Key Points
CD4 T cells play a critical role in controlling production of PF4/heparin-specific antibodies.
Abstract
Antibodies specific for platelet factor 4 (PF4)/heparin complexes are central to the pathogenesis of heparin-induced thrombocytopenia. Marginal zone B cells appear to be the source of such antibodies, but whether T-cell help is required is unclear. Here, we showed that induction of PF4/heparin-specific antibodies by PF4/heparin complexes was markedly impaired in mice depleted of CD4 T cells by anti-CD4 antibodies. Furthermore, Rag1-deficient recipient mice produced PF4/heparin-specific antibodies upon PF4/heparin challenge when reconstituted with a mixture of wild-type splenic B cells and splenocytes from B-cell–deficient (μMT) mice but not splenocytes from T- and B-cell–deficient (Rag1 knockout) mice. Lastly, mice with B cells lacking CD40, a B-cell costimulatory molecule that helps T-cell–dependent B-cell responses, displayed a marked reduction of PF4/heparin-specific antibody production following PF4/heparin challenge. Together, these findings show that helper T cells play a critical role in production of PF4/heparin-specific antibodies.
Introduction
Heparin-induced thrombocytopenia (HIT) is the most common drug-induced, antibody-mediated thrombocytopenia and occurs 3 to 6 days following heparin treatment.1,2 HIT patients develop antibodies quickly, however, which are typically undetectable in a few months.1 Platelet factor 4 (PF4)/heparin-specific antibodies, central to the pathogenesis of HIT, are predominantly of the immunoglobulin G1 (IgG1) isotype with some IgG2 in humans.2-4 IgG/PF4/heparin immune complexes bind FcγRIIA on the platelet surface and induce platelet activation, leading to thrombocytopenia and a high risk of arterial and/or venous thrombosis/thromboembolism.5,6
Long-lived mature B cells comprise 3 subsets: marginal zone (MZ), B1, and follicular B cells.7,8 The MZ subset has been shown to be critical for production of PF4/heparin-specific antibodies.9 Typically, MZ B cells produce IgM or IgG antibodies independent of T-cell help.10-12 Indeed, HIT patients have features of a T-cell–independent humoral immune response, characterized by rapid onset and decline of antibodies and apparent absence of immunologic memory.1 However, patients with severe HIT possess T cells that have a T-cell receptor with highly restricted complementarity determining region 3 regions and are responsive to PF4/heparin, suggesting a role of T cells in HIT pathogenesis.13,14 Nonetheless, direct evidence for a role of T cells in HIT pathogenesis has not been reported. Here, we describe studies to define the role of T-cell help in regulating production of PF4/heparin-specific antibodies.
Study design
Mice
Eight- to 10-week-old Rag1-deficient, CD40-deficient, μMT, and wild-type C57BL/6 mice from The Jackson Laboratory were maintained in the Biological Resource Center at the Medical College of Wisconsin (MCW). Animal protocols were approved by the MCW Institutional Animal Care and Use Committee.
In vivo depletion of CD4 T cells
Wild-type C57BL/6 mice were injected intraperitoneally with anti-mouse CD4 antibodies (clone GK1.5, 250 μg per mouse; BioXCell) or with isotype control antibodies (rat IgG2b; BioXCell) or phosphate-buffered saline (PBS) on day 0 and day 2. The efficiency of depletion was examined by flow cytometry at day 7 after the first injection, and >99% of CD4 T cells were depleted in the spleen and lymph nodes. To maintain this state, mice were further injected with GK1.5 (250 μg per mouse) on day 7 and day 14.
Immunization
PF4/heparin immunization was performed as described.9 G. Arepally (Duke University) provided mouse PF4. T-cell–dependent and –independent antigen immunizations were performed as described.9 The T-cell–dependent antigen was nitrophenyl-chicken γ globulin (NP-CGG; Biosearch Technologies) and the T-cell–independent antigen was trinitrophenyl-Ficoll (TNP-Ficoll; Biosearch Technologies).
Adoptive transfer experiment
Splenic B cells were isolated from wild-type mice by magnetic cell sorting using anti-B220–coated magnetic-activated cell sorting magnetic microbeads (Miltenyi Biotec) and then mixed 1:1 with splenocytes from μMT or Rag1-deficient mice in PBS supplemented with 2% fetal bovine serum. The mixed cells were transplanted into partially irradiated (300 rad) 8- to 10-week-old Rag1-deficient mice by IV injection (8∼10 × 106 cells per recipient). One hour after adoptive transfer, the recipients were immunized with the indicated antigens. Sera were collected at the indicated time points, and antigen-specific antibodies were measured.
Chimeric mice
Bone marrow (BM) cells from CD40-deficient or wild-type mice were mixed 1:4 with BM cells from μMT mice in PBS supplemented with 2% fetal bovine serum. The mixed cells were transplanted into lethally irradiated (1000 rad) 8- to 10-week-old μMT mice by IV injection (5 × 106 cells per recipient). Eight weeks later, the recipients were immunized with the indicated antigens. Sera were collected at the indicated time points, and antigen-specific antibodies were measured.
Statistical analysis
Statistical analysis was performed with the 2-tailed unpaired Student t test.
Results and discussion
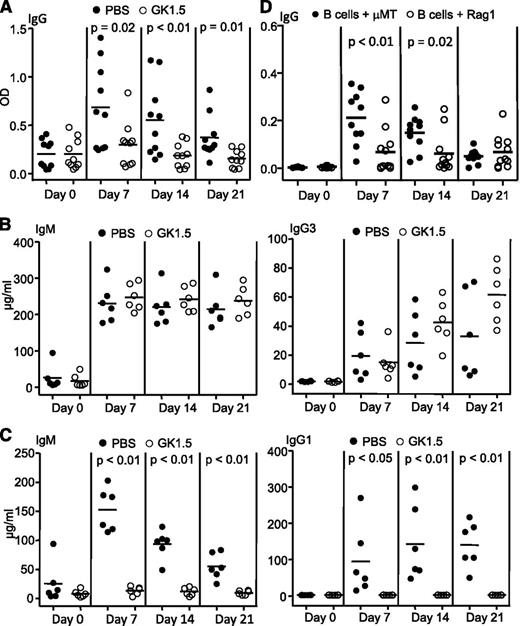
MZ B cells play a major role in producing PF4/heparin-specific antibodies.9 Typically, MZ B cells participate in T-cell–independent antibody responses.10-12 However, human patients with severe HIT possess T cells that are responsive to PF4/heparin.13,14 Here, we systematically investigated the role of T cells in production of PF4/heparin-specific antibodies in vivo. First, we examined the effect of CD4 T-cell depletion on production of PF4/heparin-specific antibodies. Wild-type mice were depleted of T cells with anti-mouse CD4 antibody GK1.5. Flow cytometry analysis demonstrated >99% deletion of CD4 T cells in spleens, lymph nodes, and blood during the entire duration of the experiment (supplemental Figure 1, available on the Blood Web site; data not shown). Following PF4/heparin challenge, production of PF4/heparin-specific antibodies was markedly reduced in these mice relative to controls (Figure 1A, supplemental Figure 2A). In the absence of CD4 T cells, B cells failed to produce any isotypes of PF4/heparin-specific IgG, including IgG2b and IgG3 (supplemental Figure 3). Of note, anti-CD4 antibody-treated mice responded normally to T-cell–independent antigen TNP-Ficoll (Figure 1B) but not T-cell–dependent antigen NP-CGG (Figure 1C), in agreement with the lack of CD4 T cells. Thus, antibody-induced depletion of CD4 T cells markedly impairs PF4/heparin-specific antibody production.
Requirement of T cells for production of PF4/heparin-specific antibodies. (A) Impaired production of PF4/heparin-specific antibodies in CD4 T-cell–depleted mice. Wild-type mice were treated with anti-mouse CD4 antibody GK1.5 or PBS, followed by immunization with PF4/heparin complexes. Sera were collected at the indicated time points after immunization and total PF4/heparin-specific IgG levels were measured by enzyme-linked immunosorbent assay (ELISA). Each dot represents a mouse and the horizontal lines indicate the mean values. Data shown are obtained from 10 mice in each group. (B) Normal T-cell–independent responses in CD4 T-cell–depleted mice. GK1.5- or PBS-treated mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) were measured by ELISA. Data shown are obtained from 6 mice in each group. (C) Impaired T-cell–dependent responses in CD4 T-cell–depleted mice. GK1.5- or PBS-treated mice were immunized with the T-cell–dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) were measured by ELISA. Data shown are obtained from 6 mice in each group. (D) Failure of adoptively transferred splenic B cells to produce PF4/heparin-specific antibodies in the absence of T cells. Splenic B cells isolated from wild-type mice were mixed with splenocytes isolated from μMT or Rag1-deficient mice at a 1:1 ratio, and then adoptively transferred into partially irradiated Rag1-deficient mice. The recipients were immunized with PF4/heparin complexes. Sera were collected at the indicated time points, and the levels of total PF4/heparin-specific IgG were measured by ELISA. Data shown are obtained from 10 recipients in each group. Each dot represents a mouse and the horizontal lines indicate the mean values.
Requirement of T cells for production of PF4/heparin-specific antibodies. (A) Impaired production of PF4/heparin-specific antibodies in CD4 T-cell–depleted mice. Wild-type mice were treated with anti-mouse CD4 antibody GK1.5 or PBS, followed by immunization with PF4/heparin complexes. Sera were collected at the indicated time points after immunization and total PF4/heparin-specific IgG levels were measured by enzyme-linked immunosorbent assay (ELISA). Each dot represents a mouse and the horizontal lines indicate the mean values. Data shown are obtained from 10 mice in each group. (B) Normal T-cell–independent responses in CD4 T-cell–depleted mice. GK1.5- or PBS-treated mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) were measured by ELISA. Data shown are obtained from 6 mice in each group. (C) Impaired T-cell–dependent responses in CD4 T-cell–depleted mice. GK1.5- or PBS-treated mice were immunized with the T-cell–dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) were measured by ELISA. Data shown are obtained from 6 mice in each group. (D) Failure of adoptively transferred splenic B cells to produce PF4/heparin-specific antibodies in the absence of T cells. Splenic B cells isolated from wild-type mice were mixed with splenocytes isolated from μMT or Rag1-deficient mice at a 1:1 ratio, and then adoptively transferred into partially irradiated Rag1-deficient mice. The recipients were immunized with PF4/heparin complexes. Sera were collected at the indicated time points, and the levels of total PF4/heparin-specific IgG were measured by ELISA. Data shown are obtained from 10 recipients in each group. Each dot represents a mouse and the horizontal lines indicate the mean values.
Next, we examined PF4/heparin-specific antibody production in immunodeficient mice reconstituted with B cells but not T cells. Wild-type splenic B cells were mixed with splenocytes from T- and B-cell–deficient Rag1-deficient mice and adoptively transferred into partially irradiated Rag1-deficient mice. The recipients were then challenged with PF4/heparin. Controls were Rag1-deficient mice that received a mixture of wild-type splenic B cells and splenocytes from B-cell–deficient μMT mice. As shown in Figure 1D, control mice responded to PF4/heparin challenge by producing PF4/heparin-specific antibodies. In contrast, mice given a mixture of wild-type splenic B cells and Rag1-deficient splenocytes barely produced PF4/heparin-specific antibodies upon PF4/heparin complex challenge (Figure 1D), but responded normally to T-cell–independent antigen TNP-Ficoll (supplemental Figure 2B). These data also show that T cells are required for PF4/heparin-specific antibody production.
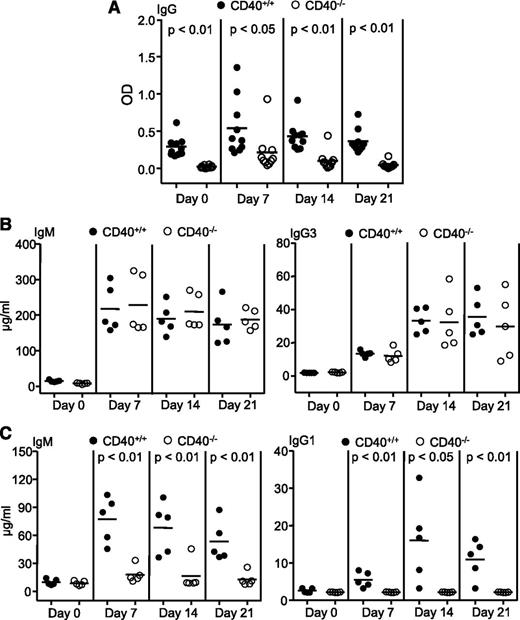
Interactions between CD40 on B cells and its ligand on CD4 T cells play a critical role in T-cell–dependent B-cell responses.15,16 To define the role of helper T cells in PF4/heparin-specific antibody response, we studied the effect of B-cell–specific CD40 deficiency. BM cells from CD40-deficient mice were mixed with BM cells from μMT mice, and transplanted into lethally irradiated μMT mice. The resulting BM chimeric mice possessing B cells derived from CD40-deficient BM cells and thus lacking CD40 failed to produce PF4/heparin-specific antibodies following PF4/heparin challenge (Figure 2A). In contrast, control BM chimeric mice that received a mixture of wild-type and μMT BM cells and thus possessed wild-type B cells responded normally to PF4/heparin challenge (Figure 2A). As expected, BM chimeric mice possessing CD40-deficient B cells responded to the T-cell–independent antigen TNP-Ficoll (Figure 2B) but not T-cell–dependent antigen NP-CGG (Figure 2C), consistent with a lack of T-cell help in these mice. Therefore, T cells provide critical help to B cells in producing PF4/heparin-specific antibodies through the CD40-CD40 ligand interaction.
Requirement of the CD40-CD40 ligand interaction between B and T cells for production of PF4/heparin-specific antibodies. BM cells from wild-type (CD40+/+) or CD40-deficient (CD40−/−) mice were mixed with BM cells from μMT mice at a 1:4 ratio, and then transplanted into lethally irradiated μMT mice. Eight weeks after transplantation, the chimeric mice were immunized with the indicated antigens. (A) Failure of chimeric mice with B-cell–specific deficiency of CD40 to produce PF4/heparin-specific antibodies. The chimeric mice were immunized with PF4/heparin complexes. Sera were collected at the indicated time points after immunization, and the levels of total PF4/heparin-specific IgG were measured by ELISA. Data shown are obtained from 10 chimeric mice at each group. (B) Normal T-cell–independent responses in chimeric mice with B-cell–specific deficiency of CD40. The chimeric mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) were measured by ELISA. Data shown are obtained from 5 mice in each group. (C) Impaired T-cell–dependent responses in chimeric mice with B-cell–specific deficiency of CD40. The chimeric mice were immunized with the T-cell–dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) were measured by ELISA. Data shown are obtained from 5 mice in each group. Each dot represents a mouse and the horizontal lines indicate the mean values.
Requirement of the CD40-CD40 ligand interaction between B and T cells for production of PF4/heparin-specific antibodies. BM cells from wild-type (CD40+/+) or CD40-deficient (CD40−/−) mice were mixed with BM cells from μMT mice at a 1:4 ratio, and then transplanted into lethally irradiated μMT mice. Eight weeks after transplantation, the chimeric mice were immunized with the indicated antigens. (A) Failure of chimeric mice with B-cell–specific deficiency of CD40 to produce PF4/heparin-specific antibodies. The chimeric mice were immunized with PF4/heparin complexes. Sera were collected at the indicated time points after immunization, and the levels of total PF4/heparin-specific IgG were measured by ELISA. Data shown are obtained from 10 chimeric mice at each group. (B) Normal T-cell–independent responses in chimeric mice with B-cell–specific deficiency of CD40. The chimeric mice were immunized with the T-cell–independent antigen TNP-Ficoll. Sera were collected at the indicated time points after immunization, and TNP-specific IgM (left) and IgG3 (right) were measured by ELISA. Data shown are obtained from 5 mice in each group. (C) Impaired T-cell–dependent responses in chimeric mice with B-cell–specific deficiency of CD40. The chimeric mice were immunized with the T-cell–dependent antigen NP-CGG. Sera were collected at the indicated time points after immunization, and NP-specific IgM (left) and IgG1 (right) were measured by ELISA. Data shown are obtained from 5 mice in each group. Each dot represents a mouse and the horizontal lines indicate the mean values.
MZ B cells recognize bacterial antigens, including those with repetitive epitopes, and produce antibodies, mainly IgM and some IgG, independent of T-cell help.10-12,17 A previous study reported that athymic nude mice fail to response to PF4/heparin challenge, indicating the involvement of T cells in HIT antibody production.18 However, athymic nude mice possess many immune defects, including impaired development of B cells.19 Thus, inability of these mice to produce PF4/heparin-specific antibodies could be due to something other than T-cell deficiency per se, including defective B cells. Here we have shown that mice fully intact except for T-cell deficiency display a severely impaired ability to generate PF4/heparin-specific antibodies and that production of such antibodies by B cells requires T-cell help mediated through the CD40-CD40 ligand interaction. Previous studies have shown that CD40-CD40 ligand interaction appears not to be required for B-cell development.16,20 However, a recent study has shown that this interaction can broadly alter the B-cell receptor repertoire during B-cell development.21 Thus, it is possible that CD40 might be required for positive selection of PF4/heparin-specific B cells during development and further research is warranted. Our previous study has shown MZ B cells are critical for production of PF4/heparin-specific antibodies.9 Although MZ B cells typically participate in T-cell–independent responses, there are exceptions to this general rule because previous studies have consistently shown that, under some circumstances, MZ B cells can interact with T cells and produce high-affinity antibodies possessing somatic hypermutation utilizing a T-cell–dependent pathway.22,23 Thus, MZ cells are functionally heterogeneous in their requirement for T-cell help. The antibody response to PF4/heparin-specific antigens represents another example of a T-cell–dependent response by this B-cell subset.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants (National Heart, Lung, and Blood Institute) PO1 HL44612 to D.W. and R01 HL13629 to R.H.A.; and (National Institute of Allergy and Infectious Diseases) R01 AI079087 to D.W.
Authorship
Contribution: Y.Z. contributed to research design, performed the research, and analyzed the results; M.Y. performed some initial experiments; A.P. critically reviewed the manuscript; R.H.A. provided intellectual input and critically reviewed the manuscript; L.Y. provided intellectual input; R.W. provided intellectual input, supervised the study, performed the research, and critically reviewed the manuscript; and D.W. conceived and supervised the study, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yongwei Zheng, Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI 53226; e-mail: yongwei.zheng@bcw.edu; and Demin Wang, Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI 53226; e-mail: demin.wang@bcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal