Key Points
A novel gain-of-function mutation in factor V leading to increased levels of TFPI and bleeding was identified by whole exome sequencing.
Factor V Amsterdam (F5 C2588G) resembles the mutation (F5 A2350G) leading to East Texas bleeding disorder.
Abstract
We investigated a small Dutch family with a bleeding diathesis, prolonged prothrombin, and activated partial thromboplastin times, in whom no classifying diagnosis was made. The 2 affected relatives had severely decreased in vitro thrombin generation, and levels of tissue factor pathway inhibitor (TFPI) were strongly increased. To identify the genetic cause of the bleeding diathesis, we performed whole exome sequencing analysis of all living relatives. We found a novel gain-of-function mutation in the F5 gene (c.C2588G), which leads to an aberrant splicing of F5 and ultimately to a short factor V protein (missing 623 amino acids from the B domain), which we called factor V Amsterdam. Factor V Amsterdam binds to TFPI, prolonging its half-life and concentration. This is the second report of an association between a shorter form of factor V and increased TFPI levels, resulting in severely reduced thrombin generation and a bleeding tendency.
Introduction
In clinical practice, we occasionally encounter patients with a bleeding tendency and prolonged coagulation screening tests, in whom a classifying diagnosis cannot be made. Here, we describe our search toward elucidating the etiology of a bleeding tendency in a mother and her son.
Study design
Subjects and samples
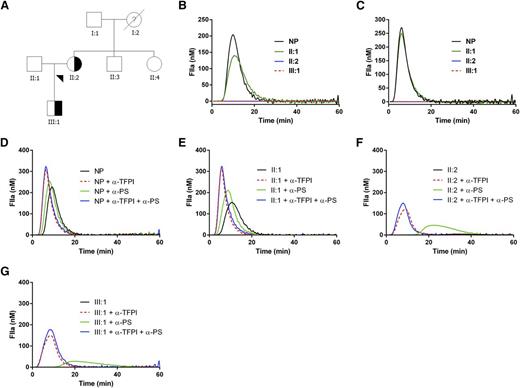
The pedigree of the family is depicted in Figure 1A. Detailed clinical features of the family are provided in supplemental Table 1, available on the Blood Web site. We collected blood from the mother (II:2) and son (III:1) with clinical manifestations of bleeding and 4 unaffected relatives. All participants provided written informed consent.
Pedigree of the family with the inherited bleeding disorder and thrombin generation in the affected relatives. (A) Pedigree of the family with the inherited bleeding disorder. Affected individuals are indicated by shapes filled half-way; unaffected individuals are indicated by nonfilled shapes; and nonfilled shapes with ? indicate disease status unknown. Blood samples were collected from all living relatives to assess coagulation parameters and ultimately to perform whole exome sequencing. (B-G) Thrombin generation in the affected relatives. Thrombin generation was initiated with (B) 1 or (C) 5 pM tissue factor in normal pooled plasma (NP), the proband (II:2), her son (III:1), and husband (II:1). Thrombin generation could be restored in the affected relatives by addition of blocking antibodies against TFPI or protein S. Thrombin generation was initiated with 1 pM tissue factor in (D) normal pooled plasma (NP), (F) the proband (II:2), (G) her son (III:1), and (E) husband (II:1) in the absence or presence of blocking antibodies against TFPI or protein S. Similar results were obtained with 5 pM tissue factor (data not shown).
Pedigree of the family with the inherited bleeding disorder and thrombin generation in the affected relatives. (A) Pedigree of the family with the inherited bleeding disorder. Affected individuals are indicated by shapes filled half-way; unaffected individuals are indicated by nonfilled shapes; and nonfilled shapes with ? indicate disease status unknown. Blood samples were collected from all living relatives to assess coagulation parameters and ultimately to perform whole exome sequencing. (B-G) Thrombin generation in the affected relatives. Thrombin generation was initiated with (B) 1 or (C) 5 pM tissue factor in normal pooled plasma (NP), the proband (II:2), her son (III:1), and husband (II:1). Thrombin generation could be restored in the affected relatives by addition of blocking antibodies against TFPI or protein S. Thrombin generation was initiated with 1 pM tissue factor in (D) normal pooled plasma (NP), (F) the proband (II:2), (G) her son (III:1), and (E) husband (II:1) in the absence or presence of blocking antibodies against TFPI or protein S. Similar results were obtained with 5 pM tissue factor (data not shown).
Coagulation testing and exome sequencing
Coagulation parameters were determined by standard methods. Thrombin generation was performed with calibrated automated thrombography. Genomic DNA was isolated from peripheral blood mononuclear cells. Direct exome sequencing of the TFPI and PROS1 genes was performed in the 2 patients and 1 unaffected relative. Whole exome sequencing data were generated for all relatives, and candidate mutations were confirmed by Sanger sequencing. Details of methods are available in supplemental Materials.
Results and discussion
Thrombin generation was measured in plasma from individuals II:1 (unaffected husband), II:2 (proband), and III:1 (affected son). Thrombin generation initiated with 1 pM tissue factor was severely affected in the proband and son (Figure 1B); this also occurred at higher concentrations of tissue factor (5 pM, Figure 1C; 20 pM, data not shown). Mixing patient plasma with normal plasma did not normalize thrombin generation, suggesting the presence of a circulating inhibitor (data not shown). Because lupus anticoagulant and deficiencies of antithrombin and protein C had been excluded in both patients and the deficiency of protein S found in the mother could not explain the bleeding tendency and was not shared by the son, we assessed levels of the natural inhibitor tissue factor pathway inhibitor (TFPI). The levels of TFPI total antigen (reference range, 51-112 ng/mL) and TFPI free antigen (reference range, 5.2-14.8 ng/mL) were strongly increased in both affected individuals: 433 and 149 ng/mL for II:2 and 312 and 109 ng/mL for III:1, respectively. Likewise, the TFPI activity (reference range, 39-149%) was also strongly increased (295% for II:2 and 271% for III:1; supplemental Table 1). Blocking anti-protein S antibodies slightly improved thrombin generation. Blocking anti-TFPI antibodies (0.33 µM, mixture of antibodies against Kunitz-1, Kunitz-2, Kunitz-3, and the C terminus of TFPIα in a 1:1:1:1 ratio) restored thrombin generation to a much larger extent than blocking anti-protein S antibodies (Figure 1D-G). Combination of blocking anti-TFPI and anti-protein S antibodies resulted in similar thrombin generation as with only anti-TFPI antibodies. To confirm that increased levels of TFPI reduced thrombin generation and hence may be causally related to the bleeding tendency in this family, we added TFPI to normal pooled plasma and observed strongly diminished thrombin generation (data not shown).
The phenotype of our family resembled the East Texas bleeding disorder,1 of which the mechanism was recently described.2 We therefore excluded the F5 2350A>G mutation known to cause this disease by targeted sequencing of this region in exon 13 of the F5 gene.2 Furthermore, sequencing of PROS1 and TFPI coding regions revealed no previously known mutations shared by the proband and son but demonstrated a single nucleotide polymorphism in the TFPI gene (rs7586970; the proband was homozygous and her son was heterozygous) that we considered to be not causal for the clinical phenotype.
Whole exome sequencing of the 6 relatives was performed, and for all samples, the mean coverage of the target region was ≥114×, and 90.9% to 93.7% of the target region was covered at ≥30×. Assuming a dominant mode of inheritance, we selected all functional rare heterozygous mutations shared by the 2 affected individuals (II:2 and III:1) but not present in any of the unaffected relatives (I:1, II:1, II:3, and II:4). The number of mutations retained after each filter step is shown in supplemental Table 2. All functional rare single nucleotide variants and insertions and deletions retained for evaluation, 79 single nucleotide variants and 8 insertions and deletions, are listed in supplemental Tables 3 and 4 along with the predicted impact on protein function, conservation scores, score of transcription factor binding sites conserved in the human/mouse/rat alignment, and mean allele frequency. An F5 mutation in exon 13, c.2588C>G with an amino acid change p.Ala863Gly and predicted as having a splicing effect, emerged as the most likely candidate to explain the phenotype.
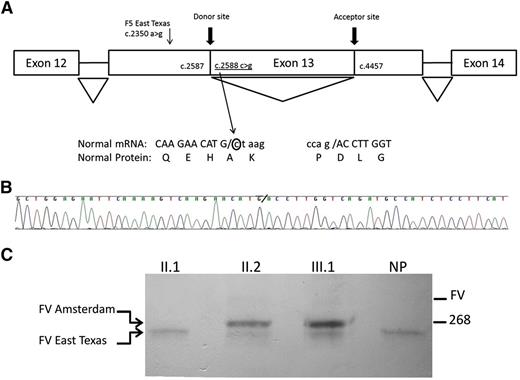
The mutation F5 C2588G [NM_000130.4:c.2588C>G, Chr1(GRCh37):g.169511740G>C] was confirmed by direct sequencing of genomic DNA of the proband and son and was absent in the unaffected relatives. This nucleotide is not conserved among species (phylogenetic P values and genomic evolutionary rate profiling++ scores are 0.039 and 0.451, respectively). The mutation would predict a change in the protein (p.Ala863Gly) but PolyPhen2, alignment with calculation of Grantham variation and Grantham deviation, sorting intolerance from tolerance, MutationTaster, and likelihood ratio test predicted a neutral impact on protein function. However, 4 of the 5 different algorithms used for splice site prediction available in Alamut software (SpliceSiteFinder-like, MaxEntScan, splice site prediction by neural network, and Human Splice Finder) predicted the creation of a strong intron donor site. To confirm that this mutation led to splicing out of part of exon 13, RNA was isolated from mononuclear cells. Sequence analysis revealed an in-frame loss of 1869 nucleotides (acceptor site at position 4457 in exon 13) in the affected individuals resulting in a variant of factor V (FV) that lacked 623 amino acids (Figure 2). Western blotting analysis of plasma of the affected individuals showed the variant form of FV, which was not present in the husband or control plasma (supplemental Figure 1).
Diagram of the novel splicing event, sequencing results of the F5 gene, and direct interaction between FV Amsterdam and TFPI. (A) cDNA sequencing from the polymerase chain reaction product of the proband revealed a sequence that is not concordant with the reference transcript from National Center for Biotechnology Information (NM_000130.4). The F5 mutation created a novel donor site at the coding position 2588, which together with an aberrant acceptor site at position 4457 of exon 13 (same as reported for the East Texas bleeding disorder) leads to a shorter F5 transcript. The new sequence is missing 1869 nucleotides in exon 13 (c.2588_4456del) that results in an in-frame loss of 623 amino acids. Diagram of the gene is not to scale. Adapted from Vincent et al.2 (B) Sequencing results of the new transcript found in the proband. The novel donor splice site is indicated by a backslash. (C) Plasma of individuals was incubated with anti-TFPI-coated beads. Bound proteins were eluted, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted with antibodies against FV. Variant forms of FV can be visualized as indicated. NP, normal plasma.
Diagram of the novel splicing event, sequencing results of the F5 gene, and direct interaction between FV Amsterdam and TFPI. (A) cDNA sequencing from the polymerase chain reaction product of the proband revealed a sequence that is not concordant with the reference transcript from National Center for Biotechnology Information (NM_000130.4). The F5 mutation created a novel donor site at the coding position 2588, which together with an aberrant acceptor site at position 4457 of exon 13 (same as reported for the East Texas bleeding disorder) leads to a shorter F5 transcript. The new sequence is missing 1869 nucleotides in exon 13 (c.2588_4456del) that results in an in-frame loss of 623 amino acids. Diagram of the gene is not to scale. Adapted from Vincent et al.2 (B) Sequencing results of the new transcript found in the proband. The novel donor splice site is indicated by a backslash. (C) Plasma of individuals was incubated with anti-TFPI-coated beads. Bound proteins were eluted, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted with antibodies against FV. Variant forms of FV can be visualized as indicated. NP, normal plasma.
At the time we performed the characterization of this family, the pathophysiology of a similar bleeding phenotype, the East Texas disorder, was reported.2 The causal mutation is a mutation in the B domain of FV (F5 A2350G, according to the latest annotated reference sequence for F5 transcript, NM_000130.4, originally reported as A2440G) that results in a shorter FV molecule. This so-called FV short forms a complex with TFPI, thereby increasing the half-life of TFPI, resulting in an increased plasma concentration. The mutation F5 C2588G, which we designated factor V Amsterdam, was in the vicinity at 238 bp downstream of the East Texas mutation.2
This is the second description of a gain-of-function mutation in F5 that is associated with bleeding in the absence of a procoagulant defect of the molecule. The mutation in the B domain results in removal of a large region including the so-called basic region. The basic region is tightly bound to an acidic region in the B domain, which is an interaction important to maintain FV in an inactive state.3,4 TFPI has a similar basic region in its C terminus that can tightly interact with the acidic region in the B domain of FV. The increased levels of TFPI in individuals with factor V Amsterdam could produce an anticoagulant effect by increasing factor Xa inhibition (with protein S), increasing factor VIIa/tissue factor inhibition, and inhibiting prothrombinase.5 We assume that the absence of the basic region in FV in both bleeding disorders promotes the interaction of TFPI and FV leading to a severe suppression of in vitro, and presumably in vivo, thrombin generation.
To demonstrate a direct interaction between TFPI and factor V Amsterdam, a pull-down experiment was performed with anti-TFPI antibodies. Visualization with FV antibodies demonstrated the presence of factor V East Texas in all tested individuals and control plasma (Figure 2C), confirming that this truncated version of FV is present in normal individuals.2 Factor V Amsterdam was only present in affected individuals. Full-length FV was hardly detected, indicating that TFPI predominantly binds to B domain truncated FV variants in which the acidic region is available for binding to the basic C terminus of TFPI.
In conclusion, we identified a novel mutation in the F5 gene designated factor V Amsterdam, leading to a deletion in the B domain of FV. This led to increased binding to and high levels of TFPI, resulting in severely reduced thrombin generation and a bleeding tendency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the relatives for participating in this research.
This work was funded in part by a grant (2010) from the Rembrandt Institute of Cardiovascular Science, Amsterdam/Leiden, The Netherlands.
Authorship
Contribution: S.M. collected clinical information, informed consent, and patient material; M.L.R.C., K.B., J.P., and J.A.M. performed the laboratory analyses; M.L.R.C. performed the bioinformatics analyses; S.M. and J.C.M.M. wrote the paper with input from all authors; and all authors made a substantial contribution to the concept and design of the study, interpreted the data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joost C. M. Meijers, Department of Experimental Vascular Medicine, Academic Medical Center, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; e-mail: j.c.meijers@amc.uva.nl.
References
Author notes
J.C.M.M. and S.M. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal