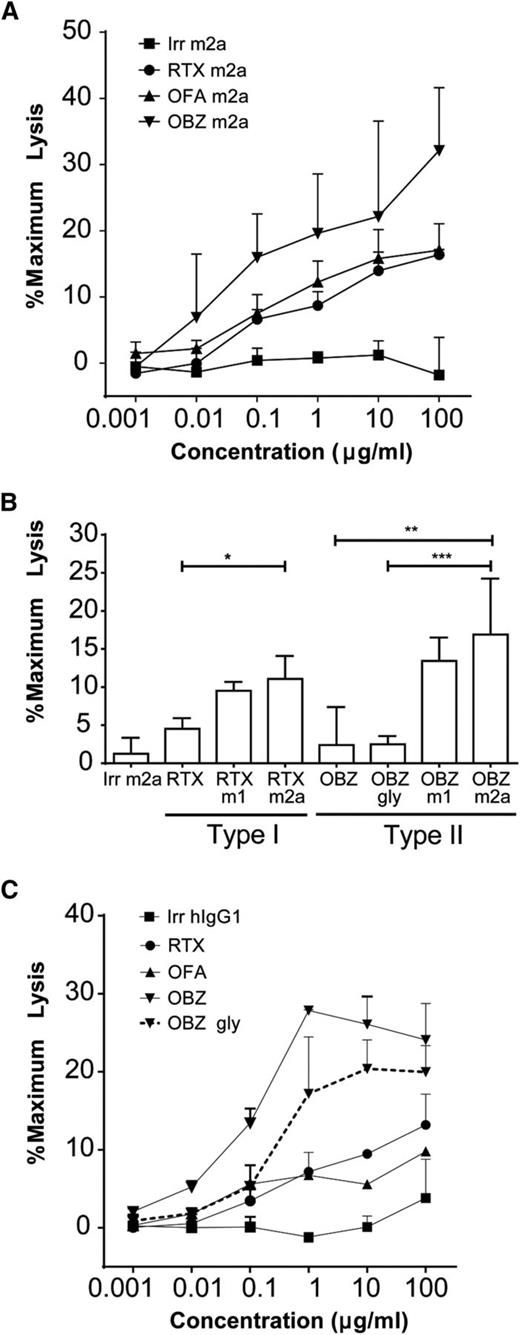
To correlate our in vitro data with the observed in vivo results, we began by assessing the efficacy of our antibodies in assays with murine effectors and then confirmed these results with human cells. We started by expanding mNK cells ex vivo in the presence of interleukin-2 (200 µg/mL). The resulting NK cells possessed a highly activated phenotype, displaying increased CD69 and FcγRIII expression (supplemental Figure 4). At all concentrations tested, the type II mAbs were able to elicit ADCC to a greater extent than were the type I mAbs (Figure 2A). This increased ADCC activity was not due to altered glycosylation because both obinutuzumab mIgG2a and mIgG1 are wild-type mAbs and not defucosylated. This observation is perhaps surprising given the relative binding levels determined in Figure 1C. Also, when we directly compared type I or II mIgG2a and mIgG1 mAbs, we observed equivalent levels of ADCC with mNK effectors (Figure 2B) in contrast to our in vivo findings, in which the mIgG1 antibodies (even type II) performed relatively poorly (supplemental Figure 1), suggesting ADCC is not the dominant mechanism of action in vivo. Furthermore, although type II hIgG1 mAbs were highly effective at depleting B cells in vivo, they demonstrated negligible ADCC activity (Figure 2B). The FcγR binding affinities indicate that in contrast to mIgG2a and mIgG1 isotypes, hIgG1 has a low affinity for mFcγRIII, but a high affinity for mFcγRI and IV (Figure 1B). Because mNK cells only express mFcγRIII,29 these data explain the inability of hIgG1 mAbs to elicit substantial ADCC with mouse effectors. Moreover, coupled with the depletions observed in hCD20 Tg mice (Figure 1A), they demonstrate that ADCC is not the dominant effector cell mechanism depleting B cells in the mouse.
ADCC of type I and II antibodies. (A) Transgenic hCD20 murine B cells were loaded with Calcein-AM and opsonized with anti-CD20 mAbs (N = 3). B cells were then cocultured with mNK cells for 2 hours at an E:T ratio of 10:1; supernatant was assayed for calcein release at 490 nm. (B) Levels of ADCC when target hCD20 murine B cells were opsonized with 10 µg/mL mAbs and cocultured with mNK cells. Statistical analyses were carried out using 1-way ANOVA with multiple comparisons and significance was accepted at *P < .05, **P < .01, and ***P < .001. (C) Primary human CLL cells were loaded with Calcein-AM and opsonized with 0.001 to 10 µg/mL anti-CD20 mAbs (N = 2). CLL cells were then cocultured with human peripheral blood mononuclear cells for 4 hours at an E:T ratio of 50:1; supernatant was assayed for calcein release at 490 nm.
ADCC of type I and II antibodies. (A) Transgenic hCD20 murine B cells were loaded with Calcein-AM and opsonized with anti-CD20 mAbs (N = 3). B cells were then cocultured with mNK cells for 2 hours at an E:T ratio of 10:1; supernatant was assayed for calcein release at 490 nm. (B) Levels of ADCC when target hCD20 murine B cells were opsonized with 10 µg/mL mAbs and cocultured with mNK cells. Statistical analyses were carried out using 1-way ANOVA with multiple comparisons and significance was accepted at *P < .05, **P < .01, and ***P < .001. (C) Primary human CLL cells were loaded with Calcein-AM and opsonized with 0.001 to 10 µg/mL anti-CD20 mAbs (N = 2). CLL cells were then cocultured with human peripheral blood mononuclear cells for 4 hours at an E:T ratio of 50:1; supernatant was assayed for calcein release at 490 nm.
We next repeated these experiments using human effector cells and primary human CLL cells as targets. Type II antibodies again showed higher levels of ADCC than type I (Figure 2C). Although obinutuzumab displayed the greatest levels of ADCC, by virtue of its glycomodification and increased affinity for hFcγRIIIa, the wild-type non-glycoengineered version also provided good levels of ADCC compared with type I antibodies, suggesting an inherent ability of type II to outperform type I antibodies in ADCC. These results were further confirmed using an hFcγRIIIa ADCC bio-reporter assay, which measures the extent of hFcγRIIIa engagement (supplemental Figure 5).
Types I and II mIgG2a and hIgG1 reagents show a similar propensity to elicit ADCP
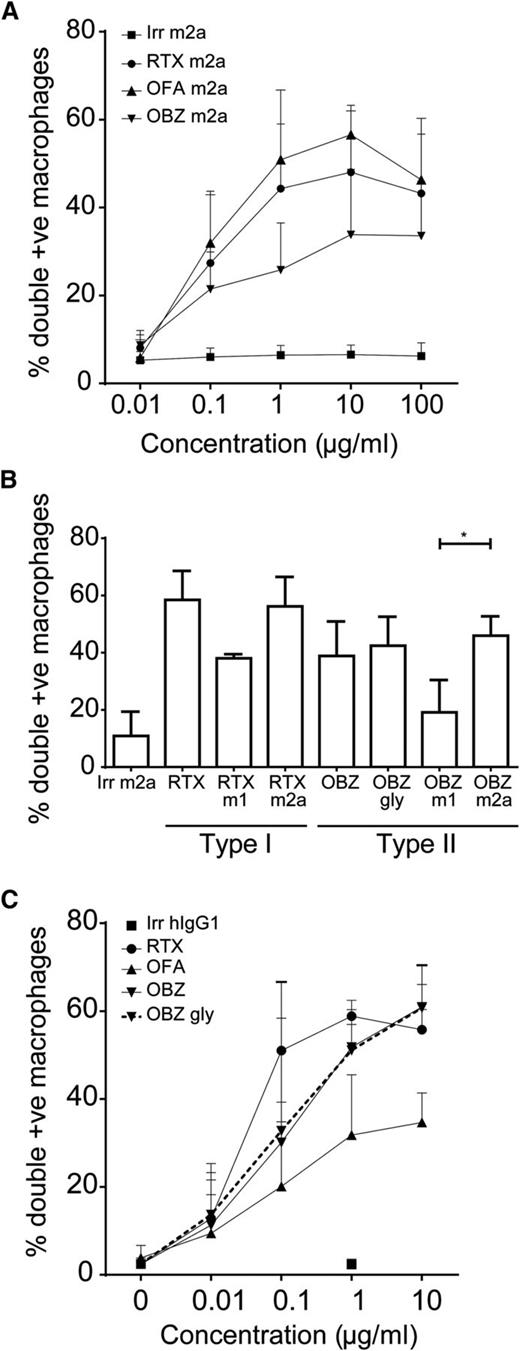
Macrophages were recently implicated as the key effector in antibody-mediated depletion.11,19,20 Because ADCC failed to adequately explain our in vivo results, we assessed the abilities of type I and II antibodies to mediate ADCP. First, we compared type I and II mIgG2a antibodies and demonstrated that all antibodies elicit phagocytosis optimally at 10 µg/mL with murine effectors (Figure 3A). In addition, type I reagents displayed a trend toward inducing greater levels of phagocytosis than type II, perhaps reflecting the higher levels of bound antibody (Figure 1C).
ADCP of type I and II antibodies. (A) Transgenic hCD20 murine B cells were carboxyfluorescein diacetate succinimidyl ester–labeled and opsonized with 0.01 to 100 µg/mL anti-CD20 mAbs (N = 5). Opsonized B cells were cocultured with murine bone marrow–derived macrophages (BMDMs) for 30 minutes to permit phagocytosis and then analyzed by flow cytometry. (B) Levels of ADCP when target hCD20 murine B cells were opsonized with 10 µg/mL mAbs and cocultured with murine BMDMs. Statistical analyses were carried out using 1-way ANOVA with multiple comparisons and significance was accepted at *P < .05. (C) Primary human CLL samples were carboxyfluorescein diacetate succinimidyl ester–labeled and opsonized with 0.01 to 10 µg/mL anti-CD20 mAbs (N = 3). Opsonized CLL B cells were cocultured with human marrow-derived macrophages (MDMs) for 60 minutes to permit phagocytosis and then analyzed by flow cytometry.
ADCP of type I and II antibodies. (A) Transgenic hCD20 murine B cells were carboxyfluorescein diacetate succinimidyl ester–labeled and opsonized with 0.01 to 100 µg/mL anti-CD20 mAbs (N = 5). Opsonized B cells were cocultured with murine bone marrow–derived macrophages (BMDMs) for 30 minutes to permit phagocytosis and then analyzed by flow cytometry. (B) Levels of ADCP when target hCD20 murine B cells were opsonized with 10 µg/mL mAbs and cocultured with murine BMDMs. Statistical analyses were carried out using 1-way ANOVA with multiple comparisons and significance was accepted at *P < .05. (C) Primary human CLL samples were carboxyfluorescein diacetate succinimidyl ester–labeled and opsonized with 0.01 to 10 µg/mL anti-CD20 mAbs (N = 3). Opsonized CLL B cells were cocultured with human marrow-derived macrophages (MDMs) for 60 minutes to permit phagocytosis and then analyzed by flow cytometry.
We next examined the impact of antibody isotype. In the case of murine targets and effectors, with type I and II reagents, the mIgG2a was superior to the mIgG1 isotype at eliciting phagocytosis (Figure 3B), correlating with the superior in vivo depletion observed (supplemental Figure 1). Furthermore, and in contrast to our ADCC data, hIgG1 antibodies showed equivalent levels of phagocytosis compared with mIgG2a antibodies for both type I and II reagents, in keeping with their similar FcγR binding profiles. When we repeated these experiments using primary human CLL cells and human macrophages (Figure 3C), type I reagents again displayed a propensity to outperform type II at suboptimal doses. Both the glycoengineered obinutuzumab and wild-type OBZ Gly displayed equivalent levels of ADCP.
Our earlier data showed that although ADCC was not elicited by hIgG1 type II mAbs with murine cells, they were efficacious in depleting target B cells in vivo. In contrast, these data demonstrate that hIgG1 mAbs were effective in ADCP assays, unlike mIgG1 equivalents. Taking into account their respective in vivo activities, these data indicate ADCP is the key effector mechanism in the mouse. However, we have also shown here that type I reagents performed better than type II reagents in ADCP assays, suggesting that type I antibodies should perform better than type II in vivo—and this is evidently not the case. Based upon our earlier observations in vitro and previous literature,11,17 we speculated that antigenic modulation may limit the effector functions of type I mAbs. To address this, we next examined the impact antigenic modulation had on both ADCC and ADCP.
Antigenic modulation limits both ADCC and ADCP with type I anti-CD20 reagents
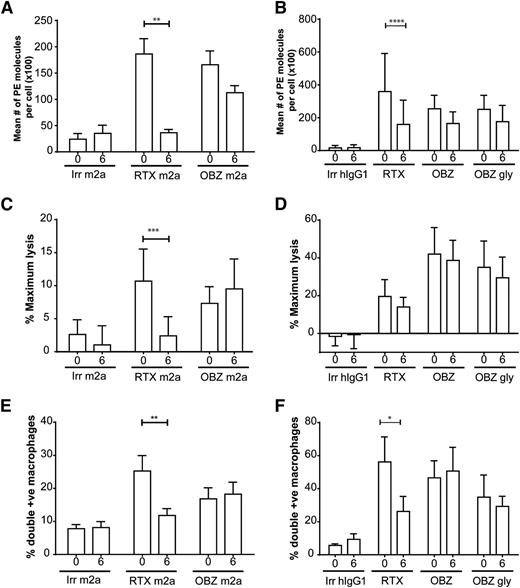
To investigate the kinetics of antigenic modulation, we stained for antibody on opsonized cells 0 to 6 hours after mAb ligation. Antibody was rapidly lost from the cell surface of hCD20 Tg B cells and primary human CLL cells with type I mAbs (Figure 4A-B), as previously reported.11
Impact of modulation on ADCC and ADCP effector mechanisms. (A-B) Surface levels of CD20 after incubation with type I and II mAbs. Target murine hCD20 (A) or primary human CLL (B) B cells were opsonized with 10 µg/mL anti-CD20 mAbs for 30 minutes or 6 hours; samples were then stained for 30 minutes with anti-mouse (A) or anti-human (B) Fc PE and assessed by a flow cytometer, using BD QuantiBRITE beads to calculate mean number of PE molecules/cell (N = 3). (C-D) Impact on ADCC, murine hCD20 (C), or primary human CLL (D) B cells were loaded with calcein-AM and incubated with anti-CD20 mAbs for either 30 minutes or 6 hours with 10 µg/mL anti-CD20 mAbs. Samples were then cocultured for either 2 hours with mNK cells (C) or 4 hours with human peripheral blood mononuclear cells (D) at an E:T ratio 10:1 and 50:1, respectively. Sample supernatant was assessed for fluorescence at 495 nm. (E-F) Impact on ADCP, murine hCD20 (E), or primary human CLL (F) B cells were stained with CFSE and incubated with 10 µg/mL anti-CD20 mAbs for either 30 minutes or 6 hours. Samples were then cocultured for 30 minutes with murine BMDMs (E) or 1 hour with human MDMs (F) and then assessed by flow cytometer. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at *P < .05, **P < .01, ***P < .001, and ****P < .001.
Impact of modulation on ADCC and ADCP effector mechanisms. (A-B) Surface levels of CD20 after incubation with type I and II mAbs. Target murine hCD20 (A) or primary human CLL (B) B cells were opsonized with 10 µg/mL anti-CD20 mAbs for 30 minutes or 6 hours; samples were then stained for 30 minutes with anti-mouse (A) or anti-human (B) Fc PE and assessed by a flow cytometer, using BD QuantiBRITE beads to calculate mean number of PE molecules/cell (N = 3). (C-D) Impact on ADCC, murine hCD20 (C), or primary human CLL (D) B cells were loaded with calcein-AM and incubated with anti-CD20 mAbs for either 30 minutes or 6 hours with 10 µg/mL anti-CD20 mAbs. Samples were then cocultured for either 2 hours with mNK cells (C) or 4 hours with human peripheral blood mononuclear cells (D) at an E:T ratio 10:1 and 50:1, respectively. Sample supernatant was assessed for fluorescence at 495 nm. (E-F) Impact on ADCP, murine hCD20 (E), or primary human CLL (F) B cells were stained with CFSE and incubated with 10 µg/mL anti-CD20 mAbs for either 30 minutes or 6 hours. Samples were then cocultured for 30 minutes with murine BMDMs (E) or 1 hour with human MDMs (F) and then assessed by flow cytometer. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at *P < .05, **P < .01, ***P < .001, and ****P < .001.
To determine which effector mechanisms were critically affected by modulation, we opsonized hCD20 Tg B cells with antibody for 0 to 6 hours and then examined them in our ADCC and ADCP assays. Murine ADCC levels were significantly (P = .0001) impacted by antigenic modulation, with a 6-hour preincubation clearly detrimental for type I but not type II reagents (Figure 4C). We then repeated these experiments with hNK effectors and primary human CLL target cells (Figure 4D). Again, a 6-hour preincubation period led to significant antigenic modulation and demonstrated a trend toward a detrimental effect on the ADCC capacity of rituximab, whereas there was no difference with type II reagents examined at the start and end of the 6-hour culture period. The differences observed with rituximab are likely not significant because the long (4 hour) target and effector incubation period used for the human ADCC assay, which would allow modulation to occur even in the 0-hour samples. We next looked into the impact of antigenic modulation on phagocytosis. Type I reagents were significantly (P < .05) affected by modulation, whereas type II reagents remained unaffected in both murine (Figure 4E) and human systems (Figure 4F).
These findings clearly demonstrate that modulation severely reduces the efficacy of key FcγR-dependent effector systems employed by directly targeting mAbs such as rituximab in both human and mouse systems against both normal and malignant B-cell targets.
Prolonged depletion of hCD20 Tg Eµ-TCL-1 B cells by type II anti-CD20 mAbs
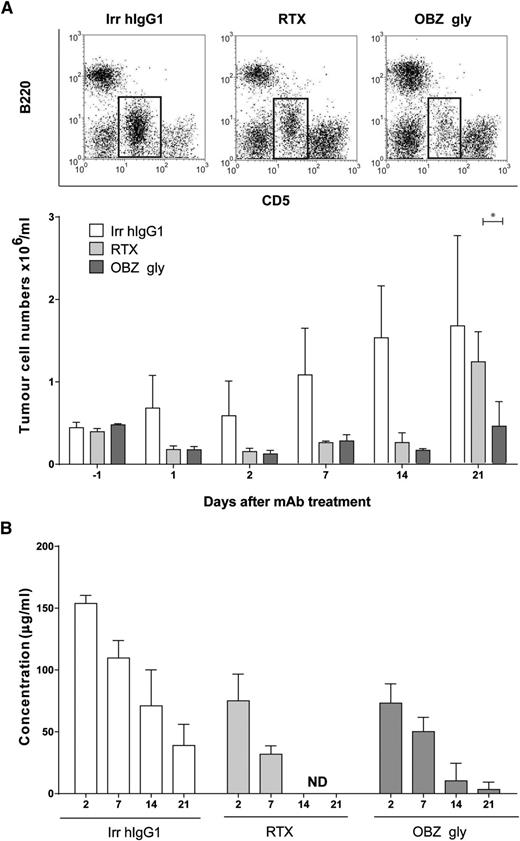
To confirm the clinical relevance of these results for hematological immunotherapy, we performed experiments in hCD20 Tg mice bearing hCD20 Tg Eµ-TCL-1 B-cell tumors, produced recently (manuscript in preparation). The Eµ-TCL-1 mouse model of CLL30 coupled to expression of hCD20 allows us to assess the ability of the clinically relevant mAbs to delete malignant cells in a fully syngeneic, immune-competent context. The data demonstrate that, as with normal hCD20 Tg B cells, hCD20 Tg Eµ-TCL-1 tumor B cells displayed significantly (P < .05) prolonged depletion when treated with type II (OBZ gly) compared with type I (rituximab) mAbs, correlating with a longer serum half-life (Figure 5). These results confirm our findings with normal B cells and demonstrate the superior effects in vivo of the type II mAbs in a clinically relevant mouse model of CLL.
In vivo depletion of Eµ-TCL-1 × hCD20 Tg leukemic B cells using type I and II antibodies. (A) Eµ-TCL-1 × hCD20 Tg splenic tumors were administered intraperitoneally to hCD20 Tg mice and treated intravenously (250 μg) with anti-CD20 mAbs when CD5+ B220low tumor B cells were clearly detectable by flow cytometry 35 to 42 days later. The percentage of circulating tumor was then measured for the following 21 days (n = 3). Example dot-plots showing tumor cell populations on day 21 (tumor indicated in the boxed area) above, with mean numbers of tumor cells/mL below. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at *P < .05. (B) The concentration of anti-CD20 mAbs in the sera of mice in panel A were determined by enzyme-linked immunosorbent assay. ND, not detectable. The results clearly show that RTX is completely lost from the sera by day 14, whereas OBZ gly remains detectable out to day 21, coincident with more prolonged tumor depletion. n = 3 mice per group. Bars represent mean ± standard deviation.
In vivo depletion of Eµ-TCL-1 × hCD20 Tg leukemic B cells using type I and II antibodies. (A) Eµ-TCL-1 × hCD20 Tg splenic tumors were administered intraperitoneally to hCD20 Tg mice and treated intravenously (250 μg) with anti-CD20 mAbs when CD5+ B220low tumor B cells were clearly detectable by flow cytometry 35 to 42 days later. The percentage of circulating tumor was then measured for the following 21 days (n = 3). Example dot-plots showing tumor cell populations on day 21 (tumor indicated in the boxed area) above, with mean numbers of tumor cells/mL below. Statistical analyses were carried out using 2-way ANOVA with multiple comparisons and significance was accepted at *P < .05. (B) The concentration of anti-CD20 mAbs in the sera of mice in panel A were determined by enzyme-linked immunosorbent assay. ND, not detectable. The results clearly show that RTX is completely lost from the sera by day 14, whereas OBZ gly remains detectable out to day 21, coincident with more prolonged tumor depletion. n = 3 mice per group. Bars represent mean ± standard deviation.
Discussion
Previously, we demonstrated that mIgG2a versions of type II anti-CD20 mAbs outperform type I and that antigenic modulation takes place more rapidly with type I antibodies.11,17 Here we show that modulation affects both FcγR-dependent effector mechanisms engaged by mAbs ADCC and ADCP, thus explaining the propensity for type II antibodies to outperform type I in vivo in both normal B-cell depletion and a model of CLL. These same effector mechanisms are similarly impacted with clinically relevant reagents targeting primary human CLL cells. Finally, based on the observation that hIgG1 was efficacious in depleting hCD20 B cells in vivo but showed little ADCC activity because of its minimal binding to mFcγRIII, we demonstrate that ADCP is the dominant effector mechanism responsible for target cell depletion in the mouse.
Previous work comparing clinically relevant antibodies have demonstrated that ofatumumab and obinutuzumab are superior to rituximab in different ways: ofatumumab displays enhanced CDC and obinutuzumab enhanced ADCC and direct cell death.31,32 Although insightful, these experiments did not take into account the potentially detrimental effect of antigenic modulation, which we show here to have a significant impact on FcγR-dependent effector mechanisms. However, work by van Meerten et al demonstrated a strong correlation between CDC and CD20 expression, whereas they found no such association with regard to ADCC.33 This suggests that antigenic modulation would perhaps have a larger impact on CDC, although we have been unable to demonstrate a significant role for CDC in mice (supplemental Figure 2).11 We and others have, however, been able to demonstrate a critical need for macrophages and ADCP (supplemental Figure 3)11,19,20 and would suggest that perhaps the levels of CD20 compared by van Meerten et al did not reach a sufficiently low level to affect FcγR mechanisms—a threshold that is crossed by significant loss of antibody from modulation.
We found that glycoengineered obinutuzumab elicited greater potency in our human ADCC assays than its wild-type counterpart, thus highlighting the benefit of defucosylation. However, we also found that, in vivo, both obinutuzumab and OBZ Gly displayed equivalent levels of B-cell depletion akin to that seen with mIgG2a. Examination of binding affinity data revealed that both hIgG1 and mIgG2a antibodies have similar affinities for mFcγRI and IV, although interestingly, obinutuzumab did have a slower dissociation rate for mFcγRIV. hFcγRIIIa is glycosylated at residue Asn162,34 with mFcγRIV glycosylated at a similar location, likely explaining this observation. Importantly, hNK cells express hFcγRIIIa, which shares higher sequence identity to mFcγRIV than mFcγRIII. Mouse FcγRIV, however, is not expressed on mNK cells and so these results may underestimate the differences that glycoengineering and also ADCC may elicit in humans.35,36 Moreover, because our SPR data only show the binding affinities of monomeric IgG, it would be interesting to determine the interaction of multimeric, complexed hIgG1 with mFcγRIII using a similar methodology to that described recently.37
As expected from SPR data, when we examined hIgG1 antibodies in assays with mouse effectors, we found that they had limited ADCC activity but high ADCP activity (equivalent to mIgG2a), demonstrating that ADCP is the dominant effector mechanism for normal and malignant B-cell targets in vivo, at least in mice. Furthermore and in direct contrast to their activity in vivo, both mouse isotypes had equivalent activity in mouse ADCC assays, but mIgG2a outperformed mIgG1 in mouse ADCP assays, correlating with the enhanced depleting activity of the mIgG2a antibodies in vivo. This conclusion is further supported by recent intravital imaging experiments that have shown liver Kupffer cells to be critical for effective antibody therapy.19,20
Intriguingly, when using human CLL cells as targets, obinutuzumab displayed equivalent levels of phagocytosis to OBZ Gly. Although obinutuzumab showed superior ADCC activity as expected, OBZ Gly also displayed substantially higher levels of ADCC than both rituximab and ofatumumab. Therefore, the superior activity of obinutuzumab over rituximab in vitro may largely reflect the inherent difference between type I and II mAbs. In keeping with these in vitro observations, our in vivo results in the hCD20 Tg Eµ-TCL-1 CLL mouse model fit well with a recently reported trial of CLL patients given chlorambucil alone or in combination with either rituximab or obinutuzumab. In this trial, patients receiving obinutuzumab and chlorambucil demonstrated improved progression-free survival and higher rates of complete response compared with those receiving rituximab and chlorambucil, although the obinutuzumab arm did receive a larger dose of antibody.38 In our CLL model, we compared identical doses of normally glycosylated hIgG1 isotype mAbs as monotherapy and still saw a benefit for OBZ Gly over rituximab, suggesting that, at least in part, some of the additional clinical benefit of obinutuzumab may be attributable to its type II character rather than enhanced FcγR interactions occurring as a result of its glycoengineered Fc.
With regard to ADCC, it is important to reconcile that there was no difference between murine type I and II antibodies, which could suggest the contribution of other FcγR-independent mechanisms. Based on our previous findings11 and those presented here, we contend that the difference between murine types I and II B-cell depletion can be accounted for by ADCP differences alone and that no FcγR-independent mechanisms are required. This conclusion is supported by the observation that C3−/− mice show no difference in B-cell depletion compared with wild-type controls (supplemental Figure 2). Recently, it has been shown that in the presence of competing IgG, obinutuzumab is superior to rituximab in ADCC experiments.9 In vivo then, in the presence of serum IgG, it may be hypothesized that obinutuzumab would be superior to OBZ Gly by virtue of its enhanced affinity for hFcγRIIIa. With regard to serum antibody titers following the administration of hIgG1 antibody, we report in Figures 1D and 5B the superior pharmacokinetics of OBZ Gly compared with rituximab and that this correlated with their observed level of normal and malignant B-cell depletion, respectively. We have previously demonstrated a similar relationship in terms of mIgG2a antibodies whereby Ritm2a showed diminished pharmacokinetics compared with tositumomab and that this also correlated with B-cell depletion in hCD20 mice.11
Rituximab ligation results in reorganization of CD20 into polarized caps; this has been proposed to augment ADCC by NK cells.39 However, here we show that type II antibodies, which do not reorganize CD20 to the same extent as rituximab, are superior at ADCC. In their study, Rudnicka et al used established B-cell lines that we have previously shown not to modulate to the same extent as primary B cells. Therefore, although rituximab-induced capping may provide superior ADCC activity, in primary cells loss of antibody from the cell surface through modulation during the 2- to 4-hour ADCC assay may overcome this beneficial effect. This observation provides further impetus for discovering means to inhibit modulation in order to improve type I anti-CD20 efficacy.
We have previously demonstrated that increased levels of FcγRIIb augments antigenic modulation by anti-CD20 antibodies.12 Given our previous findings and the results presented here, we would suggest that cells with increased surface expression of FcγRIIb would be even more affected in terms of antibody effector engagement, and so treatment with type I antibodies would be even worse with cells expressing high levels of FcγRIIb when compared with those treated with type II.
In conclusion, we demonstrate that antigenic modulation has a detrimental effect on the known FcγR-dependent effector mechanisms, leading to superior activity of nonmodulatory type II reagents in vivo. Importantly, using a variety of mAb isotypes and in vitro assays assessing effector function, we demonstrate that ADCP is the dominant effector mechanism in the mouse in vivo. Therefore, future attempts to augment immunotherapy with this class of direct targeting mAbs should focus on new avenues investigating ways of enhancing ADCP and minimizing modulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Mark Shlomchik for providing hCD20 Tg mice and Dr Christian Klein, Roche Pharmaceutical Research and Early Development, for providing material and advice on the manuscript. The authors also thank Dr Egle, Dr Pekarsky, and Prof Croce for providing the Eµ-TCL-1 mouse model and Dr Stefania Gobessi and Dimitar Efremov for advice on its use. The authors also thank the Experimental Cancer Medicine Centre–funded University of Southampton, Faculty of Medicine Human Tissue Bank (Human Tissue Authority licence 12009), for sample storage. The authors also thank Drs Francesco Forconi, Andrew Duncombe, Kathleen N. Potter, Andrew Steele, Ian Tracy, and Isla Wheatley for provision and assistance with clinical material as well as the National Blood Service Blood Transfusion unit at Southampton General Hospital.
This work was funded by Leukaemia and Lymphoma Research (grants 10055 and 12050) and Cancer Research UK (grants C34592/A12343, C1477/A10834, and C34999A/A18087).
Authorship
Contribution: T.R.W.T. helped design the research, performed experiments, analyzed results, produced figures, and wrote the paper with M.S.C. and S.A.B.; A.R., R.J.O., C.I.M., R.R.F., and L.N.D. performed experiments and produced figures; M.J.C. and K.L.C. characterized the hCD20 × Eµ-TCL-1 mouse model; P.G.H. and P.J.D. provided critical reagents for the study; and M.S.C. and S.A.B. designed the research, analyzed results, and wrote the manuscript with T.R.W.T.
Conflict-of-interest disclosure: M.S.C. acts as a consultant for BioInvent and has received research funding from them as well as from Roche. P.G.H. is an employee of Promega UK Ltd., Southampton, United Kingdom. The remaining authors declare no competing financial interests.
Correspondence: Mark S. Cragg, Antibody and Vaccine Group, Cancer Sciences Unit, University of Southampton Faculty of Medicine, Southampton General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: msc@soton.ac.uk.
References
Author notes
M.S.C. and S.A.B. are the senior authors and contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal