Abstract
Recent studies have compelled further interest in the potential pathological role of B cells in chronic graft-versus-host disease (cGVHD). In patients with cGVHD, B cells are activated and primed for survival via B-cell activating factor and B-cell receptor–associated pathways. Understanding the signaling pathways that drive immune pathology in cGVHD will facilitate the development of new strategies to selectively target aberrantly activated B cells and restore normal B-cell homeostasis after allogeneic stem cell transplantation.
Introduction
Chronic graft-versus-host disease (cGVHD) continues to be a common cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).1-3 Conventional immune suppressive therapies are often ineffective, and new therapeutic approaches are needed. The major gaps in our knowledge of cGVHD pathophysiology have recently been reviewed.4,5 Although donor T cells clearly play a critical role in this disease, it is evident that donor B cells also contribute to the immune pathology and tissue damage characteristic of cGVHD.6-11 Heightened B-cell responses in cGVHD result in marked abnormalities in B-cell homeostasis, but the mechanisms responsible for aberrant B-cell homeostasis and the inability to establish B-cell tolerance in patients with cGVHD have not been fully elucidated. Importantly, recent studies have led to a better understanding of the signaling pathways that regulate normal B-cell homeostasis and also appear to play a role in autoimmune diseases. Moreover, small-molecule inhibitors of specific B-cell signaling pathways are now available for clinical use and are being applied in the treatment of B-cell malignancies. These new agents can also be used to identify and potentially modify specific abnormalities of B-cell homeostasis. Development of clinical trials using these agents in patients undergoing allogeneic HSCT will enable the development of new strategies to target B-cell responses for prevention and treatment of cGVHD.
Establishing B-cell tolerance after allogeneic HSCT
The differentiation of mature B cells is a dynamic and highly regulated process that includes both deletion of autoreactive B cells and positive selection of B-cell clones capable of recognizing a broad repertoire of foreign antigens.12 Both B-cell activating factor (BAFF) and B-cell receptor (BCR) signaling play critical roles in this process.13,14 In healthy individuals, B-cell development begins with the continuous production of precursor B cells in the bone marrow that are exported to the periphery as a large pool of transitional B cells. Many of these B cells express autoreactive BCRs.15 Autoreactive B cells are highly BAFF dependent, and low concentrations of BAFF in the B-cell microenvironment are not sufficient to support their survival resulting in their deletion. In contrast, high levels of BAFF promote the differentiation and survival of autoreactive B cells.16,17 BCR signaling is also required for B-cell differentiation and survival, and BCR activation promotes the expression of BAFF receptors. After allogeneic HSCT, donor B-cell reconstitution occurs in the setting of ubiquitous foreign antigens and high levels of BAFF.18-20 The recovering peripheral B-cell compartment in the early post-HSCT period also contains recent bone marrow emigrants consisting of short-lived transitional B cells with high propensity for autoreactivity.21,22 Although these cells are capable of primary immune reactions and can differentiate into short-lived plasma cells, they do not take part in the germinal center (GC) reaction. This unique post-HSCT setting promotes the survival of activated, potentially allo- and autoreactive B cells that would undergo negative selection by deletion without concomitant BCR activation and BAFF receptor engagement. Nevertheless, ongoing deletion of donor-derived B cells that react with recipient tissues is imperative to prevent tissue damage, and failure to achieve B-cell tolerance is observed in patients with cGVHD.
Positive selection of potentially allo- and autoreactive B cells also likely occurs after HSCT, but this has been difficult to study because antigen targets of B- and T-cell responses remain largely unknown in cGVHD. In patients with cGVHD, antibodies to both alloantigens and nonpolymorphic (“auto”) antigens frequently develop.23-25 In cases where specific alloantigens have been defined, such as the DBY minor histocompatibility antigen, coordinated T- and B-cell responses to disparate epitopes on the target protein have been described.26,27 In these cases, T-cell responses were directed against DEAD box helicase, Y-linked (DBY) epitopes shared with DEAD box helicase, X-linked (DBX) and thus were reactive with both female donor cells and male recipient cells. In contrast, anti-DBY antibodies were directed against unique DBY epitopes not present in DBX and were therefore only reactive with male recipient cells. Although genetic disparity between donor and recipient must exist for cGVHD to develop, in murine models, transferable T-cell autoreactivity occurs following development of alloreactivity.9,28,29 Despite the presence of allo- and autoreactive antibodies, cGVHD is associated with a paucity of cells potentially important for immediate response to microbial antigens, such as B1-like cells and other protective B cells.30-32 In addition, low-intermediate affinity alloreactive B-cell clones that escape negative selection in the bone marrow likely undergo positive selection in the periphery during B-cell recovery after HSCT.
In patients with cGVHD, total B-cell ablation with anti-CD20 antibody, rituximab, results in cGVHD improvement or prevention primarily in patients capable of robust B-cell recovery after therapy.33,34 Persistence of naive B lymphopenia after rituximab therapy is associated with cGVHD development or worsening symptoms.33,35 These observations suggest that both high levels of BAFF and limited capacity to generate sufficient numbers of naïve and transitional B cells contribute to abnormal B-cell homeostasis characteristic of cGVHD. In this setting, therapeutic approaches that result in global B-cell depletion may have limited efficacy in the treatment or prevention of cGVHD.33
The cellular composition of the B-cell compartment is also critically important for the maintenance of B-cell homeostasis and immune tolerance. Supranormal numbers of naive and circulating transitional B cells after HSCT are common in patients who do not go on to develop cGVHD.20,36,37 Increased levels of κ-deleting recombination excision circles, rapid naive B-cell recovery, and low BAFF/B-cell ratios after umbilical cord blood transplantation have also been associated with a low incidence of cGVHD.35 In contrast, delayed B-cell recovery in the early post-HSCT period has been linked to both decreased numbers of precursor B cells and decreased bone marrow production of transitional B cells.20,36,38-40 Mensen et al have linked the mechanism underpinning delayed B-cell recovery to osteoblast destruction by infiltrating T cells.41 Taken together, these findings suggest that the generation of large numbers of naive and transitional B cells early after HSCT can successfully compete for available BAFF and promote deletion of alloreactive and autoreactive B cells. Alternatively, supranormal B-cell populations may also contain a significant component of regulatory B cells that promote immune tolerance through different mechanisms.42,43 Although the etiology of delayed B-cell recovery in cGVHD remains an area of active investigation, these data support the critical role of bone marrow lymphogenesis and output for the maintenance of B-cell homeostasis and immune tolerance.
Defining functionally relevant and targetable B-cell subsets in cGVHD
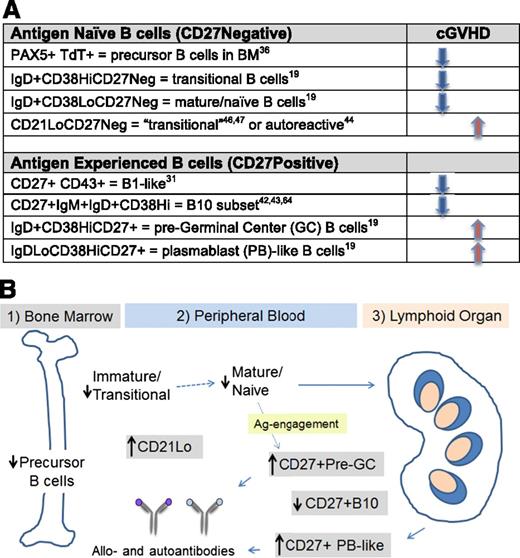
Relative B-cell lymphopenia and persistent high BAFF levels in patients with cGVHD lead to the proportional increase in 3 B-cell subsets of potential functional relevance (Figure 1). CD27−CD21low B cells represent a subset of transitional B cells that appear to be increased in patients with high BAFF/B-cell ratios and relative hypogammaglobulinemia and have been associated with cGVHD severity and response to therapy.44-48 CD27−CD21low B cells are also found in patients with common variable immune deficiency and autoimmune cytopenias and are associated with increased infections in cGVHD.49,50 The potential autoreactive capacity of transitional B cells makes it tempting to hypothesize that these cells also contribute to cGVHD pathophysiology.51,52 Utilizing cell surface markers of B-cell subsets normally found in secondary lymphoid organs,37,53 we have identified peripheral B-cell subsets prevalent in cGVHD.19 Although further studies are required to elucidate precise functional correlates for these B-cell surface phenotypes, their presence in patients with cGVHD suggests potential pathological relevance. As summarized in Figure 1, CD27+ B cell subsets with cell surface markers typically only found on healthy tonsillar pre-GC (IgD+) and post-GC (IgD−) PB-like B cells are detected in peripheral blood and proportionally increased in patients with active cGVHD. Pre-GC B cells express high levels of 2 BAFF receptors, transmembrane activator and CAML interactor and B cell maturation antigen, and these cells are increased when plasma BAFF levels are high.19 Consequently, these cells are typically absent in the peripheral blood of healthy individuals but are found in patients with autoimmune diseases.51,52 CD27+ B-cell subsets present in cGVHD appear to be functionally distinct from “healthy” B-cell subsets because they are able to constitutively produce IgG ex vivo. Thus, rather than antimicrobial recall responses, these CD27+ B cells potentially mediate antirecipient normal tissue responses.17,54 These allo- and autoreactive B cells are likely activated and regulated in both extrafollicular and GC niches in cGVHD.55 Similar murine “memory” B-cell subsets have also been identified, making it possible to examine the pathogenic role of these BCR-activated B-cell subsets in murine models.56,57
Aberrant B cells in cGVHD. (A) Cell surface phenotype of B-cell subsets altered in patients with cGVHD. Most CD27 negative (CD27Neg) B-cell subsets are proportionally decreased in active cGVHD, whereas CD21Lo CD27Neg subsets increase in association with cGVHD activity.44,47 The relative naive B lymphopenia in cGVHD has been associated with a proportional increase in 2 CD27Pos antigen-experienced populations. In contrast, B1 and B10 B-cell subsets are significantly decreased in active cGVHD.42,43 (B) Abnormalities of B-cell homeostasis in different compartments. Aberrant B-cell homeostasis in patients with cGVHD results in abnormal composition of B cells in different sites. B-cell precursors in bone marrow (1). In patients with cGVHD, the production and output of B-cell precursors is decreased. Abnormal composition of B-cell subsets in peripheral blood (2). Both transitional B cells and CD27Neg mature/naive B cells are decreased in frequency in cGVHD. Naive B cells enter secondary lymphoid organs, encounter antigen, and become activated, leading to increased proportions of several B-cell subsets of potential pathological interest. These include the “transitional-like” CD21Lo, the pre-GC IgDHiCD38HiCD27+, and the IgDLoCD38HiCD27Hi populations (all in gray shaded boxes).46 In cGVHD, extrafollicular pre-GC CD27+ B cells and post-GC plasmablast (PB)–like B cells are increased proportionally, whereas immune regulatory B10 cells are decreased. Generation of activated B cells from secondary lymphoid organs (spleen/lymph node) (3). IgDLoCD38HiCD27+ PB-like cells activated in secondary lymphoid tissues produce constitutive IgG and persist in patients with cGVHD who do not respond to B-cell depletion therapy.33
Aberrant B cells in cGVHD. (A) Cell surface phenotype of B-cell subsets altered in patients with cGVHD. Most CD27 negative (CD27Neg) B-cell subsets are proportionally decreased in active cGVHD, whereas CD21Lo CD27Neg subsets increase in association with cGVHD activity.44,47 The relative naive B lymphopenia in cGVHD has been associated with a proportional increase in 2 CD27Pos antigen-experienced populations. In contrast, B1 and B10 B-cell subsets are significantly decreased in active cGVHD.42,43 (B) Abnormalities of B-cell homeostasis in different compartments. Aberrant B-cell homeostasis in patients with cGVHD results in abnormal composition of B cells in different sites. B-cell precursors in bone marrow (1). In patients with cGVHD, the production and output of B-cell precursors is decreased. Abnormal composition of B-cell subsets in peripheral blood (2). Both transitional B cells and CD27Neg mature/naive B cells are decreased in frequency in cGVHD. Naive B cells enter secondary lymphoid organs, encounter antigen, and become activated, leading to increased proportions of several B-cell subsets of potential pathological interest. These include the “transitional-like” CD21Lo, the pre-GC IgDHiCD38HiCD27+, and the IgDLoCD38HiCD27Hi populations (all in gray shaded boxes).46 In cGVHD, extrafollicular pre-GC CD27+ B cells and post-GC plasmablast (PB)–like B cells are increased proportionally, whereas immune regulatory B10 cells are decreased. Generation of activated B cells from secondary lymphoid organs (spleen/lymph node) (3). IgDLoCD38HiCD27+ PB-like cells activated in secondary lymphoid tissues produce constitutive IgG and persist in patients with cGVHD who do not respond to B-cell depletion therapy.33
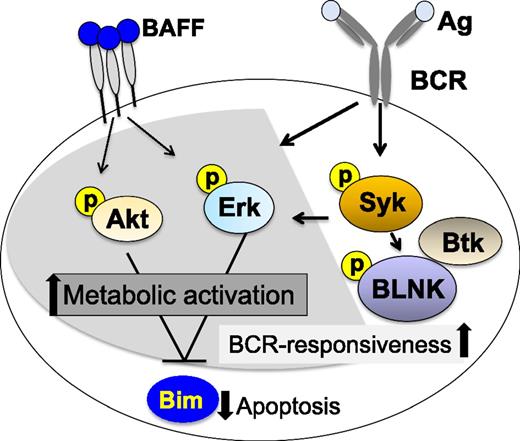
Recent studies have revealed significant increases in B-cell activation in cGVHD. Compared with B cells in patients without cGVHD, purified peripheral cGVHD B cells were found to be enlarged in size and to contain more total protein per cell, indicating heightened metabolic activity in vivo.58 These cells were also resistant to apoptotic death in vitro, and addition of exogenous BAFF further augmented cell size and attenuated cell death. Examination of B-cell signaling pathways by western blot demonstrated that the cell signaling molecules protein kinase B (Akt) and extracellular signal-regulated kinase (Erk) were constitutively activated in cGVHD B cells compared with B cells in patients without cGVHD. A relative decrease in 2 isoforms of the proapoptotic protein Bcl2-interacting mediator of cell death (BIM) was also demonstrated in cGVHD B cells, suggesting a mechanistic link between high BAFF levels, aberrant B-cell signaling, and prolonged survival of activated cells.59 Studies also demonstrated a significant increase in B-cell signaling via the BCR pathway in patients with active cGVHD after exposure to BCR ligand ex vivo. This increased responsiveness to surrogate soluble antigen was related to increased levels of effector proteins critical for downstream BCR signaling and cell activation. In these assays, spleen tyrosine kinase (Syk) and B-linker protein (BLNK) activation could be blocked using a small-molecule Syk inhibitor, fostamatinib (Rigel Pharmaceuticals), suggesting potential therapeutic use in cGVHD. Because Syk is required for positive selection of immature/transitional B cells into the recirculating B-cell pool,60 the reliance of cGVHD B cells on Syk activation affirms the importance of the BCR signaling pathway on promotion of B cells in cGVHD. The effect of BAFF and/or BCR blockade on the recovery of a functional B-cell compartment after HSCT remains to be tested.
Increased activation and survival of cGVHD B cells has also been linked to Bruton tyrosine kinase (Btk), another critical molecule in the BCR signaling pathway.61 As with Syk blockade, treatment with ibrutinib (Pharmacyclics), a Btk inhibitor, blocked Btk and downstream activation of phospholipase C gamma2 (PLCγ2) in cGVHD patient B cells, further suggesting that increased BCR activation contributes to cGVHD pathology. Figure 2 summarizes the B-cell signaling pathways that have been shown to be activated in cGVHD. Taken together, these studies suggest that both BAFF- and BCR-associated signaling pathways likely cooperate to prime cGVHD-mediating B cells for survival and antigen responsiveness.59,61,62 Although constitutive Akt and Erk signaling found in cGVHD B cells may be because of either BAFF or BCR signaling, decreased expression of Bim appears to be related primarily to BAFF signaling. Syk, BLNK, and Btk are primarily activated by BCR signaling in B cells, but Syk may also be required for BAFF signaling under certain circumstances.63 Selective targeting of B-cell signaling pathways has been shown to be an effective therapeutic approach in patients with various B-cell malignancies. The preclinical studies summarized previously suggest that selective inhibition of these pathways may also be effective in patients with cGVHD.
Aberrant B-cell signaling in active cGVHD. B cells in cGVHD exhibit increased constitutive signaling through Erk and Akt that is also associated with decreased levels of proapoptotic Bim. Aberrant BAFF-associated signaling is associated with a heightened metabolic state and resistance to apoptosis. Constitutive BCR-associated signaling in cGVHD B cells is associated with increased responsiveness to surrogate antigen ex vivo, suggesting a mechanistic link between elevated BAFF levels and aberrant B-cell survival. After initiation of BCR signaling, cGVHD B cells exhibited increased BLNK and Syk phosphorylation compared with B cells from patients without cGVHD. Shaded pathways are common to BAFF and BCR signaling. Unshaded pathways are specific for BCR signaling.
Aberrant B-cell signaling in active cGVHD. B cells in cGVHD exhibit increased constitutive signaling through Erk and Akt that is also associated with decreased levels of proapoptotic Bim. Aberrant BAFF-associated signaling is associated with a heightened metabolic state and resistance to apoptosis. Constitutive BCR-associated signaling in cGVHD B cells is associated with increased responsiveness to surrogate antigen ex vivo, suggesting a mechanistic link between elevated BAFF levels and aberrant B-cell survival. After initiation of BCR signaling, cGVHD B cells exhibited increased BLNK and Syk phosphorylation compared with B cells from patients without cGVHD. Shaded pathways are common to BAFF and BCR signaling. Unshaded pathways are specific for BCR signaling.
Evidence now also points to signaling defects in B-cell subsets with immune regulatory capacity. Interleukin-10 production by so-called B10 cells was recently found to be dampened in patients with active cGVHD. Interestingly, B10 cells produced via Toll-like receptor 9 stimulation from active cGVHD but not from non-cGVHD patients had a significantly diminished capacity to phosphorylate Erk.43 The B10 subsets identified in cGVHD include CD24HiCD27+ B cells, previously identified in healthy individuals,64 and a PB-like population, similar to one previously identified in mice.65 Both subsets are CD27+ and, like other B10 cells, likely antigen dependent (Figure 1A).66 Taken together, these recent studies reinforce the notion that function cannot be presumed based on cell surface phenotype alone. Additional studies are required to functionally distinguish B10 cells that potentially mitigate cGVHD from constitutively activated B-cell subsets that are potentially pathogenic.
In summary, the postallogeneic HSCT milieu has unique potential for production of B-cell allo- and autoreactivity. How B cells and/or the antibodies they produce potentially contribute to cGVHD initiation and progression in conjunction with T cells is an area of active investigation.8-10,67 Achievement of B-cell tolerance after HSCT is imperative, but challenging in the context of naive B-cell lymphopenia, persistent stimulation by alloantigen, and infectious and inflammatory signals.19 Current data suggest a critical breakdown in peripheral B-cell tolerance in patients with cGVHD where increased responsiveness to antigen via BCR is associated with poor recovery of the naive B-cell compartment and increased B-cell survival. Defects in the recovery of B cells with regulatory functions may also contribute to abnormal B-cell homeostasis after HSCT. New strategies to block BAFF-mediated survival, augment B-cell lymphopoiesis, and restore normal B-cell homeostasis after HSCT will require further examination in preclinical studies. Additionally, further characterization of the functional capacities of aberrantly activated B-cell subsets from cGVHD patients and testing of novel targeted agents in relevant murine models will help guide the clinical development of urgently needed preventative and curative therapies.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (K08HL107756) and National Cancer Institute (P01CA142106).
Authorship
Contribution: S.S. and J.R. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Sarantopoulos, Division of Cell Therapy and Hematologic Malignancies, Duke Cancer Institute, Box 3961 Jones Building, Room 152, Durham, NC 27710; e-mail: stefanie.sarantopoulos@duke.edu; and Jerome Ritz, Division of Hematologic Malignancies, Dana–Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jerome_ritz@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal