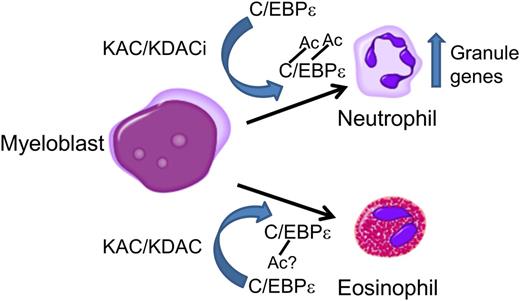
In this issue of Blood, Bartels and colleagues demonstrate that acetylation of the transcription factor CCAAT enhancer binding protein ε (C/EBPε) is essential for terminal neutrophil granulocyte differentiation.1
Acetylation of C/EBPε is essential for terminal neutrophil differentiation and function. C/EBPε is acetylated during granulocytic differentiation toward neutrophils by lysine acetylases (KAC, eg, p300) or lysine deacetylase inhibitors (KDACi, eg, nicotinamide inhibiting KDAC SIRT1). Acetylation on K121 and K198 is essential for terminal differentiation of neutrophils and expression of secondary granule proteins. Differential acetylation of C/EBPε could be important for determining differentiation along the neutrophil or eosinophil lineages.
Acetylation of C/EBPε is essential for terminal neutrophil differentiation and function. C/EBPε is acetylated during granulocytic differentiation toward neutrophils by lysine acetylases (KAC, eg, p300) or lysine deacetylase inhibitors (KDACi, eg, nicotinamide inhibiting KDAC SIRT1). Acetylation on K121 and K198 is essential for terminal differentiation of neutrophils and expression of secondary granule proteins. Differential acetylation of C/EBPε could be important for determining differentiation along the neutrophil or eosinophil lineages.
Neutrophils, the most abundant granulocytes, are essential for host innate immune defense. Neutropenia results from damage to the bone marrow or depletion or destruction of neutrophils by drugs, diseases, or congenital disorders that block neutrophil differentiation. Neutropenic individuals are extremely susceptible to bacterial infection, and febrile neutropenia increases the risk of mortality in cancer patients receiving myelosuppressive chemotherapy.2 Prophylactic use of granulocyte colony-stimulating factor (G-CSF) reduces mortality by increasing neutrophil numbers.2 A better understanding of the mechanisms that regulate granulopoiesis and terminal neutrophil differentiation could spur development of new strategies to overcome neutropenia and improve clinical outcomes.
The C/EBP transcription factor family is essential for granulopoiesis and terminal differentiation of neutrophils.3 C/EBPα is predominantly expressed in immature myeloid cells, and lack of expression leads to a block at the myeloblast stage.3 C/EBPβ is expressed from the metamyelocyte stage and on during maturation.3 C/EBPβ-deficient mice display normal granulocyte differentiation and steady-state levels of neutrophils but are unable to produce neutrophils in response to cytokine exposure or infection during “emergency” granulopoiesis.4 C/EBPε is expressed at the promyelocyte to myelocyte stage, and knockout mice lack neutrophils and eosinophils because of a block at this stage.3 Interestingly, mutations in the CEBPE gene account for some cases of human neutrophil-specific granule disease, a rare congenital disorder, characterized by increased circulating, immature neutrophils, and recurrent pyogenic infections.5 Also, induction of C/EBPε by G-CSF is important for inducing neutrophil maturation.6
Expression of C/EBPε is restricted primarily to the myeloid lineage in 4 protein isoforms of 32, 30, 27, and 14 kDa. C/EBPε32/30 activates transcription, C/EBPε27 represses transcription, and C/EBPε14 acts as a dominant negative.1 The contribution of these different isoforms toward granulocytic differentiation remains largely unknown as is the importance of posttranslational modifications. Phosphorylation, sumoylation, and acetylation modulate the transcriptional activities of several C/EBP family members. C/EBPα is phosphorylated at several sites, and depending on the amino acid residue that is phosphorylated, granulocyte differentiation can be either blocked or increased. Also, sumolyation of lysine residues in C/EBPα inhibits granulocyte gene expression.7 Acetylation of C/EBPβ is functionally linked to transcriptional activity, but its impact on granulopoiesis is unknown.1 Phosphorylation of C/EBPε on threonine 75, which is located in the transactivation domain, leads to increased DNA binding and transcriptional activity, and sumolyation of a lysine residue 121 (K121) within repression domain I enhances transcriptional activation by C/EBPε.1
A potential role for acetylation in regulating neutrophil production and differentiation was suggested by these authors as lysine deacetylase inhibitors (KDACi) affect myeloid differentiation.8 Skokowa et al demonstrated that nicotinamide phosphoribosyltransferase (NAMPT) mediates G-CSF-triggered granulopoiesis by increasing activity levels of the lysine deacetylase (KDAC) sirtuin 1 (SIRT1), levels of C/EBPα and -β, and expression of G-CSF and G-CSF receptor in vitro through the production of NAD+.9 Further, treatment of healthy human volunteers with nicotinamide, a substrate for NAMPT, increases circulating neutrophil levels. Subsequently, Kyme et al showed that nicotinamide treatment increases expression of C/EBPε32/30, acetylation of lysine residues on C/EBPε32/30, and expression of the lactoferrin and cathelicidin antimicrobial peptide genes.10 Also, nicotinamide treatment augments neutrophil killing and enhances clearance of Staphylococcus aureus both in vitro and in vivo; however, the importance of C/EBPε32/30 acetylation in mediating these outcomes has not been determined in either study.9,10
The current study extends these findings and provides the first experimental evidence that acetylation at specific lysines (K121 and K198) is essential for C/EBPε32/30 transcriptional activity and terminal differentiation of neutrophils (see figure).1 In a series of experiments, they demonstrated acetylation of C/EBPε32/30 in cell culture and CD34+ umbilical cord blood cells after G-CSF-induced neutrophil differentiation. They showed that C/EBPε32/30 acetylation status is regulated by SIRT1 and p300 (a lysine acetylase, KAC) and that nicotinamide, an inhibitor of lysine deacetylase SIRT1, increases levels of acetylated C/EBPε32/30 in CD34+ umbilical cord blood cells differentiated toward neutrophils (see figure). Acetylation was required for transcriptional activity as a “lysine” dead version of C/EBPε32/30 (all 15 lysines mutated to arginine) lost activity. Mass spectrometry analysis identified 4 acetylated lysines: 2 in repression domain I (K100 and K121), 1 lysine between repression domain II and the basic region (K198), and 1 in the basic region (K202). Mutational analysis revealed that K121 and K198 were required for transcriptional activation and that loss of K121 acetylation decreased DNA binding. A functional role for K121 and K198 acetylation in neutrophil differentiation was demonstrated by retroviral transduction of lysine mutants into CD34+ umbilical cord blood cells differentiated into neutrophils. The lysine dead C/EBPε32/30 dramatically reduced the percentage of mature neutrophils by 10-fold and lactoferrin protein levels by threefold. Adding back K121 and K198 increased the percentage of mature neutrophils and restored levels of lactoferrin protein to nearly that observed for the wild-type C/EBPε32/30. Previously, the authors observed that overexpression of wild type C/EBPε32/30 in hematopoietic progenitor cells promotes eosinophil differentiation at the expense of neutrophils and C/EBPε27 and C/EBPε14 block eosinophil differentiation.1 The authors observed this phenomenon with retroviral transduction of the wild-type C/EBPε32/30, but the R121/198K mutant restored neutrophil differentiation, and no increase in eosinophil precursors was observed. One conclusion could be that acetylation at these lysines is selectively important for differentiation along either the neutrophil or eosinophil lineages (see figure).1 A limitation of the current study is that the conclusions are based on in vitro experiments. Future studies in a mouse model could provide more definitive evidence that acetylation of C/EBPε is essential during granulopoiesis in vivo. For example, one could introduce loss of function (K to A) or gain of function (K to Q, an “acetylation mimic”) mutations in the endogenous Cebpe gene by genome editing and determine the impact on terminal neutrophil differentiation.
Prior studies with nicotinamide suggest a potentially important function for acetylation of C/EBP members in boosting neutrophil numbers and function.9,10 The current study’s novel findings provide for the first time the important experimental evidence that acetylation of C/EBPε is critical for terminal neutrophil differentiation. These findings establish a foundation for future work toward developing therapies that more effectively and specifically manipulate neutrophil production and function with the goal of treating neutropenia and neutrophil dysfunction.
Conflict-of-interest disclosure: The author declares no competing financial interests.