In this issue of Blood, Kapur et al show that C-reactive protein (CRP) enhances IgG-mediated phagocytosis of platelets and patients with immune thrombocytopenia (ITP) have elevated CRP levels, which predicted slower platelet recoveries and bleeding severity.1
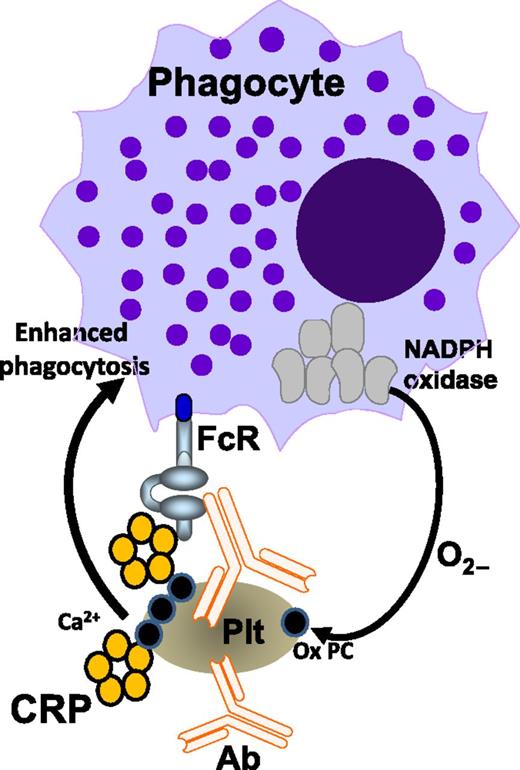
Binding of antiplatelet antibodies (Ab) to platelets (Plt) triggers platelet oxidation, which also requires the phagocyte NADPH oxidase system. Platelet oxidation exposes oxidized phosphorylcholine (Ox PC) residues from the platelet membrane, providing a Ca2+-dependent binding platform for CRP. CRP subsequently boosts antibody-mediated platelet phagocytosis via phagocytic FcRs. The figure is based on Figure 7 in the article by Kapur et al that begins on page 1793.
Binding of antiplatelet antibodies (Ab) to platelets (Plt) triggers platelet oxidation, which also requires the phagocyte NADPH oxidase system. Platelet oxidation exposes oxidized phosphorylcholine (Ox PC) residues from the platelet membrane, providing a Ca2+-dependent binding platform for CRP. CRP subsequently boosts antibody-mediated platelet phagocytosis via phagocytic FcRs. The figure is based on Figure 7 in the article by Kapur et al that begins on page 1793.
Antibody-mediated platelet destruction in fetal or neonatal alloimmune thrombocytopenia (FNAIT) and ITP occurs primarily through engagement of immunoglobulin (Ig)G opsonized platelets with activating Fc receptors (FcRs) on the surface of phagocytes in the spleen and liver, resulting in phagocytosis and thrombocytopenia.2 In FNAIT, for example, the antiplatelet IgG antibodies cross the placenta and target the fetal platelets, which can result in serious complications such as intracerebral hemorrhage. Antibody titer has been shown to be related to fetal platelet counts3 ; however, this correlation is far from perfect, as there are many cases not attributed to antibody titer alone,4 suggesting other factors such as IgG Fc-fucosylation may be involved.5 Even more striking, in ITP, there is no test that reliably predicts bleeding outcomes or severity of bleeding. Thus, a tool for the prediction of both FNAIT and ITP severity is highly warranted.
Kapur et al describe a series of elegant studies in which a serum factor was found to enhance IgG-mediated responses (respiratory burst and phagocytosis) against platelets.1 The involvement of the complement cascade was excluded, and the factor was found to be present in healthy sera and appeared to be acting against platelets in a calcium-dependent manner. As such, CRP was convincingly identified as the serum factor that enhanced IgG-mediated phagocytosis of platelets. CRP belongs to the pentraxin family, which contains a calcium-dependent ligand binding site and is a major acute-phase protein and a known ligand for FcRs.6 CRP is significantly upregulated during infections (from <0.05 to >500 mg/L), but is also present in healthy sera, albeit at a lower concentrations (∼0.8 mg/L in healthy adult volunteers).6 There was an intriguing correlation between CRP concentrations in 14 different healthy sera and antibody-mediated phagocytic activity against platelets.1 In addition, purified CRP was clearly shown to enhance antibody-mediated phagocytic responses against platelets, and CRP depletion and repletion experiments corresponded to IgG-mediated phagocytic activity against platelets.
Several experiments were performed to elucidate the working mechanism of CRP in IgG-mediated targeting of platelets. Platelet activation was shown to be involved, but not via the platelet FcγRIIa. Using pneumococcal cell wall polysaccharide (CWPS), which binds to the phosphorylcholine-ligand binding site on CRP, the authors demonstrated an abrogation of the IgG-enhancing serum effect in a phagocytic assay. Furthermore, using a novel innovative approach of cellular surface plasmon resonance, CRP binding to platelets was further explored. CRP was absorbed on sensor surface spots on a chip and IgG-opsonized platelets were circulated over the chip, measuring the specific interaction of platelets to CRP with high sensitivity. IgG-opsonized platelets demonstrated increased binding to CRP compared with isotype control (nonbound) platelets. Importantly, the binding of platelets to CRP was completely blocked by coinjection of CWPS, suggesting that CRP binds to platelet-membrane phosphorylcholine exposed after platelet activation induced by the antiplatelet antibodies. The nature of this platelet activation was further explored by using both inhibitors and an enhancer of oxidation. Platelet oxidation was found to be associated with increased binding to CRP and, using oxidation inhibitors in the platelet phagocytosis assay, the authors additionally demonstrated a role for the phagocyte reduced NAD phosphate (NADPH) oxidase system.
The aforementioned experiments were conducted with antibodies from maternal FNAIT sera (anti–HPA-1a antibodies), as well as a human monoclonal FNAIT antibody, B2G1. As CRP levels were found to be elevated in both FNAIT and ITP patients and the fact that oxidative stress molecules have been implicated in ITP as well,7,8 the authors further analyzed CRP in a clinical trial investigating the effect of intravenous immunoglobulin (IVIg) in children with newly diagnosed ITP (treatment with or without IVIG in children with acute ITP [TIKI study NTR1563]). Intriguingly, within a week, IVIg treatment led to a significant decrease of CRP levels, increased platelet numbers, and clinically decreased bleeding severity. From a diagnostic perspective, patients with elevated CRP levels at diagnosis took longer to normalize their platelet counts after 3 months. This remarkable finding could perhaps be used to determine which children with newly diagnosed ITP are in need of treatment.
In other analogous types of experiments, it has been previously shown that the gram-negative bacterial endotoxin lipopolysaccharide could also enhance IgG-mediated platelet phagocytosis in vitro,9 as well as induce a potent thrombocytopenic effect in vivo.10 It was suggested that the enhanced phagocytosis occurs via interactions between the phagocyte FcR and Toll-like receptors resulting in a synergistic intracellular signaling cascade, thus enhancing platelet phagocytosis and in vivo clearance. Whether these results have a relationship to the mechanism of CRP enhancement of phagocytosis is, however, still unknown.
In conclusion, CRP is a novel pathogenic cofactor that enhances IgG-mediated phagocytic responses resulting in thrombocytopenia (see figure). It appears that, on binding of antiplatelet antibodies, platelet oxidation occurs, which results in exposure of platelet membrane phosphorylcholine. This process is independent of complement or platelet FcγRIIa, but requires the phagocyte NADPH oxidase system. CRP subsequently binds to the platelet phosphorylcholine in a calcium-dependent manner and boosts the uptake and degradation of opsonized platelets via phagocytic FcRs. The data suggest that CRP may be an important factor that could explain the frequently observed aggravation of ITP on infections. Future studies may expand these fascinating observations and further investigate CRP effects on IgG-mediated phagocytosis in other diseases such as those associated with anti-red blood cell antibodies. The diagnostic potential of CRP in these disorders should be validated, and perhaps targeting CRP, either directly or via oxidation inhibitors, may be a promising therapeutic approach at least in patients with IgG antiplatelet antibodies.
Conflict-of-interest disclosure: The author declares no competing financial interests.