In this issue of Blood, Khorashad et al1 show that genetic (eg, short hairpin RNA [shRNA]-mediated) or pharmacologic (eg, KPT-330 [selinexor]) inhibition of nucleocytoplasmic protein trafficking restored sensitivity to tyrosine kinase inhibitors (TKIs) and impaired clonogenic potential of chronic myeloid leukemia (CML) cell lines with BCR-ABL1 kinase-independent TKI resistance (see figure).
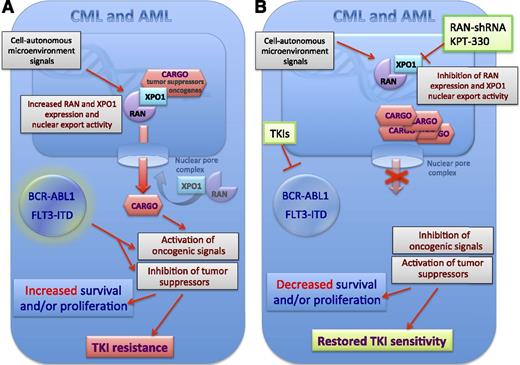
Mechanisms of oncogene kinase-independent resistance to TKI therapy in CML with wild-type BCR-ABL1 and FLT3-ITD+ AML. The expression of the nuclear export factors RAN and XPO1 was found to be increased in CML and AML in an oncogene kinase-independent manner and likely through either signals generated by the microenvironment or by cell-autonomous BCR-ABL or FLT3-ITD kinase-independent signals (A). This results in increased nuclear export of their cargo that also is comprehensive of oncogenic signal transducers (eg, SET) and tumor-suppressor proteins (eg, p53) that if delocalized become activated and inactivated, respectively. Such an effect together with the BCR-ABL1- or FLT3-ITD-generated oncogenic signals will both enhance proliferation and/or survival of myeloid progenitors. Interestingly, Khorashad et al1 show that the activity of XOP1 and RAN seems to specifically control those oncogene kinase-independent molecular events leading to resistance to TKI-induces apoptosis. Khorashad et al1 reported that inhibition of nuclear export by downregulation of RAN or pharmacologic inhibition of XPO1 activity by the clinically relevant karyopherin inhibitor KPT-330 results in nuclear accumulation of their cargo and, consequently, restored sensitivity of leukemic progenitors to TKI-induced inhibition of proliferation and/or survival and restoration of sensitivity to TKI treatment (B).
Mechanisms of oncogene kinase-independent resistance to TKI therapy in CML with wild-type BCR-ABL1 and FLT3-ITD+ AML. The expression of the nuclear export factors RAN and XPO1 was found to be increased in CML and AML in an oncogene kinase-independent manner and likely through either signals generated by the microenvironment or by cell-autonomous BCR-ABL or FLT3-ITD kinase-independent signals (A). This results in increased nuclear export of their cargo that also is comprehensive of oncogenic signal transducers (eg, SET) and tumor-suppressor proteins (eg, p53) that if delocalized become activated and inactivated, respectively. Such an effect together with the BCR-ABL1- or FLT3-ITD-generated oncogenic signals will both enhance proliferation and/or survival of myeloid progenitors. Interestingly, Khorashad et al1 show that the activity of XOP1 and RAN seems to specifically control those oncogene kinase-independent molecular events leading to resistance to TKI-induces apoptosis. Khorashad et al1 reported that inhibition of nuclear export by downregulation of RAN or pharmacologic inhibition of XPO1 activity by the clinically relevant karyopherin inhibitor KPT-330 results in nuclear accumulation of their cargo and, consequently, restored sensitivity of leukemic progenitors to TKI-induced inhibition of proliferation and/or survival and restoration of sensitivity to TKI treatment (B).
It is increasingly the case that a deeper knowledge of the crosstalk between leukemic stem and progenitor cells and their bone marrow microenvironment is needed to fully understand how the latter influences leukemia development, progression, and resistance to targeted therapies. This also appears to be true for those cases of CML that show BCR-ABL1 kinase-independent resistance to TKI therapy.
Given that missense mutations in the BCR-ABL1 kinase domain explain only 30% to 40% of clinical imatinib resistance cases, and that ∼20% to 40% of newly diagnosed CML patients eventually require alternative therapies due to TKI intolerance or resistance,2 the understanding of the still largely unclear molecular events regulating drug resistance in patients without BCR-ABL1 mutations appears to be of extreme clinical relevance. As also indicated by Khorashad et al,1 this is true not only for CML but also for those cases of acute myeloid leukemia (AML) showing FLT3-independent resistance to FLT3-targeting drugs.3
Mechanistically, Khorashad et al1 have identified signaling pathways associated with BCR-ABL1 kinase-independent TKI resistance by performing a lentiviral shRNA library screen on K562 cells (K562S, imatinib sensitive) and an imatinib-resistant derivative line (K562R) that maintains viability despite suppression of BCR-ABL1 kinase activity. Genes with a potential role in resistance were selected based on criteria designed to minimize false-positive results. The RAS-related nuclear protein RAN and the karyopherin β family member XPO1 (exportin-1, also called chromosome maintenance protein 1 [CRM1]), two interacting proteins with key functions in nucleocytoplasmic transport, were among the top 5 candidates, suggesting a role for these factors and corresponding signal transduction pathways in BCR-ABL1–independent TKI resistance (see figure).
RAN is involved in the transport of proteins across the nuclear envelope by interacting with karyopherins and changing their ability to bind or release cargo molecules. Cargo proteins containing nuclear localization signals are bound by importins and transported into the nucleus. Inside the nucleus, RAN–guanosine triphosphate (GTP) binds to importin and releases the import cargo. Cargo that needs to exit the nucleus into the cytoplasm binds to exportin in a ternary complex with RAN-GTP. Upon hydrolysis of RAN-GTP to RAN–guanosine diphosphate (GDP) outside the nucleus, the complex dissociates and the export cargo is released. Khorashad et al1 found that RAN and XPO1 synergize to promote nucleocytoplasmic trafficking of cargo proteins through the nuclear pore complex. Although binding of XPO1 to either RAN or cargo protein alone is weak, simultaneous binding of RAN and cargo to XPO1 increases its affinity to both by 1000-fold (see figure). Notably, XPO1-mediated nucleocytoplasmic protein trafficking regulates the function of tumor suppressors and oncogenes (eg, SET, PP2A, p53, p21, p27, NF-κB, Mcl-1, myc, Rb, BRCA1, APC, NMP1, and FoxO3a) that play an important role in survival and proliferation of normal and cancer cells, including different types of lymphoid and myeloid and acute and chronic leukemias (reviewed in Turner et al4 and Tan et al5 ).
Interestingly, Khorashad et al1 also identified through the shRNA library screen many other pathways whose roles in TKI resistance are yet to be experimentally validated. Among these pathways are genes involved in proteasomal protein degradation, chromatin remodeling, protein biosynthesis, cell-cycle regulation, apoptosis, antioxidation, ubiquitination, and DNA repair. In particular, 5 of the top 50 genes (PSMA1, UBE1, NEDD8, PSMD3, and PSMD1) are associated with proteasome-dependent protein degradation, which has been implicated in TKI resistance of leukemic stem and progenitor cells.6 Thus, nuclear export and signaling linking the stem/progenitor cell to the microenvironment will further elucidate BCR-ABL–independent signaling in CML and AML.
XPO1/RAN-mediated export was implicated in many types of solid tumors and hematologic malignancies.7-10 Given that XPO1 is a critical regulator of cell proliferation and survival, which is not only overexpressed but also described as a poor prognostic factor in different hematologic malignancies, it is not surprising that different inhibitors of XPO1-mediated export through the nuclear pore complex have been developed. Among these, the selective inhibitors of nuclear export (SINE; Karyopharm Therapeutics) are leptomycin B–based small molecules that irreversibly bind to Cys528 in the cargo-binding groove of XPO1 to prevent XPO1-cargo interactions (see figure). Previous studies have shown that the closely related SINE compounds KPT-251, KPT-276, and KPT-330 have strong antileukemic activity and minimal and acceptable adverse effects in acute myelogenous leukemia and CML in blastic transformation.8-10 Notably, the clinically relevant XPO1 inhibitor KPT-330 leads to apoptosis and impairment of leukemic clonogenic potential of leukemic but not normal CD34+ progenitors and significantly increased survival of leukemic mice. Mechanistically, KPT-330 altered the subcellular localization of leukemia-regulated factors, including RNA-binding heterogeneous nuclear ribonucleoprotein A1 and the oncogene SET, thereby inducing reactivation of protein phosphatase 2A tumor suppressor and inhibition of BCR-ABL1 in CML blast crisis cells. Because XPO1 is important for leukemic cell survival, KPT-330 may represent an alternative therapy for TKI-refractory Ph+ leukemias.8 Thus, the notion that RAN/XPO1 activity controls oncogene kinase-independent drug resistance in both AML and CML1 further supports the use of the available XPO1 inhibitors in therapeutic protocols for those patients. Notably, the SINE KPT-330 is currently in clinical trials for advanced hematologic malignancies and solid tumors (NCT01607892 and NCT01607905). Furthermore, the work of Khorashad et al1 opens the gateway to characterize microenvironment-generated signals responsible for altered XPO1 expression/activity and, consequently, to develop strategies to efficiently counteract drug resistance in AML as well as in those cases of CML not responding to TKI monotherapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.