Key Points
Chronic graft-versus-host disease is associated with a global Breg defect.
This defect is particularly accentuated in the CD24hiCD27+ Breg compartment.
Abstract
Interleukin 10 (IL-10)–producing B cells (regulatory B cells [Bregs]) regulate autoimmunity in mice and humans, and a regulatory role of IL-10–producing plasma cells has been described in mice. Dysfunction of B cells that maintain homeostasis may play a role in the pathogenesis of chronic graft-versus-host disease (cGVHD) after allogeneic stem cell transplantation. Here, we found a relation between decreased Breg frequencies and cGVHD severity. An impaired ability of B cells to produce IL-10, possibly linked to poor signal transducer and activator of transcription 3 and extracellular signal-regulated kinase phosphorylation, was found in patients with active cGVHD. IL-10 production was not confined to a single B-cell subset, but enriched in both the CD24hiCD27+ and CD27hiCD38hi plasmablast B-cell compartments. In vitro plasmablast differentiation increased the frequency of IL-10–producing B cells. We confirmed that allogeneic transplant recipients had an impaired reconstitution of the memory B-cell pool. cGVHD patients had less CD24hiCD27+ B cells and IL-10–producing CD24hiCD27+ B cells. Patients with cGVHD had increased plasmablast frequencies but decreased IL-10–producing plasmablasts. These results suggest a role of CD24hiCD27+ B-cell and plasmablast-derived IL-10 in the regulation of human cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is the leading cause of morbi-mortality after allogeneic hematopoietic stem cell transplantation (AHSCT).1 GVHD prevention by means of adoptive transfer of regulatory T cells (Tregs)2,3 and cGVHD treatment by in vivo induction of Tregs by low-dose interleukin 2 (IL-2)4,5 may be effective. The exact role of IL-10–producing regulatory B cells (Bregs) in cGVHD is yet to be understood. Bregs have been shown to downmodulate adaptive6 or innate immune responses7 in mice and humans. In cGVHD, B cells are essentially recognized as positive regulators of inflammation.1,8,9 Increased B-cell receptor responsiveness was found in cGVHD patients.10 B-cell homeostatic defects were described in cGVHD, including elevated B-cell activating factor of the tumor necrosis factor (TNF) family (BAFF)/B-cell ratios,11-15 expansion of CD21lo B cells,16 reduced CD5+ B1-like cell numbers,17 decreased CD27+ memory B cells, and hypogammaglobulinemia.18 BAFF concentrations correlated with increased circulating pre-germinal center (GC) B-cell and post-GC plasmablast cell counts in patients with cGVHD.11 B-cell depletion with rituximab prevented cGVHD in humans.19 Patients with cGVHD frequently have circulating antibodies reactive to recipient cells.1,9,20
Besides their functions in antibody secretion, cytokine and chemokine production, and antigen presentation, B cells exhibit regulatory properties in several human autoimmune diseases including systemic lupus erythematosus,21 rheumatoid arthritis,22 primary Sjögren syndrome,7 and immune thrombocytopenia,23 all of which share certain clinical features with human cGVHD.1 Depending on the disease, the phenotypic markers and in vitro stimulation used, different B-cell subsets are enriched in Bregs, including CD24hiCD27+ B cells7 and CD24hiCD38hi transitional B cells.21,24 GC reactions may play a major role in the pathogenesis of cGVHD in mice.25-27 GC-derived “plasmablast-like” B cells are increased in human cGVHD.11 Recent studies have shown that the latter cell subset bears regulatory properties and may be the main IL-10–producing B-cell subset in mice.28-31
Discrepancies in the cell surface antigens studied and a lack of consensual definitions of the subset phenotypes limit the direct comparison of human B-cell subpopulation analyses. It is generally admitted that most human memory B cells are characterized by the expression of CD2732-35 and that human plasmablasts display a CD20loCD24−CD27hiCD38hi phenotype.33,35-39
Here, we prospectively studied Bregs and B-cell subsets in 69 AHSCT recipients. Our results suggest that Bregs are enriched in both the CD24hiCD27+ B-cell subset and CD24−CD27hiCD38hi plasmablast cell compartments. In vitro differentiation of human B cells into plasmablasts and plasma cells increased IL-10–producing B-cell numbers. Allogeneic transplant recipients with cGVHD had decreased Bregs and IL-10–producing plasmablast counts as compared with AHSCT recipients with no cGVHD. This suggests a novel role of CD24hiCD27+ B-cell– and plasmablast-derived IL-10 in the regulation of human cGVHD.
Patients and methods
Patients
This prospective study was conducted at Saint-Louis Hospital from November 2010 to December 2014. The diagnosis and staging of cGVHD were made at study inclusion using National Institutes of Health (NIH) criteria.40 Patients with active cGVHD were included at the time of first cGVHD flare before starting cGVHD treatment, or at any time of cGVHD relapse under cGVHD treatment. Relapse was defined as an increase of at least 1 point, and response (“remission”) a decrease of at least 1 point, in the NIH global severity score. Patient characteristics are detailed in Table 1. All samples were collected following written informed consent according to the Declaration of Helsinki. This study received the agreement of the local ethics committee.
Patient characteristics
| Characteristic . | AHSCT recipients without GVHD, n = 22 . | All HSCT recipients with cGVHD, n = 47 . | HSCT recipients with active cGVHD, n = 28 . | HSCT recipients with cGVHD in remission, n = 19 . | P* . |
|---|---|---|---|---|---|
| Male sex, n (%) | 14 (64) | 27 (57) | 15 (54) | 12 (63) | .56 |
| Age at study inclusion, mean (range), y | 41 (19-69) | 47 (20-66) | 47 (26-63) | 46 (20-66) | .89 |
| Time from AHSCT, mean (range), mo | 41 (2-146) | 44 (2-319) | 31 (2-83) | 65 (4-319) | .07 |
| Hematologic disease, n (%) | |||||
| Hodgkin/NHL/CLL/MM | 0/2/3/1 (27) | 4/6/0/4 (30) | 3/5/0/1 (21) | 1/1/0/3 (26) | |
| AML/ALL | 3/2 (23) | 11/8 (40) | 5/3 (32) | 6/5 (56) | |
| MDS/MPS | 3/3 (27) | 8/5 (28) | 7/4 (39) | 1/1 (11) | |
| Aplastic anemia/Hemoglobinopathy | 4/1 (23) | 0/1 (2) | 0/0 | 0/1 (5) | |
| Conditioning: MAC/RIC, n (%) | 1045/12 (55) | 11 (23)/36 (77) | 7 (25)/21 (75) | 4 (21)/15 (79) | |
| Donor type: sibling/MUD, n (%) | 8 (36)/14 (64) | 18 (38)/29 (62) | 11 (39)/17 (61) | 7 (37)/12 (63) | |
| Immunosuppressors at study inclusion, n (%) | 6 (27) | 40 (85) | 22 (79) | 18 (95) | .21 |
| Systemic corticosteroids, n (%) | 1 (5) | 36 (77) | 20 (71) | 16 (84) | .48 |
| Mean dose of steroids (equivalent to prednisone, mg/j) | 20 (0-120) | 24 (0-120) | 13 (0-60) | .41 | |
| Ciclosporin/tacrolimus, n (%) | 5/0 (23) | 15/2 (36) | 11/0 (39) | 4/2 (32) | .76 |
| MMF/MTX, n (%) | 0 | 11/0 (23) | 5 /0 (18) | 6/0 (32) | .31 |
| Rapamycin/everolimus, n (%) | 1 (5) | 1/1 (4) | 1 /0 (4) | 0/ 1 (5) | 1 |
| Extracorporeal photopheresis, n (%) | 0 | 5 (11) | 5 (18) | 0 | .07 |
| Imatinib, n (%) | 0 | 3 (6) | 2 (7) | 1 (5) | 1 |
| cGVHD NIH global severity score, n (%) | NA | ||||
| Mild | 10 (21) | 2 (7) | 8 (42) | .008 | |
| Moderate | 8 (17) | 4 (14) | 4 (21) | .69 | |
| Severe | 29 (62) | 22 (79) | 7 (37) | .006 | |
| cGVHD organ involvement, n (%) | NA | ||||
| Skin | 43 (91) | 24 (86) | 19 (100) | .13 | |
| Lung | 10 (21) | 7 (25) | 3 (16) | .71 | |
| Digestive tract | 15 (32) | 10 (36) | 5 (26) | .54 |
| Characteristic . | AHSCT recipients without GVHD, n = 22 . | All HSCT recipients with cGVHD, n = 47 . | HSCT recipients with active cGVHD, n = 28 . | HSCT recipients with cGVHD in remission, n = 19 . | P* . |
|---|---|---|---|---|---|
| Male sex, n (%) | 14 (64) | 27 (57) | 15 (54) | 12 (63) | .56 |
| Age at study inclusion, mean (range), y | 41 (19-69) | 47 (20-66) | 47 (26-63) | 46 (20-66) | .89 |
| Time from AHSCT, mean (range), mo | 41 (2-146) | 44 (2-319) | 31 (2-83) | 65 (4-319) | .07 |
| Hematologic disease, n (%) | |||||
| Hodgkin/NHL/CLL/MM | 0/2/3/1 (27) | 4/6/0/4 (30) | 3/5/0/1 (21) | 1/1/0/3 (26) | |
| AML/ALL | 3/2 (23) | 11/8 (40) | 5/3 (32) | 6/5 (56) | |
| MDS/MPS | 3/3 (27) | 8/5 (28) | 7/4 (39) | 1/1 (11) | |
| Aplastic anemia/Hemoglobinopathy | 4/1 (23) | 0/1 (2) | 0/0 | 0/1 (5) | |
| Conditioning: MAC/RIC, n (%) | 1045/12 (55) | 11 (23)/36 (77) | 7 (25)/21 (75) | 4 (21)/15 (79) | |
| Donor type: sibling/MUD, n (%) | 8 (36)/14 (64) | 18 (38)/29 (62) | 11 (39)/17 (61) | 7 (37)/12 (63) | |
| Immunosuppressors at study inclusion, n (%) | 6 (27) | 40 (85) | 22 (79) | 18 (95) | .21 |
| Systemic corticosteroids, n (%) | 1 (5) | 36 (77) | 20 (71) | 16 (84) | .48 |
| Mean dose of steroids (equivalent to prednisone, mg/j) | 20 (0-120) | 24 (0-120) | 13 (0-60) | .41 | |
| Ciclosporin/tacrolimus, n (%) | 5/0 (23) | 15/2 (36) | 11/0 (39) | 4/2 (32) | .76 |
| MMF/MTX, n (%) | 0 | 11/0 (23) | 5 /0 (18) | 6/0 (32) | .31 |
| Rapamycin/everolimus, n (%) | 1 (5) | 1/1 (4) | 1 /0 (4) | 0/ 1 (5) | 1 |
| Extracorporeal photopheresis, n (%) | 0 | 5 (11) | 5 (18) | 0 | .07 |
| Imatinib, n (%) | 0 | 3 (6) | 2 (7) | 1 (5) | 1 |
| cGVHD NIH global severity score, n (%) | NA | ||||
| Mild | 10 (21) | 2 (7) | 8 (42) | .008 | |
| Moderate | 8 (17) | 4 (14) | 4 (21) | .69 | |
| Severe | 29 (62) | 22 (79) | 7 (37) | .006 | |
| cGVHD organ involvement, n (%) | NA | ||||
| Skin | 43 (91) | 24 (86) | 19 (100) | .13 | |
| Lung | 10 (21) | 7 (25) | 3 (16) | .71 | |
| Digestive tract | 15 (32) | 10 (36) | 5 (26) | .54 |
AHSCT, allogeneic hematopoietic stem cell transplantation; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; cGVHD, chronic graft-versus-host disease; CLL, chronic lymphocytic leukemia; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; MMF, mycophenolate mofetil; MPS, myeloproliferative syndrome; MTX, methotrexate; MUD, matched unrelated donor; NA, not applicable; NHL, non-Hodgkin lymphoma; NIH, National Institutes of Health; RIC, reduced-intensity conditioning.
Active cGVHD vs remission.
Human cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by density gradient centrifugation over lymphocyte separating medium (LSM; PAA Laboratories).
Cell culture and B-cell IL-10 production
Total PBMCs were cultured at 2 × 106/mL for 72 hours in RPMI 1640 medium (Life Technologies) supplemented with 10% human AB serum (Jacques Boy Biotechnologies Institute, Reims, France), 1% penicillin/streptomycin and 1% glutamate (Sigma-Aldrich) (complete RPMI medium), and stimulated with CpG-B (3 μg/mL; Invivogen), or CpG-B and recombinant human CD40 ligand (CD40L) (1 μg/mL; R&D Systems). Phorbol myristate acetate (PMA) (10 ng/mL; Sigma), ionomycin (1 μg/mL; Life Technologies), and brefeldin A (10 μg/mL; Sigma) were added during the last 5 hours of cell culture. Cells were harvested, washed, and incubated for 20 minutes at 4°C with fixable viability dye eFluor 506 (eBioscience), washed again and stained with anti-CD19-PerCP-Cy5.5 clone Hib19 monoclonal antibody (mAb) (eBioscience), washed, and fixed/permeabilized for 20 minutes at 4°C with an intracellular staining kit (Becton Dickinson). Cells were then incubated for 30 minutes at 4°C with 5 μL of phycoerythrin (PE)-Cy7–conjugated anti-human IL-10 mAb clone JES3-9D7 (Biolegend) or control isotype-matched antibody. The data were acquired on a FACSCanto II cytometer (Becton Dickinson) and analyzed with FlowJo software.
Phenotypic characterization of IL-10–producing B cells
IL-10–producing B cells were characterized by flow cytometry using in vitro stimulation, permeabilization procedure, and intracellular IL-10 staining as described in the previous paragraph. The following mouse mAbs to human cell surface antigens were used: anti-CD19-PerCP-Cy5.5 clone Hib19, anti-CD27–allophycocyanin (APC)–eFluor 780 clone LG7F9 (eBioscience), anti-CD19–fluorescein isothiocyanate (FITC) clone Hib19, anti-CD24-FITC clone ML5, anti-CD38-APC clone HIT2, anti-CD20-PerCP clone 2H7, anti–immunoglobulin D (IgD)–BD Horizon V450 clone IA6-2, anti-CD5-APC clone UCHT2, anti-CD43-FITC clone 1G10, anti-CD70-PE clone Ki24 (BD Pharmingen), anti-IgM-PE clone PJ2-22H3 (Miltenyi Biotec), anti-CD138-PE clone B-A38 (Beckman Coulter).
B-cell phenotypic studies
Total PBMCs from cGVHD patients, AHSCT recipients with no cGVHD, and healthy controls were stained with fixable viability dye eFluor 506 (eBioscience), anti-CD19-PerCP-Cy5.5 clone Hib19, anti CD27-APC-eFluor 780 clone LG7F9 (eBioscience), anti-CD24-FITC clone ML5, anti-CD38-APC clone HIT2, and anti-IgD-BD Horizon V450 clone IA6-2 (BD Pharmingen).
Signaling assays
PBMCs were stimulated by incubation with either recombinant human IL-21 (200 ng/mL; R&D Systems) or CpG-B (10 μg/mL; Invivogen) for 2 hours at 37°C in complete RPMI 1640 medium. Unstimulated PBMCs were incubated for 2 hours at 37°C in complete RPMI 1640 medium as negative controls. Cells were fixed/permeabilized using the BD Phosflow kit (Becton Dickinson) and stained with anti-CD19-PerCP-Cy5.5 clone Hib19 (eBioscience), 4/P–signal transducer and activator of transcription 3 (STAT3) (pY705)–Alexa Fluor 647 and extracellular signal-regulated kinase 1/2 (ERK1/2) (pT202/pY204)–Alexa Fluor 488 (Becton Dickinson). Analyses were performed with identical fluorescence-activated cell sorter (FACS) settings. One patient from each group was studied on the same day, that is, 1 healthy subject, 1 allogeneic transplant recipient without cGVHD, and 1 cGVHD patient were studied on the same day, to reduce any batch effect.
In vitro plasmablast differentiation
Unstimulated B cells were isolated from fresh PBMCs using magnetic cell separation (Miltenyi Biotec). B-cell purity was always higher than 95% as assessed by flow cytometry. Purified B cells were cultured in complete Iscove modified Dulbecco medium (IMDM) and stimulated for 10 days with crosslinked CD40L, CpG-B, IL-2, IL-6, IL-10, IL-15, and interferon-α (IFN-α) in a 3-step sequential differentiation method as described by Jourdan et al.41 At days 1, 4, 7, and 10, B-cell phenotype on freshly harvested cells, and IL-10 production by B cells after 5-hour PMA + ionomycin in vitro stimulation, were assessed by flow cytometry as described in “Cell culture and B-cell IL-10 production.”
Statistics
All values are expressed as medians. Values are plotted with their median and interquartile range and compared between groups with Prism software (GraphPad) by Fisher exact test for categorical variables, 2-tailed Mann-Whitney U test to compare continuous variables in 2 sample groups, or the Kruskal-Wallis nonparametrical analysis of variance (ANOVA) test to compare >2 sample groups simultaneously. Correlations were assessed using a nonparametric Spearman test. P ≤ .05 was considered statistically significant.
Results
Deficient IL-10–producing Breg compartment in cGVHD patients
Various in vitro reagents have been used to detect IL-10–producing Bregs in humans including Toll-like receptor 9 (TLR9) agonists (CpG) and CD40 agonists (CD40L).6,7 We evaluated IL-10–producing Bregs in healthy humans, AHSCT recipients without cGVHD, AHSCT patients with active cGVHD, and cGVHD in remission using 2 in vitro stimulation methods (CpG ± CD40L).
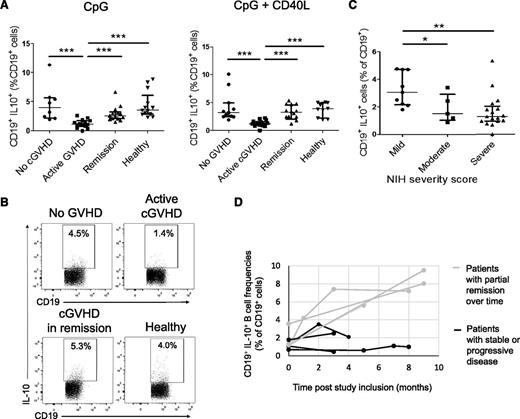
After CpG stimulation, Breg frequencies were significantly decreased in active cGVHD patients compared with the other groups. In line with Khoder et al,24 we found significantly lower Bregs in patients with active cGVHD than in healthy donors (1.1% vs 3.5%, P < .0001), patients with no GVHD (1.1% vs 4.0%, P = .0004), and patients with cGVHD in remission (1.0% vs 2.6%, P = .0001) (Figure 1A, left panel, B). Similar differences were noted using in vitro dual stimulation with CpG + CD40L (Figure 1A, right panel). Considering that the differences in Breg frequencies could be linked to other factors than to the cGVHD status, we compared the baseline characteristics between the patient subgroups. There was no significant difference in the proportion of patients receiving immunosuppressive therapy, or in the mean dose of steroids received by the patients, between patients with active cGVHD and patients with cGVHD in remission (Table 1). There was no relation between the daily doses of steroids received and the Breg frequencies (supplemental Figure 1, see supplemental Data available on the Blood Web site).
Deficient IL-10–producing Breg compartment in cGVHD patients. Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, cGVHD in remission, and healthy donors were cultured for 66 hours with CpG-B (3 μg/mL) or CpG-B and recombinant human CD40L (1 μg/mL) followed by restimulation with PMA + ionomycin in the presence of brefeldin A during the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in CD19+ cells by flow cytometry. CD19+IL-10+ cells in proportion of total live CD19+ cells in AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy blood donors (A) after CpG stimulation (left panel) and after CpG + CD40L stimulation (right panel). (B) Representative dot plots showing the proportion of CD19+IL-10+ cells after CpG in an AHSCT recipient without GVHD, a patient with active cGVHD, a patient with cGVHD in remission, and a healthy donor. (C) Frequencies of CD19+IL-10+ cells in proportion to live CD19+ cells after CpG stimulation in patients with cGVHD (active or in remission, derived from panel A) according to the NIH global severity score (mild vs moderate and severe cGVHD). (D) Longitudinal follow-up of Breg frequencies in cGVHD patients. Patients were included at the time of active cGVHD and prospectively followed during the course of immunosuppressive treatment. An increase in Breg frequencies was observed in patients achieving a partial remission of cGVHD over time, whereas no or little change was observed in patients with stable or worsening symptoms.
Deficient IL-10–producing Breg compartment in cGVHD patients. Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, cGVHD in remission, and healthy donors were cultured for 66 hours with CpG-B (3 μg/mL) or CpG-B and recombinant human CD40L (1 μg/mL) followed by restimulation with PMA + ionomycin in the presence of brefeldin A during the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in CD19+ cells by flow cytometry. CD19+IL-10+ cells in proportion of total live CD19+ cells in AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy blood donors (A) after CpG stimulation (left panel) and after CpG + CD40L stimulation (right panel). (B) Representative dot plots showing the proportion of CD19+IL-10+ cells after CpG in an AHSCT recipient without GVHD, a patient with active cGVHD, a patient with cGVHD in remission, and a healthy donor. (C) Frequencies of CD19+IL-10+ cells in proportion to live CD19+ cells after CpG stimulation in patients with cGVHD (active or in remission, derived from panel A) according to the NIH global severity score (mild vs moderate and severe cGVHD). (D) Longitudinal follow-up of Breg frequencies in cGVHD patients. Patients were included at the time of active cGVHD and prospectively followed during the course of immunosuppressive treatment. An increase in Breg frequencies was observed in patients achieving a partial remission of cGVHD over time, whereas no or little change was observed in patients with stable or worsening symptoms.
The only factor that differed significantly between the “Active cGVHD” and “Remission” group was the cGVHD severity. Patients with active cGVHD had a higher cGVHD severity score than patients with cGVHD in remission (79% had severe cGVHD vs 37%, P = .006) (Table 1). In the whole group of cGVHD patients (active or in remission), patients with moderate and severe cGVHD had lower Breg frequencies (1.3% and 1.5% in the “moderate” and “severe” groups) than patients with mild cGVHD (3.0%, P = .06 and P = .004, respectively) (Figure 1C). Altogether, these data indicate that the differences in the Breg frequencies between patients with active cGVHD and patients with cGVHD in remission are more closely linked to the cGVHD status than to other baseline characteristics.
To further investigate any possible effect of corticosteroids on the differences in Breg frequencies observed in vivo, we prospectively analyzed Breg frequencies in 6 patients treated with systemic corticosteroids (mean dose of oral prednisone, 57 mg, range: 30-100) for various pathologic conditions before and after (mean, 11 days) the initiation of the treatment. There was no significant difference between the Breg frequencies before (median, 7.3%) and after treatment (6.5%, P = .32) (supplemental Figure 2). Last, we restudied Breg frequencies in 8 patients with cGVHD from the initial cohort after they had received immunosuppressive treatments for an extended period of time (mean, 6 months; range, 3-9). The mean change in patients with remission of cGVHD was +440% (range, +120% to +700%) vs + 20% (range, −60% to +60%) in patients with stable or worsening symptoms (Figure 1D). These data indicate that corticosteroids alone did not significantly impact Breg frequencies in the absence of change in the clinical activity of the disease.
The dynamics of Bregs could be evaluated in 5 patients treated with extracorporeal photopheresis (ECP) for steroid-refractory cGVHD. In these 5 patients who had a favorable response to ECP, Breg frequencies increased at 1 month after ECP start (M1, median Breg cell frequency, 3.6%) compared with baseline (M0, median, 1.1%). Breg frequencies had further increased at M3 (median, 5.2%) compared with M1 (P = .003) (supplemental Figure 3). Taken together, these results suggest a role of IL-10–producing Bregs in the regulation of cGVHD after AHSCT.
Alteration of STAT3 and Erk signaling in B cells from cGVHD patients
Bregs are not confined to a single cell subset and IL-10 competence remains the best marker to define human Bregs.6,7,21 B-cell IL-10 production may be triggered by CpG-B6,7 as well as IL-21.42 Intracellular signaling pathways that are critical for human B-cell IL-10 production include STAT321,43 and the Erk kinase.43 We evaluated Erk and STAT3 pathways in total CD19+ B cells from AHSCT patients with and without cGVHD and in healthy donors. Thus, these experiments potentially reflect the activation of Bregs.
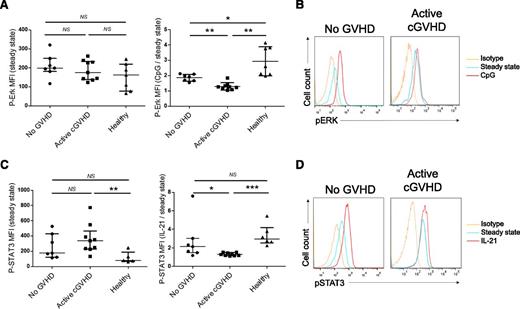
The basal level of phosphorylated Erk (without in vitro activation) was not significantly different between AHSCT recipients with or without cGVHD and healthy donors (Figure 2A, left panel). After incubation with CpG-B, significant phosphorylation of Erk was observed in healthy donors (phospho-Erk median fluorescence intensity [MFI] ratio activated/basal: 2.6) and in AHSCT recipients without cGVHD (1.9), which was less important in patients with active cGVHD (1.3, P = .001) (Figure 2A, right panel, B). Of note, all patients in the cGVHD group in this experiment were receiving immunosuppressive therapies, 4 of them receiving more than what is equivalent to 10 mg of prednisone per day. Patients receiving the highest doses of steroids did not have impaired phosphorylation of Erk (supplemental Figure 4). Higher basal levels of phospho-STAT3 were seen in active cGVHD patients compared with healthy donors and AHSCT recipients without cGVHD (Figure 2C, left panel). IL-21 induced significant phosphorylation of STAT3 in all healthy donors (phospho-STAT3 MFI ratio activated/basal: 3.0) and to a lesser extent in AHSCT recipients without cGVHD (2.1). Patients with active cGVHD had significantly lower phosphorylated STAT3 after IL-21 treatment (phospho-STAT3 MFI ratio activated/basal: 1.3), as compared with patients with no cGVHD (P = .01) and to healthy donors (P < .001) (Figure 2C, right panel, D).
Alteration of STAT3 and Erk signaling in B cells from cGVHD patients. (A) Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, and healthy donors were left unstimulated (medium alone, left) or stimulated with CpG-B 10 μg/mL for 2 hours at 37°C (right), fixed, permeabilized, and incubated with anti-CD19 and phospho-Erk 1/2 mAbs. Phospho-Erk MFIs in CD19+ cells are shown at baseline (left) and after CpG stimulation as a ratio activated/baseline phospho-Erk MFI (right). (B) Representative histograms of phospho-Erk MFI in CD19+ cells at baseline (blue), after CpG stimulation (red), isotypic control (yellow) in an AHSCT recipient with no GVHD and a cGVHD patient. (C) Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, and healthy donors were left unstimulated (medium alone, left) or stimulated with recombinant human IL-21 200 ng/mL for 2 hours at 37°C (right), fixed, permeabilized, and incubated with anti-CD19 and phospho-STAT3 mAbs. Phospho-STAT3 MFI in CD19+ cells are shown at baseline (left) and after IL-21 stimulation as a ratio activated/baseline phospho-STAT3 MFI (right). (D) Representative histograms of phospho-STAT3 MFI in CD19+ cells at baseline (blue), after IL-21 stimulation (red), isotypic control (yellow) in an AHSCT recipient with no GVHD and a cGVHD patient.
Alteration of STAT3 and Erk signaling in B cells from cGVHD patients. (A) Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, and healthy donors were left unstimulated (medium alone, left) or stimulated with CpG-B 10 μg/mL for 2 hours at 37°C (right), fixed, permeabilized, and incubated with anti-CD19 and phospho-Erk 1/2 mAbs. Phospho-Erk MFIs in CD19+ cells are shown at baseline (left) and after CpG stimulation as a ratio activated/baseline phospho-Erk MFI (right). (B) Representative histograms of phospho-Erk MFI in CD19+ cells at baseline (blue), after CpG stimulation (red), isotypic control (yellow) in an AHSCT recipient with no GVHD and a cGVHD patient. (C) Whole PBMCs from AHSCT recipients with no GVHD, active cGVHD, and healthy donors were left unstimulated (medium alone, left) or stimulated with recombinant human IL-21 200 ng/mL for 2 hours at 37°C (right), fixed, permeabilized, and incubated with anti-CD19 and phospho-STAT3 mAbs. Phospho-STAT3 MFI in CD19+ cells are shown at baseline (left) and after IL-21 stimulation as a ratio activated/baseline phospho-STAT3 MFI (right). (D) Representative histograms of phospho-STAT3 MFI in CD19+ cells at baseline (blue), after IL-21 stimulation (red), isotypic control (yellow) in an AHSCT recipient with no GVHD and a cGVHD patient.
IL-10 production is enriched in the CD24hiCD27+ and the CD24−CD27hiCD38hi plasmablast B-cell pools in healthy patients and in AHSCT recipients with or without cGVHD
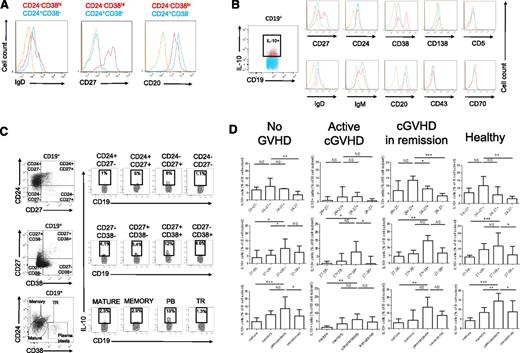
Previous studies showed that Bregs were significantly increased either in the CD24hiCD38hi transitional B-cell population21 or in the CD24hiCD27+ B-cell compartment.7 In our previous work, we did not identify a particular IL-10–producing B-cell compartment after CpG sole stimulation but an enrichment of these cells in the CD27hi and CD38hi B-cell pools.6 We evaluated the phenotype of IL-10–producing B cells in healthy individuals, and AHSCT recipients with and without cGVHD after CpG in vitro stimulation using a backgating strategy on IL-10+ vs IL-10− B cells. Sixty-six hours of CpG stimulation induced a slight increase of the CD38 expression and decrease of the CD24 expression by B cells but no significant changes in the proportions of the different B-cell subsets (supplemental Figure 5). As HSCT patients may have abnormal B-cell markers due to disturbed reconstitution of the immune system,44 we wanted to confirm that the CD19+CD24−CD38hi population expressed other markers usually used to identify plasmablast cells. The CD24−CD38hi B-cell subset was CD20loIgD−CD27hi, consistent with a plasmablastic phenotype (Figure 3A). As shown in Figure 3B (taken from a healthy donor), IL-10–producing B cells were not exclusively restricted to the CD27+ or CD38+ compartment. IL-10–producing B cells were enriched in the CD27hi, CD38hi compartments as compared with IL-10− cells. IL-10+ cells did not significantly overexpress CD20, CD24, or CD138. As expected, CD138+ cells were extremely rare in peripheral blood.
IL-10 production is enriched in the CD24hiCD27+ and the CD24−CD27hiCD38hi plasmablast B-cell pools in healthy patients and in AHSCT recipients with or without cGVHD. Whole PBMCs were cultured for 66 hours with CpG-B (3 μg/mL, left panel) followed by restimulation with PMA + ionomycin in the presence of brefeldin A the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in live CD19+ cells by flow cytometry. (A) Histograms of the expression of IgD, CD27, and CD20 by CD19+CD24−CD38hi plasmablasts (red) and CD19+CD24+CD38− cells (blue) vs control isotype (orange) in CD19+ cells from a representative AHSCT recipient. (B) Determination of the cell surface phenotype of human IL-10+ B cells. Representative histograms of the expression of CD27, CD24, CD38, CD138, CD5, IgD, IgM, CD20, CD43, CD70, in IL-10+ B cells (red) vs IL-10− B cells (blue) and control isotype (orange) in a healthy donor. (C) Dot plots of CD19+IL-10+ cells in B-cell subsets defined by the expression of CD24 and CD27 (top panel), CD27 and CD38 (middle), CD24 and CD38 (bottom panel) in a representative allogeneic transplant recipient with no cGVHD. Dot plots are gated on live CD19+ cells. (D) IL-10+ cells in proportion to each B-cell subset defined by the expression of CD24 and CD27 (top panel), CD27 and CD38 (middle), CD24 and CD38 (bottom panel) in healthy donors (n = 14), patients with no cGVHD (n = 9), patients with active cGVHD (n = 6), and cGVHD in remission (n = 8). Dot plots are gated on live CD19+ cells. PB, plasmablast; TR, transitional B cell.
IL-10 production is enriched in the CD24hiCD27+ and the CD24−CD27hiCD38hi plasmablast B-cell pools in healthy patients and in AHSCT recipients with or without cGVHD. Whole PBMCs were cultured for 66 hours with CpG-B (3 μg/mL, left panel) followed by restimulation with PMA + ionomycin in the presence of brefeldin A the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in live CD19+ cells by flow cytometry. (A) Histograms of the expression of IgD, CD27, and CD20 by CD19+CD24−CD38hi plasmablasts (red) and CD19+CD24+CD38− cells (blue) vs control isotype (orange) in CD19+ cells from a representative AHSCT recipient. (B) Determination of the cell surface phenotype of human IL-10+ B cells. Representative histograms of the expression of CD27, CD24, CD38, CD138, CD5, IgD, IgM, CD20, CD43, CD70, in IL-10+ B cells (red) vs IL-10− B cells (blue) and control isotype (orange) in a healthy donor. (C) Dot plots of CD19+IL-10+ cells in B-cell subsets defined by the expression of CD24 and CD27 (top panel), CD27 and CD38 (middle), CD24 and CD38 (bottom panel) in a representative allogeneic transplant recipient with no cGVHD. Dot plots are gated on live CD19+ cells. (D) IL-10+ cells in proportion to each B-cell subset defined by the expression of CD24 and CD27 (top panel), CD27 and CD38 (middle), CD24 and CD38 (bottom panel) in healthy donors (n = 14), patients with no cGVHD (n = 9), patients with active cGVHD (n = 6), and cGVHD in remission (n = 8). Dot plots are gated on live CD19+ cells. PB, plasmablast; TR, transitional B cell.
Multiple color staining of IL-10+ vs IL-10− B cells revealed a significantly higher frequency of IL-10+ B cells within both the CD27+ B-cell subsets (Figure 3C, higher panel), and the CD27hiCD38hi and CD24−CD38hi B-cell subsets (Figure 3C, lower panels), the last phenotype being consistent with a plasmablast B-cell population.
We next determined the relative frequency of IL-10–producing B cells in each B-cell subset defined by the CD24/CD27/CD38 antigens. As shown in Figure 3D, the highest frequencies of IL-10–producing B cells were found within the CD24hiCD27+, CD24−CD38hi, and CD27hiCD38hi plasmablast B-cell subsets in AHSCT recipients without cGVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors.
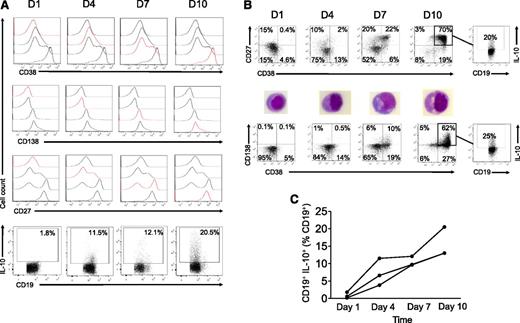
Plasmablasts and long-lived plasma cells can be expanded in vitro from purified human circulating B cells using CpG, CD40L, IL-2, IL-6, IL10, IL-15, and IFN-α in a 3-step/10-day sequential procedure.41 The in vitro differentiation of human B cells into human plasmablasts resulted in a high rate of cell death but the resulting population corresponded phenotypically (CD27hiCD38hiCD138+/−) and morphologically (Figure 4A-B) to plasmablasts and plasma cells. Strikingly, the median frequencies of IL-10–producing B cells were 0.6% at day 1, 6.6% at day 4, 9.7% at day 7, and 13.0% at day 10 (P = .04) (Figure 4C). The highest frequencies of IL-10–producing cells in B-cell subsets were found at day 10 within CD27hiCD38hi plasmablastic (up to 20%) and CD38+CD138+ plasma cell–like (up to 25%) B cells.
In vitro plasmablast and plasma cell differentiation increases B-cell IL-10 production. Purified B cells from a healthy donor were cultured for 4 days with crosslinked recombinant human CD40L, CpG-B, IL-2, IL-10, and IL-15, followed by a 3-day culture with IL-2, IL-6, IL-10, and IL-15 and a 3-day plasma cell differentiation with IL-6, IL-15, and IFN-α as described.41 B-cell surface phenotypes (on freshly harvested cells) and intracellular IL-10 (after 5-hour PMA + ionomycin stimulation) were assessed by flow cytometry at days 1, 4, 7, and 10. Data are representative of 3 experiments. (A) Histograms of the expression of CD27, CD38, and CD138 by cultured B cells at days 1, 4, 7, and 10 (top panels). CD19+IL-10+ cells in proportion to total live CD19+ cells at the corresponding time points (bottom panel). (B) B-cell phenotypes and morphology (after May-Grünwald-Giemsa staining of the cytospinned cells) and IL-10 production by the expanded CD27hiCD38hiCD138+ B-cell pool at day 10. (C) CD19+IL-10+ cells as determined by flow cytometry in proportion of live CD19+ at days 1, 4, 7, and 10 in 3 independent experiments.
In vitro plasmablast and plasma cell differentiation increases B-cell IL-10 production. Purified B cells from a healthy donor were cultured for 4 days with crosslinked recombinant human CD40L, CpG-B, IL-2, IL-10, and IL-15, followed by a 3-day culture with IL-2, IL-6, IL-10, and IL-15 and a 3-day plasma cell differentiation with IL-6, IL-15, and IFN-α as described.41 B-cell surface phenotypes (on freshly harvested cells) and intracellular IL-10 (after 5-hour PMA + ionomycin stimulation) were assessed by flow cytometry at days 1, 4, 7, and 10. Data are representative of 3 experiments. (A) Histograms of the expression of CD27, CD38, and CD138 by cultured B cells at days 1, 4, 7, and 10 (top panels). CD19+IL-10+ cells in proportion to total live CD19+ cells at the corresponding time points (bottom panel). (B) B-cell phenotypes and morphology (after May-Grünwald-Giemsa staining of the cytospinned cells) and IL-10 production by the expanded CD27hiCD38hiCD138+ B-cell pool at day 10. (C) CD19+IL-10+ cells as determined by flow cytometry in proportion of live CD19+ at days 1, 4, 7, and 10 in 3 independent experiments.
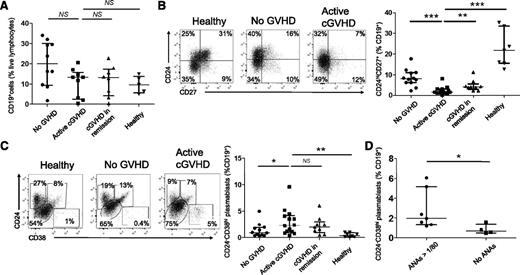
Defective CD24hiCD27+ B-cell and enriched plasmablast B-cell pools in cGVHD patients
To further characterize B-cell homeostasis after AHSCT, we analyzed the CD24+CD38+ transitional, the CD24hiCD27+, and the CD24−CD38hi plasmablast B-cell pools in cGVHD patients. Patients with either active cGVHD or in remission of cGVHD tended to have a lower proportion of CD19+ B cells to live lymphocytes (13.4% and 12.9%, respectively) than AHSCT recipients with no cGVHD (20%) (Figure 5A). Despite comparable follow-up lengths (mean follow-up without cGVHD 41 months vs 44 months with cGVHD), the proportion of the CD24hiCD27+ B-cell subset was significantly reduced in active cGVHD patients (1.6%) in comparison with cGVHD patients in remission (4.1%, P = .005), AHSCT patients without cGVHD (8.1%, P = .0002), and healthy donors (21.8%, P = .0003) (Figure 5B). This finding is consistent with a defective CD24hiCD27+ B-cell reconstitution in cGVHD patients.45
Defective CD24hiCD27+ B-cell and enriched plasmablast B-cell pools in cGVHD patients. (A) Percentages of CD19+ cells in proportion to live lymphocytes in AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors. (B) Dot plots of the expression of CD24 and CD27 by live CD19+ cells in a representative healthy donor, patient with no GVHD, and with cGVHD (left panel). Proportions of CD24hiCD27+ B cells in live CD19+ cells from allogeneic recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors (right panel). (C) Dot plots of the expression of CD24 and CD38 by live CD19+ cells in a representative healthy donor, patient with no GVHD, and with cGVHD (left panel). Proportions of CD24−CD38hi B cells in live CD19+ cells from AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors (right panel). (D) Proportions of CD24−CD38hi B cells in live CD19+ cells from patients with cGVHD (active or in remission) according to the presence or absence of ANAs (titer ≥1/80).
Defective CD24hiCD27+ B-cell and enriched plasmablast B-cell pools in cGVHD patients. (A) Percentages of CD19+ cells in proportion to live lymphocytes in AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors. (B) Dot plots of the expression of CD24 and CD27 by live CD19+ cells in a representative healthy donor, patient with no GVHD, and with cGVHD (left panel). Proportions of CD24hiCD27+ B cells in live CD19+ cells from allogeneic recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors (right panel). (C) Dot plots of the expression of CD24 and CD38 by live CD19+ cells in a representative healthy donor, patient with no GVHD, and with cGVHD (left panel). Proportions of CD24−CD38hi B cells in live CD19+ cells from AHSCT recipients with no GVHD, patients with active cGVHD, patients with cGVHD in remission, and healthy donors (right panel). (D) Proportions of CD24−CD38hi B cells in live CD19+ cells from patients with cGVHD (active or in remission) according to the presence or absence of ANAs (titer ≥1/80).
In contrast, cGVHD patients had a relative expansion of the CD24−CD38hi plasmablast B-cell pool (2.3% vs 0.4% in healthy donors; P = .001, 1.0% in AHSCT recipients without cGVHD; P = .05, and 1.9% in patients with cGVHD in remission; P = .78) (Figure 5C). These results are in line with previously published data in autoimmune diseases reporting an increase in the plasmablast B-cell population.46 Some cGVHD patients may develop connective tissue disease-like manifestations of cGVHD, leading us to compare plasmablast frequencies among B cells in cGVHD patients with antinuclear antibodies (ANA titer >1/80) and without ANAs. The frequency of plasmablasts was greatly increased in the ANA group (2.0%) vs cGVHD patients with no ANAs (0.7%, P = .02) (Figure 5D).
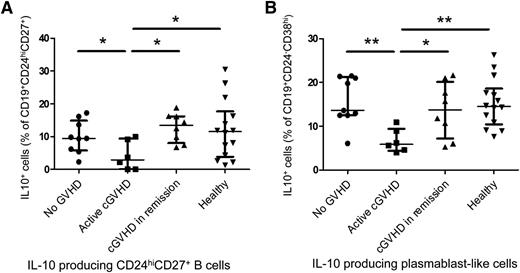
Patients with cGVHD have defective CD24hiCD27+ and plasmablast-like IL-10–producing B-cell compartments
The CD24hiCD27+ B-cell population can inhibit monocyte activation in humans.7 After demonstrating that this B-cell population was enriched in IL-10–producing B cells in healthy and AHSCT patients, we compared CD24hiCD27+IL-10+ B-cell frequencies between the groups of patients. As depicted in Figure 6A, patients with active cGVHD had decreased CD24hiCD27+ IL-10–producing B-cell frequencies (2.9%), in proportion to CD24hiCD27+ cells, compared with patients with cGVHD in remission (13.5%, P = .02), healthy patients (11.5%, P = .04), allogeneic recipients with no cGVHD (9.5%, P = .05). IL-10–producing plasmablast frequencies were lower in cGVHD patients (5.9%) than in healthy donors (14.5%, P = .002), patients with cGVHD in remission (13.8%, P = .04), and allogeneic recipients with no cGVHD (13.6%, P = .006) (Figure 6B).
Patients with cGVHD have defective CD24hiCD27+ and plasmablast-like IL-10–producing B-cell compartments. Whole PBMCs were cultured for 66 hours with CpG-B (3 μg/mL, left panel) followed by restimulation with PMA + ionomycin in the presence of brefeldin A the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in live B-cell subsets by flow cytometry. (A) Proportions of CD19+CD24hiCD27+IL-10+ cells in percentages of live CD19+CD24hiCD27+ cells in healthy donors, patients with active cGVHD, cGVHD in remission, and AHSCT recipients with no history of cGVHD. (B) Proportions of CD19+CD24−CD38hiIL-10+ plasmablast-like cells in percentages of live CD19+CD24−CD38hi cells in healthy donors, patients with active cGVHD, cGVHD in remission, and AHSCT recipients with no history of cGVHD.
Patients with cGVHD have defective CD24hiCD27+ and plasmablast-like IL-10–producing B-cell compartments. Whole PBMCs were cultured for 66 hours with CpG-B (3 μg/mL, left panel) followed by restimulation with PMA + ionomycin in the presence of brefeldin A the last 5 hours of culture, fixed, permeabilized, and intracellular IL-10 was measured in live B-cell subsets by flow cytometry. (A) Proportions of CD19+CD24hiCD27+IL-10+ cells in percentages of live CD19+CD24hiCD27+ cells in healthy donors, patients with active cGVHD, cGVHD in remission, and AHSCT recipients with no history of cGVHD. (B) Proportions of CD19+CD24−CD38hiIL-10+ plasmablast-like cells in percentages of live CD19+CD24−CD38hi cells in healthy donors, patients with active cGVHD, cGVHD in remission, and AHSCT recipients with no history of cGVHD.
Discussion
The pathophysiology of cGVHD involves impaired regulatory mechanisms of tolerance between recipient tissues and donor-derived immunity. Treg homeostasis is altered in cGVHD and adoptive transfer or in vivo induction of Tregs may prevent or improve the symptoms of cGVHD.2-5 Our understanding of the function of Bregs in this disease is limited. IL-10–producing B cells have been shown to inhibit CD4 T-cell proliferation,6 TNF-α and IFN-γ production,21 and monocyte activation7 in in vitro coculture experiments.
In this prospective study of 69 AHSCT recipients including 47 patients with cGVHD, we found a striking Breg defect in cGVHD. CD24hiCD27+ and plasmablast B cells were the 2 compartments most enriched in Bregs. The Breg defect was associated with a disturbed B-cell homeostasis, including CD24hiCD27+ B-cell deficiency and increased CD24−CD38hi plasmablast cells. This Breg defect was marked in the CD24hiCD27+ and plasmablast B-cell compartments. The IL-10 B-cell frequencies were influenced by the cGVHD activity and severity rather than by the immunosuppressive therapies delivered at the time of sampling. The specific ability of CD24hiCD27+ IL-10–producing B cells and IL-10–producing plasmablast cells to regulate the alloimmune response will require further functional experiments.
We showed that B cells from patients with cGVHD were refractory to CpG and IL-21 stimulation, 2 signaling pathways that are critical for B-cell IL-10 production in mice42 and humans.43 B cells from cGVHD patients had decreased phosphorylation of Erk in response to CpG stimulation. Beside its role in inducing IL-10 production42,43 in B cells, IL-21 produced by human follicular helper T cells and CD4 Th17 cells is also critical for human plasmablast differentiation.47-50 Elevated levels of IL-2151 and IL-21–producing cells52 have been found in human cGVHD and a deleterious role of IL-21 has been demonstrated in mouse models of cGVHD.25,52 Accordingly, we found relatively high basal levels of phosphorylated STAT3 as well as increased circulating plasmablasts in cGVHD patients in our study.
Breg frequencies were increased after ECP treatment in 5 patients. ECP possibly induces Tregs through incompletely elucidated mechanisms involving the induction of apoptotic cells.53 Such a role in the induction of Bregs has not been demonstrated to date, although BAFF levels have been linked to the response of cGVHD to ECP.54 A validation in a higher number of patients would be of interest.
In a recent study by Khoder et al mostly focused on healthy subjects, IL-10–producing Bregs were found to be enriched in the CD24hiCD38hi transitional and IgM+CD27+ “IgM memory” B-cell compartments in healthy donors. The results in the 11 patients with cGVHD in this same study also suggested that IL-10–producing B cells were less frequent in cGVHD patients than in healthy controls and AHSCT recipients without cGVHD.24 Although we detected a significant concentration of Bregs in the CD27+ B-cell compartment, we could not replicate the findings of specific enrichment either in the transitional or in the CD5+ B-cell compartment as previously described.55,56 Several factors may explain these discrepancies. First, our study was focused on cGVHD patients and included a higher number of patients: 47 patients vs 11 in the study by Khoder et al. Second, the stimulations used to study B-cell IL-10 production were different. Khoder et al used CD40L-transfected fibroblasts, whereas we used CpG, a TLR9 agonist, with or without recombinant human CD40L, as established by our group6,57 and others.7,23,43,58,59
In vitro–induced CD38hiCD138+ plasma cells were able to secrete large quantities of IL-10. These results are consistent with the work by Shen et al29 and Matsumoto et al31 who showed that CD138+ plasma cells and plasmablast cells were the main IL-10 producers in mice with experimental autoimmune encephalomyelitis. In vitro–expanded IL-10–producing B cells markedly inhibited disease symptoms when transferred into mice with established autoimmune disease.42 IL-10 production by human plasmablasts has not been examined in detail, although a recent study suggested that human plasmablasts were a major source of B-cell–derived IL-10.31 Previous studies provided evidence that IL-10–producing B cells had a transcriptional profile close to that of antibody-secreting cells and overexpressed PRDM1, IRF4, AICDA, and CD27 in human60 and in murine models.61 Our results show that human plasmablasts are enriched in IL-10–producing B cells. Maseda et al have shown that Bregs produce autoantibodies in mice61 and it would be interesting to know whether this also happens in humans, especially in the context of cGVHD. The role of in vitro–expanded IL-10–producing Bregs in the prevention or treatment of human GVHD also merits further investigation.
In conclusion, our results suggest a role for plasmablast and C24hiCD27+ B-cell–derived IL-10 and Bregs in the regulation of human cGVHD. The imbalance between CD24hiCD27+ B cells and plasmablasts provides evidence that altered GC reactions may participate in the defective tolerance in cGVHD. GC reactions are hardly accessible for studies in humans, but have indeed been shown to play a role in mouse models of cGVHD.25,26 Further mechanistic studies will define the accurate role of the different B cells subsets in regulating the alloimmune response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Michel Jourdan (INSERM Unité 1040, Institut de Recherche en Biothérapie, Hôpital Saint-Eloi, Montpellier) and Dr Paul A. Blair (Centre for Rheumatology, University College London, United Kingdom) for valuable technical advice, as well as the patients who contributed to this study.
This work was supported by a grant from the Société Française de Dermatologie and Association d'Entraide aux Greffés de Moëlle Osseuse. A.d.M. was supported by Institut National du Cancer/Institut Thématique Multi-Organismes Cancer.
Authorship
Contribution: A.d.M. and J.-D.B. performed experiments and designed, interpreted, analyzed, and wrote the manuscript; H.L.B. provided advice and technical assistance; V.D. provided technical help; D.B. performed experiments and analyzed results; M. Robin., A.O., N.P., E.H., J.-B.M., M. Branchtein, M. Rybojad., D.M., F.S.d.F., A. Bergeron., A.X., R.I., N.D., R.P.d.L., A.X., and M. Bagot provided patient samples and commented on the manuscript; and M. Bagot, A. Bensussan., and G.S. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerard Socie, Hematology/Immunology/Oncology Division, Transplant Program, INSERM UMRS 1160, University Paris VII, AP-HP, Saint Louis Hospital, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: gerard.socie@sls.aphp.fr; and Jean-David Bouaziz, Hôpital Saint Louis, INSERM U976, Equerre Bazin, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: jean-david.bouaziz@sls.aphp.fr.
References
Author notes
A.d.M. and J.-D.B. contributed equally to this study.
A. Bensussan and G.S. contributed equally to this study.