Abstract
Treatment of Hodgkin lymphoma is associated with 2 major types of risk: that the treatment may fail to cure the disease or that the treatment will prove unacceptably toxic. Careful assessment of the amount of the lymphoma (tumor burden), its behavior (extent of invasion or specific organ compromise), and host related factors (age; coincident systemic infection; and organ dysfunction, especially hematopoietic, cardiac, or pulmonary) is essential to optimize outcome. Elaborately assembled prognostic scoring systems, such as the International Prognostic Factors Project score, have lost their accuracy and value as increasingly effective chemotherapy and supportive care have been developed. Identification of specific biomarkers derived from sophisticated exploration of Hodgkin lymphoma biology is bringing promise of further improvement in targeted therapy in which effectiveness is increased at the same time off-target toxicity is diminished. Parallel developments in functional imaging are providing additional potential to evaluate the efficacy of treatment while it is being delivered, allowing dynamic assessment of risk during chemotherapy and adaptation of the therapy in real time. Risk assessment in Hodgkin lymphoma is continuously evolving, promising ever greater precision and clinical relevance. This article explores the past usefulness and the emerging potential of risk assessment for this imminently curable malignancy.
Introduction
Over the past 60 years, continuous improvement in the management of Hodgkin lymphoma has brought clinicians and patients to an era in which the large majority of patients are cured, regardless of disease presentation.1-7 This improvement in outcome has brought a new obligation to those who wish to optimally manage this previously lethal malignancy: an obligation to maintain very high cure rates while simultaneously minimizing toxicity, especially persistent late toxicity, which may permanently reduce the quality of life of survivors or even cause their death. A fine balance must be maintained in which maximal effectiveness of treatment, which presently is built around multiagent chemotherapy and judicious use of radiation, is maintained while minimizing exposure to interventions associated with major late toxicity. In brief, clinicians must recommend just enough treatment to achieve the greatest efficacy and yet induce the least harm. Careful assessment of risk is an essential part of achieving this balance. Such risk assessment must, in turn, address multiple factors, of which some are intrinsic to the host, others are related to tumor burden and tumor biology, and lastly, several are evaluable at diagnosis and determinable as the treatment course unfolds (Figure 1). Full appreciation of important factors that increase the risk of treatment failure or the likelihood of undesirable, potentially avoidable acute or late toxicity and how these risks can be minimized is essential to optimal management of Hodgkin lymphoma today. This review examines these risk factors and identifies strategies that minimize their impact on our patients. It is necessary to acknowledge, however, that important risk-altering biological characteristics may remain undescribed at present but be identified and become important with further research.
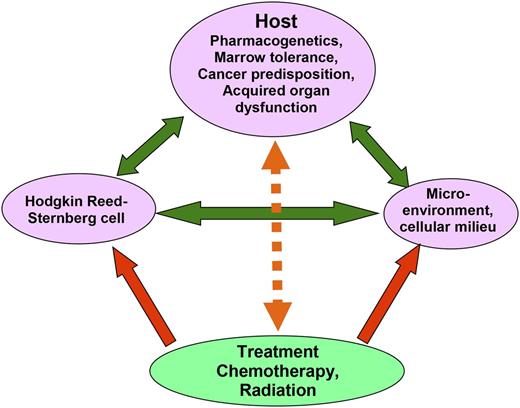
Complex interaction affecting risk of treatment failure for patients with newly diagnosed Hodgkin lymphoma. Green arrows show how the malignant Hodgkin Reed-Sternberg cells interact with preexisting host factors, including cancer predisposition, pharmacogenetics, and acquired organ dysfunction, each of which may enhance malignant cell survival or interfere with effective treatment delivery. In addition, the Hodgkin Reed-Sternberg cells manipulate cells in their microenvironment, inducing release of growth-enhancing and immune-suppressing cytokines. Treatment (red arrows) reduces the risk of treatment failure by exerting direct cytotoxicity on the Hodgkin Reed-Sternberg cells, by interrupting the stimulation of tumor cell growth encouraged by microenvironmental cells, and by restoring an effective immune response. Treatment effectiveness is modulated (orange arrow) by host factors, with some (eg, good performance status, young age) increasing host tolerance for higher dose treatment and therefore effectiveness, and others (eg, organ dysfunction, coincident HIV infection) diminishing treatment effectiveness.
Complex interaction affecting risk of treatment failure for patients with newly diagnosed Hodgkin lymphoma. Green arrows show how the malignant Hodgkin Reed-Sternberg cells interact with preexisting host factors, including cancer predisposition, pharmacogenetics, and acquired organ dysfunction, each of which may enhance malignant cell survival or interfere with effective treatment delivery. In addition, the Hodgkin Reed-Sternberg cells manipulate cells in their microenvironment, inducing release of growth-enhancing and immune-suppressing cytokines. Treatment (red arrows) reduces the risk of treatment failure by exerting direct cytotoxicity on the Hodgkin Reed-Sternberg cells, by interrupting the stimulation of tumor cell growth encouraged by microenvironmental cells, and by restoring an effective immune response. Treatment effectiveness is modulated (orange arrow) by host factors, with some (eg, good performance status, young age) increasing host tolerance for higher dose treatment and therefore effectiveness, and others (eg, organ dysfunction, coincident HIV infection) diminishing treatment effectiveness.
Risk factors intrinsic to the patient
Many studies have identified patient-related risk factors that impact outcome of treatment of individuals with Hodgkin lymphoma. Table 1 lists those risk factors most relevant to current-day management, including age, gender, HIV infection, and prior organ compromise such as pulmonary disease related to smoking and cardiac dysfunction reflecting underlying coronary artery disease. Each of these factors has a profound, highly significant effect on outcome; however, not all can be altered or addressed effectively using currently available interventions. Previously acquired pulmonary compromise, usually related to cigarette smoking, may necessitate omission of bleomycin from primary treatment. Deciding when to drop bleomycin is made more challenging due to the lack of useful objective screening assessment tools. Formal pulmonary function testing, even with inclusion of carbon monoxide diffusion capacity, is quite unreliable at identifying patients at risk for significant bleomycin toxicity.8-10 The decision to omit bleomycin must be made on clinical grounds. I have found that a useful rule of thumb is to consider the potential impact of a relatively rapid loss of 30% to 40% of current respiratory reserve. If a patient appears, based on a review of current activity levels and exercise tolerance, capable of absorbing that much loss of lung function from current pulmonary reserve, bleomycin can be safely, but still carefully, included in planned chemotherapy. Such a patient has adequate reserve to tolerate pulmonary injury if it occurs. When I do not think such a loss could be endured safely, I omit bleomycin at least until pulmonary reserve improves, as may happen if the compromise was due to the Hodgkin lymphoma, perhaps reflecting a large mediastinal mass or lung involvement; or permanently if prior damage from smoking or occupational exposure appears irreversible. Concern has been expressed that coincident use of bleomycin and neutrophil growth factors may exacerbate bleomycin-related pulmonary toxicity.11 However, 2 well-conducted studies, a retrospective review12 and a prospective clinical trial,13 have failed to substantiate this suspicion. Neutrophil growth factors should be used sparingly in the management of Hodgkin lymphoma14 ; however, when they are necessary, there is no need to avoid them due to concern over coincident use of bleomycin.
Risk factors affecting outcome of treatment of patients with Hodgkin lymphoma that are intrinsic to the patient
| Risk factor . | Frequency, % . | 5-y OS, % . | P . | Reference . |
|---|---|---|---|---|
| Age | 27, 33, 38-42, 111-117 | |||
| >45 y | 34 | 96 vs 77 | <.0001 | |
| >60 y | 18 | 95 vs 64 | <.0001 | |
| >79 y | 2 | 91 vs 34 | <.0001 | |
| Male gender | 55 | 91 vs 88 | .018 | 27, 33, 39-42 |
| HIV infection | 1.2 | 89 vs 41 | <.0001 | 16, 17, 20-27 |
| Prior reduced lung function, major | 5-10 | * | Not applicable | BCCA “experience” |
| Prior reduced cardiac function | 5-10 | * | Not applicable | BCCA “experience” |
| Risk factor . | Frequency, % . | 5-y OS, % . | P . | Reference . |
|---|---|---|---|---|
| Age | 27, 33, 38-42, 111-117 | |||
| >45 y | 34 | 96 vs 77 | <.0001 | |
| >60 y | 18 | 95 vs 64 | <.0001 | |
| >79 y | 2 | 91 vs 34 | <.0001 | |
| Male gender | 55 | 91 vs 88 | .018 | 27, 33, 39-42 |
| HIV infection | 1.2 | 89 vs 41 | <.0001 | 16, 17, 20-27 |
| Prior reduced lung function, major | 5-10 | * | Not applicable | BCCA “experience” |
| Prior reduced cardiac function | 5-10 | * | Not applicable | BCCA “experience” |
Frequency with which the risk factor was encountered and single-variable impact (5-year OS; % absent vs % present) in a large sample (n = 1443) of consecutively diagnosed, unselected patients with Hodgkin lymphoma in British Columbia between 1998 and 2013. Note: a steady improvement in overall survival has occurred across this time interval.
BCCA, British Columbia Cancer Agency; OS, overall survival.
Presence of these risk factors reduces OS by ∼20% (hazard rate for overall survival: ∼0.75).
Underlying cardiac disease may similarly affect the safety of chemotherapy, in this case, the use of anthracyclines. A prior history of congestive heart failure or ongoing evidence of impaired cardiac reserve such as a left ventricular ejection fraction <50% should prompt careful consideration of the risk that exposure to doxorubicin or doxorubicin plus mediastinal radiation will worsen underlying cardiomyopathy. In such cases, careful serial monitoring of ventricular function must be included in the patient’s assessments during treatment, and omission of the anthracyclines and substitution with an alternative chemotherapeutic agent such as etoposide should be considered.
Another aspect of organ function affecting risk is that of bone marrow tolerance for exposure to cytotoxic agents. This risk emerges clearly in studies focused on the relationship between gender and bone marrow function. Because myelosuppression is reflected in number and depth of episodes of neutropenia, and female gender correlates with increased sensitivity to marrow suppression, these studies typically demonstrate that women have more episodes of neutropenia and deeper and more prolonged nadirs in neutrophil counts compared with men given the same doses of chemotherapy.15 Because depth and length of myelosuppression reflect biological potency of chemotherapy agents, patients who experience greater myelosuppression (in this case, women) have better outcomes, reflecting the more effective dosing of the chemotherapy.
Coincident infection with HIV alters the behavior of Hodgkin lymphoma, leading to more frequent systemic symptoms, earlier spread to extranodal tissue, and markedly decreased failure-free survival (FFS) and OS rates.16-19 There is now ample evidence that the use of highly active antiretroviral treatment (HAART) not only reduces the incidence of HIV-associated Hodgkin lymphoma but HAART plus vigorous supportive care employing prophylactic anti-Pneumocystis, antifungal, and antiherpesvirus antibiotics; neutrophil growth factors; and comprehensive social intervention substantially improve outcome in patients with coincident Hodgkin lymphoma and HIV infection. Most studies indicate that such interventions reduce the risk of death by at least 50%.16,17,20-27
A final factor relevant to treatment of Hodgkin lymphoma is older age, which has quite consistently been noted to have an adverse impact, although the threshold for its impact has varied across studies from age 45 years to >70 years. The challenge when considering age is that, in many ways, it is simply a proxy for physiological function. Ignoring age places the patient at exaggerated risk, but unduly emphasizing it risks undertreatment. In addition to assessment of specific organ function, such as cardiac or pulmonary function as discussed above, a reasonable and practical approach to adjusting treatment based on age is to start treatment with a modest dose reduction by 20% to 30% of the myelosuppressive chemotherapy agents and to escalate to full doses with subsequent cycles, seeking to reach the maximum that can be achieved without undue toxicity as early in overall treatment as feasible.
Knowledge of relevant patient-specific risk factors is important in the crafting of optimal treatment. Thus, treatment outcome for Hodgkin lymphoma patients with certain patient-specific risk factors indicating a diminished prognosis or signaling specific organ dysfunction can be substantially improved by employing individualized interventions.
Risk factors related to tumor burden and tumor biology
Risk factors related to tumor burden and tumor biology can be roughly divided into 2 categories; the first being older assessments describing global clinical factors and laboratory tests, which often reflect not only disease-specific factors but also, indirectly, patient-specific characteristics; the second being newer assessments based on specific biological characteristics of the lymphoma itself.
Risk factors describing global clinical factors and laboratory tests
The most obvious clinical risk factor impacting outcome for patients with Hodgkin lymphoma is stage of disease, which is equally obviously reflective of net tumor burden. The most recent version of the staging system (the Cotswold revision of the Ann Arbor system) includes a basic measure of tumor bulk: the diameter of the largest single tumor mass.28,29 There is universal agreement that stage affects risk of treatment failure and must be considered in treatment planning. Patients are most often divided into 2 groups: those with limited-stage disease, typically including those with stage I or II disease; and those with advanced-stage (stage III or IV) disease. Often, especially in Europe, additional substaging focuses on specific risk factors such as bulk of tumor, presence of B symptoms, number of involved nodal groups, and certain laboratory measurements such as erythrocyte sedimentation rate, and assigns patients to favorable or unfavorable substages of limited disease.30,31 Because stage and substage directly determine planned duration of treatment, with limited-stage disease typically treated using 2 to 4 cycles of chemotherapy and advanced-stage disease using 6 or more cycles, this aspect of tumor burden as a distinct risk factor for treatment failure is intrinsically acknowledged in the modern treatment of Hodgkin lymphoma.
Over several decades extending from the 1970s to the 1990s, clinical factors impacting prognosis and outcome of patients with Hodgkin lymphoma were described in many studies, some of which focused on all patients, whereas others attempted to identify factors specific to stage of disease or define subsets of patients such as those with specific histologic subtypes.27,30,32-43 The extent to which these clinical factors impact patient outcomes has diminished substantially as the effectiveness of interventions has improved. Obvious and clinically important changes in the accuracy and diminished relevance of clinical risk factors can be readily seen when one examines the impact of the factors needed to assign a score using the pivotal International Prognostic Factors Project (IPFP) score.42 The IPFP was an international effort coordinated by the German Hodgkin Study Group, in which investigators assembled data on a large number of potentially relevant prognostic factors and treatment outcomes from 25 Hodgkin lymphoma treatment centers or cooperative groups for 5141 patients with advanced-stage Hodgkin lymphoma treated primarily (>75%) with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD)44-46 as delivered in the late 1980s and early 1990s. In the final multivariable analysis, the IPFP identified 7 independently prognostic factors, shown in Table 2, each of which contributed approximately equally to the impact on freedom from progression (FFP) and OS: age, gender, stage, hemoglobin level, serum albumin level, peripheral blood white blood cell count, and degree of peripheral blood lymphopenia. Given 1 point for each factor present, patients were assigned a score from 0 to 7, which resulted in a wide range of outcomes measured as 5-year FFP and 5-year OS rates. For example, the investigators found that a patient with no adverse factors (IPFP score 0) had 5-year FFP and 5-year OS rates of 84% and 89%, respectively, whereas a patient with 4 factors (IPFP score 4) had 5-year FFP and 5-year OS rates of 51% and 61%, respectively. The strengths of the IPFP were the large number of patients examined, the international participation, ready availability in standard clinical practice of the factors identified, and the use of ABVD as the primary treatment in the large majority of patients, although the inclusion of some patients treated with regimens other than ABVD somewhat weakens the strength of this latter observation. As useful as this index has proven, there are several limitations in the original publication: 446 patients were excluded due to age outside the range from 15 to 65 years or receipt of noncurative chemotherapy; 40% of the included patients also received treatment with radiation; 25% of the patients had stage I or II disease; all 7 variables included in the final score were available for only 1618 (34%) of the patients, forcing the statisticians to interpolate the missing variables based on assumptions about their probable distribution; and, lastly and most importantly, the patients were primarily treated in the 1980s and early 1990s, before secondary treatment with high-dose chemotherapy and autologous hematopoietic stem cell transplantation (ASCT) came into wide use for patients with relapse or primary progression despite ABVD.47-55
Prognostic factors with independent impact on outcome for patients with advanced-stage Hodgkin lymphoma identified in the IPFP
| Factor . | Criterion . | Frequency, % . |
|---|---|---|
| Age | >44 y | 21 |
| Gender | Male | 61 |
| Stage | IV | 42 |
| Serum albumin, g/L | <40 | 35 |
| Hemoglobin, g/L | <105 | ∼20* |
| WBC count ×109/L | >15 | 19 |
| Lymphocyte count ×109/L | <0.6 or <8% of total WBC | 21 |
| Factor . | Criterion . | Frequency, % . |
|---|---|---|
| Age | >44 y | 21 |
| Gender | Male | 61 |
| Stage | IV | 42 |
| Serum albumin, g/L | <40 | 35 |
| Hemoglobin, g/L | <105 | ∼20* |
| WBC count ×109/L | >15 | 19 |
| Lymphocyte count ×109/L | <0.6 or <8% of total WBC | 21 |
WBC, white blood cell.
Value estimated from primary publication.
Much has changed in the management of patients with advanced-stage Hodgkin lymphoma since the analysis underlying the IPFP scoring system was conducted. Diagnostic imaging has improved, first with the introduction of faster computed tomographic scanning, with its ability to provide finer and more exact detail, and later with the introduction of functional imaging based on fluorodeoxyglucose positron emission tomography (FDG-PET). Improved imaging not only increases the accuracy of disease identification but also introduces the artifact of stage migration, which in turn improves apparent treatment outcomes.56 Chemotherapy dose delivery has improved coincident with the widespread use of neutrophil growth factors, although the necessity to employ such growth factors has frequently been questioned.14 Diagnostic accuracy has improved, reducing the modest but still important number of patients with poorer-prognosis non-Hodgkin lymphomas such as anaplastic large-cell lymphoma57,58 and T-cell/histiocyte-rich large B-cell lymphoma58-60 erroneously included in series of patients thought to have classical Hodgkin lymphoma. Lastly, and most importantly, as described above, ASCT has been universally adopted as standard secondary, potentially curative, treatment for patients who have relapse after ABVD or primary progression during ABVD. The combined impact of these mitigating factors (stage migration due to improved imaging, improved dose delivery, more accurate diagnoses, and wide use of ASCT) can be seen when one examines the outcome achieved by primary treatment with ABVD in major clinical trials and large single-institution series over the years from the late 1980s through the early 2000s (Table 3). The 5-year OS rate has increased from just over 70% to approximately 90% despite the nominal use of exactly the same chemotherapy regimen (ABVD).
Improvement in 5-year OS rates from the late 1980s to the 2000s
| 5-y OS, % . | Year of publication . | Reference . |
|---|---|---|
| 73 | 1992 | 45 |
| 78 | 1998 | 42 |
| 82 | 2003 | 46 |
| 83 | 2003 | 118 |
| 86 | 2008 | 119 |
| 84 | 2009 | 6 |
| 90 | 2009 | 5 |
| 91 | 2012 | 7 |
| 88 | 2013 | 1 |
| 5-y OS, % . | Year of publication . | Reference . |
|---|---|---|
| 73 | 1992 | 45 |
| 78 | 1998 | 42 |
| 82 | 2003 | 46 |
| 83 | 2003 | 118 |
| 86 | 2008 | 119 |
| 84 | 2009 | 6 |
| 90 | 2009 | 5 |
| 91 | 2012 | 7 |
| 88 | 2013 | 1 |
Improvement in 5-year OS rates after primary treatment with ABVD or equivalent chemotherapy for advanced-stage Hodgkin lymphoma seen in serial major international clinical trials and large single-institution series.
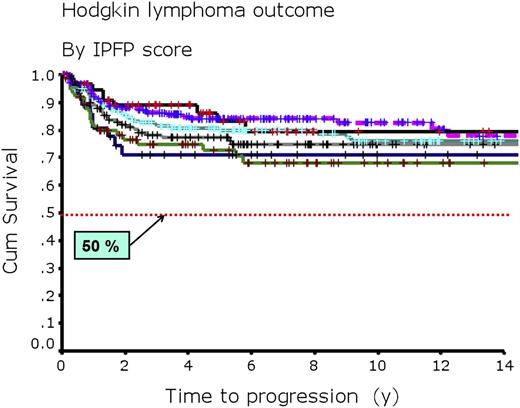
The impact of this apparent improvement in outcome after primary treatment with ABVD for advanced-stage Hodgkin lymphoma on the usefulness of the IPFP scoring system is evident in the results we have seen at the British Columbia Cancer Agency (BCCA) (Table 4; Figure 2).7 Table 4 shows a comparison of outcomes for subgroups of patients with varying IPFP scores as seen in the original IPFP report42 and in our single-institution experience of 579 consecutive patients treated with ABVD or equivalent chemotherapy.7 Figure 2 shows updated FFP curves for 675 consecutive patients treated with ABVD or equivalent chemotherapy at the BCCA through 2009 broken down by IPFP score. These results show that the 42% spread in the 5-year FFP rate, which ranged from 84% to 42% in the original publication for patients with a score of 0 compared with those with a score ≥5, has markedly narrowed to a 17% spread, ranging from 83% to 66%, using ABVD today. Even more importantly, presently, for the 94% of patients with advanced-stage Hodgkin lymphoma who present with IPFP scores of 0 to 4, the 5-year OS rate has improved to approximately 90%. Clearly, the usefulness of the IPFP score has diminished markedly with time. The same is true for the individual factors that make up the IPFP score and those that have been described in multiple other publications addressing clinical prognostic factors. This change reflects the general principle that as overall treatment strategies improve, the impact of individual (and even aggregated) prognostic factors diminishes. With even the worst subsets of patients (such as the small group [6%] of patients with an IPFP score of ≥5) having a likelihood of cure, with ABVD exceeding 65% and 5-year OS rates >85%, prognostic models based on clinical factors such as those used in the IPFP scoring system no longer have useful clinical relevance. We must search for a different approach to estimating risk.
Five-year FFP and 5-year OS rates according to IPFP scores
| IPFP score . | Number of patients (%) . | BCCA 5-y FFP, % . | IPFP report 5-y FFP, % . | BCCA 5-y OS, % . | IPFP report 5-y OS, % . |
|---|---|---|---|---|---|
| 0 | 48 (8.3) | 83 ± 6 | 84 ± 4 | 98 ± 2 | 89 ± 2 |
| 1 | 166 (28.7) | 86 ± 3 | 77 ± 3 | 97 ± 2 | 90 ± 2 |
| 2 | 157 (27.1) | 80 ± 3 | 67 ± 2 | 92 ± 2 | 81 ± 2 |
| 3 | 109 (18.8) | 74 ± 4 | 60 ± 3 | 87 ± 4 | 78 ± 3 |
| 4 | 62 (10.7) | 67 ± 6 | 51 ± 4 | 85 ± 5 | 61 ± 4 |
| ≥5 | 37 (6.4) | 66 ± 8 | 42 ± 5 | 74 ± 8 | 56 ± 5 |
| Reference | 7 | 42 | 7 | 42 |
| IPFP score . | Number of patients (%) . | BCCA 5-y FFP, % . | IPFP report 5-y FFP, % . | BCCA 5-y OS, % . | IPFP report 5-y OS, % . |
|---|---|---|---|---|---|
| 0 | 48 (8.3) | 83 ± 6 | 84 ± 4 | 98 ± 2 | 89 ± 2 |
| 1 | 166 (28.7) | 86 ± 3 | 77 ± 3 | 97 ± 2 | 90 ± 2 |
| 2 | 157 (27.1) | 80 ± 3 | 67 ± 2 | 92 ± 2 | 81 ± 2 |
| 3 | 109 (18.8) | 74 ± 4 | 60 ± 3 | 87 ± 4 | 78 ± 3 |
| 4 | 62 (10.7) | 67 ± 6 | 51 ± 4 | 85 ± 5 | 61 ± 4 |
| ≥5 | 37 (6.4) | 66 ± 8 | 42 ± 5 | 74 ± 8 | 56 ± 5 |
| Reference | 7 | 42 | 7 | 42 |
A comparison of the 5-year outcome seen in 579 consecutive patients treated with ABVD for advanced-stage Hodgkin lymphoma at the BCCA and the projected outcome seen in patients included in the IPFP.
Time to progression for 675 consecutive adult patients with advanced-stage Hodgkin lymphoma. Adult patients with advanced-stage Hodgkin lymphoma treated with ABVD or equivalent chemotherapy at the BCCA through 2009 by IPFP score. A score of 0 is indicated by a solid black line (n = 57); a score of 1 by a dashed purple line (n = 185); a score of 2 by a turquoise solid line (n = 186); a score of 3 by a solid gray line (n = 133); a score of 4 by a solid blue line (n = 76); and a score 5 to 7 by a solid green line (n = 38). Cum, cumulative.
Time to progression for 675 consecutive adult patients with advanced-stage Hodgkin lymphoma. Adult patients with advanced-stage Hodgkin lymphoma treated with ABVD or equivalent chemotherapy at the BCCA through 2009 by IPFP score. A score of 0 is indicated by a solid black line (n = 57); a score of 1 by a dashed purple line (n = 185); a score of 2 by a turquoise solid line (n = 186); a score of 3 by a solid gray line (n = 133); a score of 4 by a solid blue line (n = 76); and a score 5 to 7 by a solid green line (n = 38). Cum, cumulative.
Risk factors reflecting specific biologic characteristics
Risk factor assessment systems for cancer based on global clinical factors and/or laboratory tests are fundamentally crude in that they reflect a mix of intrinsic host factors such as age, comorbid conditions, gender, and others, plus factors that primarily but not exclusively reflect tumor burden, such as largest mass size, stage, number of extranodal sites, and constitutional symptoms. Even specific clinical laboratory tests such as hemoglobin level, lactate dehydrogenase level, serum albumin level, erythrocyte sedimentation rate, and degree of peripheral blood lymphopenia blend tumor and patient characteristics, obscuring the contribution of intrinsic tumor biology to disease behavior and treatment outcome. It is appealing to hope that examination of specific biologic characteristics of the malignant cells themselves may be more informative and less confusing to interpret. Additionally, identification of factors unique to malignant cells may provide attractive specific targets for therapeutic intervention that promise to improve the therapeutic index of treatment by concentrating the treatment effect on the malignant cells and sparing normal cells.
A large and steadily growing number of variously specific biologic characteristics of Hodgkin lymphoma (biomarkers) have been identified to have an apparent impact on risk (Table 5).61-88 These biomarkers are of several types: antigens expressed on the Hodgkin Reed-Sternberg cells, antigens expressed on circulating lymphocytes, antigens expressed on microenvironmental cells within the tumor and associated biologically with the Hodgkin Reed-Sternberg cells, presence of Epstein-Barr virus in the Hodgkin Reed-Sternberg cells, circulating biomarkers detectable in the serum, specific gene expression and miRNA profiles obtained by analysis of biopsied tumors, and specific germline polymorphisms. All are of interest; however, different subsets are relevant to risk assessment for clinical management in different ways. Increased expression of antigens expressed by Hodgkin Reed-Sternberg cells, including aberrant T-cell antigens, FOXP3, CD20, BCL-XL, and p53, as well as loss of HLA class II markers, can be assessed at the time of diagnosis and may predict a worse outcome. In most, but not all, studies that have focused on their presence, increased numbers of macrophages within the tumor microenvironment measured by various immunohistochemical markers, especially CD68 and CD163, reproducibly identified patients with higher risk of relapse and higher risk of eventual death from Hodgkin lymphoma. Elevated levels of specific serum biomarkers, including TARC, galectin-1, CD163, IL-10, IL-10 receptor, IL-6, CD30, TNF, TNF receptor, CD4, CD8, CD25, and CD54, have been reported to be associated with a worse prognosis. A polygene gene expression profile, including approximately 15 genes, performed on tumor biopsies and, therefore, primarily reflecting microenvironmental cells, appears to identify a subset of Hodgkin lymphoma patients with a markedly higher risk of treatment resistance.62,69 Lastly, certain germline polymorphisms of IL-10, IL-6, and NPAT may be associated with poorer prognosis (IL-10 and IL-6) or risk of development of nodular lymphocyte–predominant Hodgkin lymphoma (NPAT).71 Several challenges arise, however, as we try to turn these interesting biological observations into clinically relevant biomarkers. Most problematic is the lack of wide validation of the significance of these biomarkers, which typically have been demonstrated in only small series of selected patients and have not been reproducibly shown to be significant in multiple independent series of patients. Additionally, many of these biomarkers, especially those detected in serum or those expressed as surface markers on Hodgkin Reed-Sternberg cells, are not independent in their impact on prognosis. Rather, they travel together such that elevated levels or elevated expression of one is often associated with elevation of several others.
Biomarkers with potential impact on outcome in patients treated for Hodgkin lymphoma
| Factor . | Impact on prognosis . | Reference . |
|---|---|---|
| Assessment of Hodgkin Reed-Sternberg cells | ||
| Aberrant T-cell antigen expression* | Negative | 61 |
| FOXP3 expression* | Negative | 66 |
| CD20 expression* | Negative | 66 |
| BCL-XL* | Negative | 77 |
| p53* | Negative | 77 |
| HLA class II, loss* | Negative | 75 |
| Presence of Epstein-Barr virus | Negative | 82-86 |
| Assessment of microenvironmental or circulating nonneoplastic cells, cytokines, and membrane-associated antigens | ||
| Fibroblast growth factor 2† | Negative | 67 |
| Syndecan-1† | Negative | 67 |
| Tumor-associated macrophages‡ | Negative | 68, 70,72, 87, 88 |
| CD68 expression‡ | Negative | 66 |
| Serum TARC, elevated | Negative | 63, 65 |
| Serum galectin-1, elevated | Negative | 64, 79 |
| Serum CD163, elevated | Negative | 65 |
| Serum IL-10, elevated | Negative | 73, 76, 78 |
| Serum IL-10 receptor, elevated | Negative | 76 |
| Serum IL-6, elevated | Negative | 76 |
| Serum CD30, elevated | Negative | 76, 78 |
| Serum TNF, elevated | Negative | 76 |
| Serum TNF receptor, elevated | Negative | 76 |
| Serum CD4, elevated | Negative | 78 |
| Serum CD8, elevated | Negative | 78 |
| Serum CD25, elevated | Negative | 78 |
| Serum CD54, elevated | Negative | 78 |
| Gene expression and miRNA profiling reflecting the tumor microenvironment | ||
| Gene expression profiling | Positive or negative | 62, 69 |
| Global miRNA levels, including MIR21, MIR30E, MIR30D, and MIR92B | Positive or negative | 80, 81 |
| Host germline polymorphisms and mutations | ||
| IL-10-specific polymorphism 592AA | Negative | 74 |
| IL-6-specific polymorphism 174GG | Negative | 74 |
| Germline NPAT mutation | Marker for risk of nodular lymphocyte–predominant Hodgkin lymphoma | 71 |
| Factor . | Impact on prognosis . | Reference . |
|---|---|---|
| Assessment of Hodgkin Reed-Sternberg cells | ||
| Aberrant T-cell antigen expression* | Negative | 61 |
| FOXP3 expression* | Negative | 66 |
| CD20 expression* | Negative | 66 |
| BCL-XL* | Negative | 77 |
| p53* | Negative | 77 |
| HLA class II, loss* | Negative | 75 |
| Presence of Epstein-Barr virus | Negative | 82-86 |
| Assessment of microenvironmental or circulating nonneoplastic cells, cytokines, and membrane-associated antigens | ||
| Fibroblast growth factor 2† | Negative | 67 |
| Syndecan-1† | Negative | 67 |
| Tumor-associated macrophages‡ | Negative | 68, 70,72, 87, 88 |
| CD68 expression‡ | Negative | 66 |
| Serum TARC, elevated | Negative | 63, 65 |
| Serum galectin-1, elevated | Negative | 64, 79 |
| Serum CD163, elevated | Negative | 65 |
| Serum IL-10, elevated | Negative | 73, 76, 78 |
| Serum IL-10 receptor, elevated | Negative | 76 |
| Serum IL-6, elevated | Negative | 76 |
| Serum CD30, elevated | Negative | 76, 78 |
| Serum TNF, elevated | Negative | 76 |
| Serum TNF receptor, elevated | Negative | 76 |
| Serum CD4, elevated | Negative | 78 |
| Serum CD8, elevated | Negative | 78 |
| Serum CD25, elevated | Negative | 78 |
| Serum CD54, elevated | Negative | 78 |
| Gene expression and miRNA profiling reflecting the tumor microenvironment | ||
| Gene expression profiling | Positive or negative | 62, 69 |
| Global miRNA levels, including MIR21, MIR30E, MIR30D, and MIR92B | Positive or negative | 80, 81 |
| Host germline polymorphisms and mutations | ||
| IL-10-specific polymorphism 592AA | Negative | 74 |
| IL-6-specific polymorphism 174GG | Negative | 74 |
| Germline NPAT mutation | Marker for risk of nodular lymphocyte–predominant Hodgkin lymphoma | 71 |
IL, interleukin; miRNA, microRNA; TARC, thymus and activation-regulated chemokine; TNF, tumor necrosis factor.
Presence detected by immunohistochemistry on tumor Hodgkin Reed-Sternberg cells.
Presence detected by immunohistochemistry on circulating peripheral blood CD30-positive cells.
Presence detected by immunohistochemistry on tumor microenvironment cells.
Although there remain challenges due to lack of validation and/or cross-association among the many potentially important biomarkers for increased risk of treatment resistance in Hodgkin lymphoma, some of these biomarkers have emerged as more attractive candidates to signal higher risk. In particular, the increased presence of tissue-infiltrating macrophages, whether measured by immunohistochemistry66,68,70,72 or implied by specific gene expression profiles,62,69 appears to be a reproducible and powerful negative prognostic factor. Appropriately, the presence of increased numbers of tissue-infiltrating macrophages is now being examined in prospective clinical trials to see whether this finding can be sufficiently reproducibly demonstrated to justify its use to identify patients for either reduction of treatment because macrophage numbers are very low or escalation of treatment because they are very high. Certain serum markers also seem promising, especially serum TARC, galectin-1, and IL-10, because their prognostic impact has been validated independently63-65,73,76,78,79 and because they can be readily measured not only at diagnosis, when their prognostic importance can be assessed, but also serially during treatment to determine whether they can reliably identify patients whose treatment response is proving to be suboptimal. Finally, examining the overall gene expression profile detectable in biopsy tissue involved with Hodgkin lymphoma, which necessarily profiles the gene expression of the host reactive cells and not the malignant Hodgkin Reed-Sternberg cells, appears capable of separating a minority (29%) of patients with a sixfold worse prognosis from a majority (71%) with a much more favorable prognosis using modern chemotherapy.62
Risk factors that become recognizable during treatment
Treatment of Hodgkin lymphoma is typically delivered over several months, and treatment of advanced-stage disease often takes at least 6 to 8 months to complete. It is therefore attractive to try to identify risk factors during treatment that signal a higher likelihood that treatment will fail so that a change in (or an addition to) treatment can be considered. Attempts to find such an assessable factor in the past have proven unreliable. Speed of response has been assessed with the hope that rapid responders would do well and that a change in treatment plan would improve outcomes for slow responders, but this has not proven reliable. Quality of response early in the delivery of multiple cycles of chemotherapy is conceptually similar and is discussed below. Lastly, the absence of a complete response at the end of planned chemotherapy may identify patients with higher risk of relapse. Unfortunately, in the past, response assessment relying on such techniques as gallium, magnetic resonance, and even computed tomographic imaging has run afoul of the tendency of Hodgkin lymphoma to be associated with residual, sometimes large fibronecrotic masses, even when viable tumor cells have been eradicated. Thus, slow or incomplete response, as previously measured with historically available imaging techniques, has not reliably identified poor-prognosis patients, nor has intensification of chemotherapy dosing or addition of radiation based on speed or quality of response proven effective at reducing treatment failures.89-96 This situation may now be changing, however, with the wide availability of functional imaging using FDG-PET. Further complicating interpretation of the available literature is the possibility that FDG-PET during treatment, so-called interim PET, may have different usefulness in the management of limited-stage Hodgkin lymphoma compared with advanced-stage disease.
Interim PET as a risk factor for limited-stage Hodgkin lymphoma
Current management of adults with limited-stage Hodgkin lymphoma (stage IA or IIA, nonbulky [greatest diameter <10 cm]), typically consisting either of brief chemotherapy followed by involved-field or involved-nodal radiation or of only brief chemotherapy, cures almost all patients, and secondary treatment rescues many of those who relapse, virtually eliminating death from Hodgkin lymphoma in this population.97,98 For that reason, the current focus of clinical research is on identifying subsets of patients with limited-stage disease for whom treatment can be de-escalated without forfeiting effectiveness, perhaps by reducing the number of chemotherapy cycles or eliminating the radiation. Substantial consistency of results is apparent in the reported experiences employing interim PET after 2 to 3 cycles of ABVD for patients with limited-stage Hodgkin lymphoma (Table 6).99-102 Approximately 80% to 85% of patients reach an FDG-PET-negative state after 2 to 3 cycles of ABVD, and such patients have an approximately 90% likelihood of remaining free of relapse, even when radiation is omitted from their management. Thus, patients with a negative FDG-PET scan after 2 cycles of ABVD are at low risk of treatment failure. The prognostic value of a positive FDG-PET scan is less clear because most investigators have chosen to change treatment modality based on it, switching to radiation (Table 7), leaving both the scan’s usefulness in indicating a need to use radiation and its impact on risk unclear.
Selected large studies reporting interim PET scan results in patients with limited-stage Hodgkin lymphoma treated with ABVD
| No. of patients . | Cycles of chemotherapy . | Negative interim PET scan . | Reference . | |
|---|---|---|---|---|
| n . | % . | |||
| 571 | 3 | 426 | 75 | 102 |
| 441 | 2 | 381 | 86 | 99 |
| 221 | 2 | 183 | 83 | updated from 101 |
| 80 | 2-4 | 70 | 87 | 100 |
| No. of patients . | Cycles of chemotherapy . | Negative interim PET scan . | Reference . | |
|---|---|---|---|---|
| n . | % . | |||
| 571 | 3 | 426 | 75 | 102 |
| 441 | 2 | 381 | 86 | 99 |
| 221 | 2 | 183 | 83 | updated from 101 |
| 80 | 2-4 | 70 | 87 | 100 |
Modifiable risk factors and effective interventions affecting outcome in the treatment of Hodgkin lymphoma
| Risk factor . | Frequency, % . | Effective interventions . | Reference . |
|---|---|---|---|
| Reduced pulmonary function, major | ∼5-10 | Omit bleomycin | 120, 121 |
| Left ventricular ejection fraction <50% | ∼5-10 | Omit doxorubicin; consider substitution with etoposide | * |
| HIV infection | 1.2† | Vigorous supportive care with appropriate antibiotics and neutrophil growth factors | 16, 17, 20-27 |
| Positive interim PET scan, limited-stage disease | 15-20 | Involved-field or involved-nodal radiation | 99-102 |
| Risk factor . | Frequency, % . | Effective interventions . | Reference . |
|---|---|---|---|
| Reduced pulmonary function, major | ∼5-10 | Omit bleomycin | 120, 121 |
| Left ventricular ejection fraction <50% | ∼5-10 | Omit doxorubicin; consider substitution with etoposide | * |
| HIV infection | 1.2† | Vigorous supportive care with appropriate antibiotics and neutrophil growth factors | 16, 17, 20-27 |
| Positive interim PET scan, limited-stage disease | 15-20 | Involved-field or involved-nodal radiation | 99-102 |
Frequency with which the risk factor was encountered in a large sample (n = 1443) of consecutively diagnosed, unselected patients with Hodgkin lymphoma in British Columbia between 1998 and 2013.
Interim PET as a risk factor for advanced-stage Hodgkin lymphoma
The accuracy and usefulness of interim PET as a risk factor in patients with advanced-stage Hodgkin lymphoma are considerably less clear than for limited-stage patients.103-110 ABVD is the only multiagent chemotherapy program for which interim PET has been evaluated extensively. Table 8 shows the outcome for patients treated with ABVD for advanced-stage Hodgkin lymphoma and compares results for those with a positive interim PET scan during chemotherapy to those whose interim PET scan had become negative. A negative interim PET scan is found in approximately 80% of patients and appears to be strongly predictive for a favorable outcome. In addition, a negative interim PET scan appears to override or at least rival the prognostic impact of the IPFP score. However, for the approximately 20% of patients with a positive interim PET scan, the positive result’s impact is much less clear, even when the chemotherapy regimen is not altered. Reported FFS rates range from zero to almost 40% (Table 8). Furthermore, it remains quite unclear whether the negative prognostic impact of a positive interim PET scan can be overcome by changing the treatment approach. Preliminary observations that a switch to an escalated regimen of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone after a positive interim PET scan may as much as double 2- to 3-year FFS rates require confirmation.106 Once again, similarly to its value in limited-stage Hodgkin lymphoma, the primary usefulness of an interim PET scan in the assessment of patients with advanced-stage Hodgkin lymphoma appears to be in consistently identifying patients who have a low risk of eventual treatment failure with strong negative predictive impact after a negative interim PET scan; however, the usefulness of a positive interim PET scan remains problematic, with its value in assessing risk obscured by the potential impact of altering treatment midway though management. Thus, in both situations in which we can extract instructive observations, the interim PET scan seems most valuable for its ability to identify low risk of treatment failure; the value of a positive interim PET scan remains undetermined.
Selected large studies reporting interim PET scan results in patients treated with 6 to 8 cycles of ABVD for advanced-stage Hodgkin lymphoma
| No. of patients . | Outcome by interim PET scan result . | Reference . | |||
|---|---|---|---|---|---|
| Negative . | Positive . | ||||
| n (%) . | FFS, % (y) . | n (%) . | FFS, % (y) . | ||
| 260 | 215 (83) | 95 (3) | 45 (17) | 28 (3) | 103, 104 |
| 260 | 210 (81) | 95 (2) | 50 (19) | 13 (2) | 105 |
| 160 | 137 (86) | 92 (2) | 23 (14) | * | 106 |
| 77 | 61 (79) | 96 (2) | 16 (21) | 0 (2) | 107 |
| 91 | 77 (85) | 73 (3) | 14 (15) | 38 (3) | 110 |
| No. of patients . | Outcome by interim PET scan result . | Reference . | |||
|---|---|---|---|---|---|
| Negative . | Positive . | ||||
| n (%) . | FFS, % (y) . | n (%) . | FFS, % (y) . | ||
| 260 | 215 (83) | 95 (3) | 45 (17) | 28 (3) | 103, 104 |
| 260 | 210 (81) | 95 (2) | 50 (19) | 13 (2) | 105 |
| 160 | 137 (86) | 92 (2) | 23 (14) | * | 106 |
| 77 | 61 (79) | 96 (2) | 16 (21) | 0 (2) | 107 |
| 91 | 77 (85) | 73 (3) | 14 (15) | 38 (3) | 110 |
Interim PET scans were performed after 2 cycles of chemotherapy.
Positive interim PET scan outcome is not interpretable due to change in chemotherapy.
Risk-adapted treatment of Hodgkin lymphoma
The assessment of risk has 3 basic purposes in the management of serious disease such as Hodgkin lymphoma. First, risk assessment establishes reasonable assumptions in terms of prognosis, aligning the expectations of the patient, the patient’s family, and the treating physicians and, often, especially in the case of Hodgkin lymphoma, providing solid justification for optimism that the disease will be permanently eradicated. Second, identification of some risks may suggest specific additions to or alterations of the treatment plan that can meaningfully alter the risk of treatment failure. An example of such a modifiable risk factor is coincident HIV infection, with the need to add markedly enhanced supportive care and coincident HAART. Third, isolation of risk factors based on specific biological characteristics of the disease may suggest avenues for the development of targeted therapy that can focus its impact exclusively on the malignancy (or on the malignancy and the elements in the microenvironment providing a growth advantage) and avoid the negative impact of off-target toxicity, a characteristic all too often retained by conventional chemotherapeutic agents and radiation treatments. The clinical, biological, and imaging-related risk factors described in this article reach across this spectrum of purposes for assessing risk in Hodgkin lymphoma.
Acknowledgments
The author thanks his clinical colleagues at the BCCA and the physicians of British Columbia for their continued support and referral of patients; and Ms Suman Singh for help with maintenance of the BCCA Lymphoid Cancer Database.
Authorship
Contribution: J.M.C. composed the entire article.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Joseph M. Connors, Center for Lymphoid Cancer, British Columbia Cancer Agency, 600 West 10th Ave, Vancouver, BC, Canada V5Z 4E6; e-mail: jconnors@bccancer.bc.ca