Key Points
Epitopes in antigen-armed antibodies that target B-cell receptors are efficiently presented by B lymphoma cells to cytotoxic CD4+ T cells.
Memory T cells activated by AgAbs ex vivo are able to kill targeted B lymphoma cells.
Abstract
The treatment of non-Hodgkin lymphomas has benefited enormously from the introduction of monoclonal antibody-based therapies. However, the efficacy of these treatments varies with lymphoma subtypes and typically decreases with subsequent relapses. Here, we report on antigen-armed antibodies (AgAbs) as a potential treatment of B-cell lymphoma. AgAbs include antigens from ubiquitous pathogens, such as Epstein-Barr virus (EBV), that persist in their host and elicit strong lifelong T-cell responses. They act as vectors by introducing antigen directly into tumor cells to induce an antigen-specific CD4+ T-cell response against these cells. We have fused antibodies targeting human B-cell surface receptors (CD19-22) to immunodominant T-cell antigens from EBV proteins, including EBNA1, EBNA3B, and EBNA3C. Exposure of EBV-transformed B cells and of Burkitt lymphoma cells to AgAbs led to antigen presentation, T-cell recognition, and target cell killing. The efficiency of AgAb action paralleled the abundance of the targeted molecules on lymphoma cells as well as their HLA class II expression levels. AgAbs can also induce activation and proliferation of EBV-specific memory CD4+ T cells ex vivo. These studies show the potential of AgAbs as an effective therapeutic strategy against B-cell lymphomas.
Introduction
B-cell lymphomas represent approximately 90% of all non-Hodgkin lymphomas in Western countries.1,2 They include diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, and Burkitt lymphoma (BL).2 The development of monoclonal antibodies, such as rituximab, to target specific markers on B lymphoma cells has completely revolutionized treatment and improved patient prognosis.3,4 Rituximab adheres to CD20 and orchestrates cell death via complement- and antibody-mediated cellular cytotoxicity and by sensitizing malignant B cells to concomitant chemotherapy.5,6 Current treatment regimens now generally include combinations of conventional cancer treatments, typically including cytostatic drugs, and monoclonal antibodies directed against molecules expressed on the surface of lymphoma cells.7,8 Unfortunately, however, not all lymphoma types are sensitive to antibody treatment and those that are frequently become resistant.9 For this reason, alternative antibody molecules are under development—including antibodies coupled to various oncolytic substances such as toxins, radionuclides, and cytotoxic drugs.10 These armed antibodies are substantially more active than their classical counterparts, but so far do not represent a curative treatment.
Antigen-armed antibodies (AgAbs) are antibodies fused to antigens first described by Lunde et al, who demonstrated that antigen could be delivered to antigen-presenting cells, including dendritic and B cells, using antibody as a targeting vehicle and that this resulted in activation of antigen-specific T cells, both in vitro and in vivo.11,12 Diverse antigens could be used in AgAbs, including the hemagglutinin protein of influenza virus, hen egg lysozyme, and ovalbumin.13 Therefore, AgAbs represent a promising vaccine strategy for viral and bacterial pathogens such as Epstein-Barr virus (EBV), HIV, and pneumonic plague14-19 as well as cancer vaccines using specific tumor antigens, such as MAGE-A3.20 Most studies have focused on dendritic cells by targeting cell surface receptors, such as DEC-205, Langerin, and Clec9a. However, Leung and colleagues demonstrated that antibodies directed against DEC-205 were also effective in targeting activated EBV-transformed B cells (lymphoblastoid B-cell lines, LCLs).19 LCLs express DEC-205 at similar levels to dendritic cells and efficiently present targeted EBV nuclear antigen 1 (EBNA1) and EBV-latent membrane protein 1 (LMP1) to EBNA1- and LMP1-specific CD4+, and to a lesser extent, CD8+ T-cell clones in vitro.19
The effectiveness of the AgAb strategy in inducing a T-cell response against the viral antigen it carries could be adapted to specifically target B lymphoma cells. This requires an antibody that efficiently targets B-cell lymphoma cells and induces presentation of the antigen it carries at the surface of the tumor cells to elicit a specific immune response. We have generated AgAbs that target various B-cell receptors and contain immunodominant T-cell epitopes from multiple EBV proteins. EBV is a ubiquitous gamma herpesvirus for which more than 90% of the adult human population is seropositive.21 By choosing epitopes from a virus such as EBV, we can ensure that the vast majority of the patient population will have circulating memory T cells that are specific to the epitopes contained in the AgAbs. In this study, we present our findings that LCLs and various BL cell lines effectively present antigen to cytotoxic CD4+ T cells after AgAb treatment targeted to the CD19, CD20, CD21, and CD22 B-cell receptors. This study demonstrates the potential of the AgAb treatment strategy in treating B-cell lymphomas.
Materials and methods
Primary cells and cell lines
Cell lines included HEK293,22 LCLs generated by transformation of B cells with EBV,23 and human EBV− (Awia, DG75,24 Yakobo) and EBV+ (AG876) BL cell lines (Awia, Yakobo, and AG876 kindly provided by Prof A. Rickinson). Hybridoma cell lines included THB-5 (αCD21),25 1F5 (αCD20; kindly provided by Dr G. Moldenhauer), and Cy34.1.2 (α-mouse CD22) (ATCC). Mononuclear cells were purified from blood buffy coats by density gradient centrifugation. Cells were grown in RPMI medium supplemented with 10% fetal calf serum. EBV-specific CD4+ T-cell clones were isolated from EBV-seropositive donors and cultivated as described previously.23,26 Ethical approval to use sera from voluntary donors was obtained from the Ethikkommission of the Medizinische Fakultät Heidelberg (S-36/2011).
Plasmids
Generation and expression of the plasmids containing the heavy- and light-chain genes of anti-human CD19-, CD20-, CD21-, and CD22-specific antibodies with and without EBV epitopes as well as of the different controls is described in the supplemental Methods on the Blood Web site, together with western blot and flow cytometry.
T-cell antigen recognition assays
Antigen presentation by LCLs and B lymphoma cell lines was tested in T-cell antigen recognition assays, as described previously.23 Briefly, B cells (5 × 104) were incubated with medium only, peptides, or antibodies for 24 hours. These were subsequently mixed for 18 hours with autologous EBV-specific CD4+ T-cell clones (1 × 105). Interferon-γ (IFN-γ) released into the supernatants was quantified by enzyme-linked immunosorbent assay (ELISA) system (R&D Systems).
Cytotoxicity assay
The ability of antigen-specific T cells to kill their targets was determined in a 4-hour chromium-51 (51Cr) release assay, as described previously.27 Target cells (1.5 × 105) were treated for 24 hours with peptide or AgAbs, or were untreated, and subsequently labeled with 51Cr (30 μCi). Each treatment group was divided into a negative control (medium only, spontaneous release), a positive control (treatment with 1% Triton-X 100, maximum release), and several test reactions containing increasing effector T cell to target cell (E:T) ratios. Effector T cells (EBV antigen-specific T-cell clones) were added at E:T ratios ranging from 1:1 to 50:1. Supernatants were harvested after 4 hours and cell lysis was determined by 51Cr release, as measured by a Microbeta Trilux liquid scintillation counter (PerkinElmer). The percentage of specific release was calculated as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) ×100. All experimental groups were performed in triplicate.
Generation and expansion of 5H11-specific T-cell clones ex vivo
Peripheral blood mononuclear cells (PBMCs) were isolated from blood of an EBV-positive donor whose HLA class II genotype was compatible with the LCLs used as antigen-presenting cells using density gradient centrifugation. A total of 5 × 106 PBMCs in 2 mL of AIM-V medium (Life Technologies) without interleukin-2 were pulsed with 1.3 ng of CD21-specifc 5H11-coupled antibody. After 2 weeks, 5 × 106 fresh PBMCs isolated from the same donor were pulsed with 1.3 ng of aCD21-5H11 antibody, irradiated (40 Gy), and mixed with the previously isolated PBMCs in AIM-V medium with 10 U/mL interleukin-2. The same procedure was repeated biweekly. After the seventh stimulation round, limiting dilution cloning was performed as described by seeding 1, 3, or 6 cells/well and using haplotype-matched irradiated (80 Gy) LCLs loaded with 5H11 peptide as stimulators.14,17 Outgrowing T-cell clones were expanded to perform T-cell assays with the aCD21-5H11 antibody.
Results
Recombinant AgAbs target B-cell surface receptors on LCLs
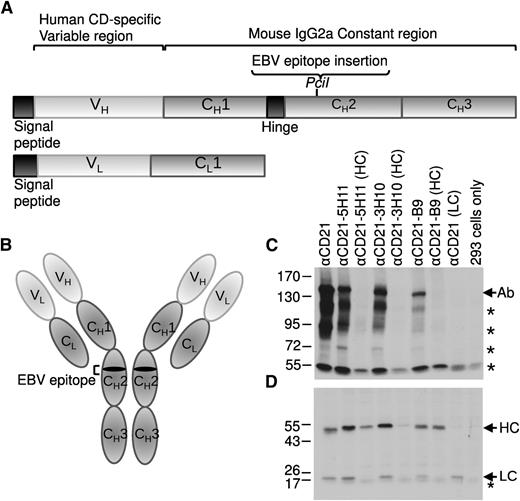
We generated a panel of eukaryotic expression plasmids encoding the heavy and light chains of mouse immunoglobulin G2a (IgG2a) antibodies directed against the B-cell surface receptors CD19, CD20, CD21, and CD22 (Figure 1A). To produce AgAbs (Figure 1B), various EBV epitopes were introduced into the CH2 region of the antibody heavy chains (Table 1). The heavy and light chains of the antibodies were expressed and assembled into whole antibody molecules in vitro after transfection of 293 cells (Figure 1C-D). Native antibodies (not containing EBV epitopes) were expressed at relatively high levels in vitro. Incorporation of the epitope into the antibody heavy chains resulted in some inhibition of whole antibody assembly, but did not appear to negatively affect heavy chain expression (Figure 1C-D). The degree of inhibition varied depending on the epitope; insertion of the 5H11 and 3H10 epitopes of EBNA3C resulted in only a small decrease in antibody, whereas insertion of the B9 epitope of EBNA3B led to dramatically less antibody (Figure 1C; Table 1). Similar expression patterns were observed for the CD19-, CD20-, and CD22-specific antibodies (data not shown). The functionality of these antibodies (without epitopes) in binding to LCLs was determined by in vitro flow cytometry analysis (supplemental Figure 1). All antibodies bound effectively to LCLs, but not to control 293 cells, with the exception of αCD21, which showed some low-level binding to 293 cells (supplemental Figure 1). It has previously been shown that 293 cells endogenously express CD21 at a low level, or a protein that cross-reacts with αCD21 antiserum.28 These data indicate the ability of the AgAbs to target antigen to B cells. To determine the potential of the LCLs to present AgAb-targeted antigen to either CD4+ or CD8+ T cells, we also determined the expression of HLA class I and II molecules on the cell surface by flow cytometry analysis (supplemental Figure 1). LCLs expressed high levels of both HLA class I and II.
Generation of recombinant antibodies containing EBV epitopes. (A) Schematic representation of the immunoglobulin heavy chain (HC) and light chain (LC) genes showing the PciI restriction enzyme site used for insertion of the EBV epitope sequences into the CH2 region of the HC gene. (B) The fully assembled antibody molecule is composed of 2 copies of the LC and 2 copies of the HC containing the EBV epitope. Plasmids expressing the HC and LC of the anti-CD21 antibody (with and without EBV epitopes) were transfected into 293 cells. A western blot analysis of transfected 293 cell extracts was performed under (C) native or (D) reduced conditions for αCD21 antibodies, including epitopes from the EBNA3C (5H11 and 3H10) and the EBNA3B (B9) proteins. An antibody specific to mouse immunoglobulin HC and LC was used to visualize the antibody proteins. In panel C, Ab represents the complete antibody molecule containing 2 HC and 2 LC and * represents other variations of the HC and LC complexes. In panel D, * represents a nonspecific band present in all samples.
Generation of recombinant antibodies containing EBV epitopes. (A) Schematic representation of the immunoglobulin heavy chain (HC) and light chain (LC) genes showing the PciI restriction enzyme site used for insertion of the EBV epitope sequences into the CH2 region of the HC gene. (B) The fully assembled antibody molecule is composed of 2 copies of the LC and 2 copies of the HC containing the EBV epitope. Plasmids expressing the HC and LC of the anti-CD21 antibody (with and without EBV epitopes) were transfected into 293 cells. A western blot analysis of transfected 293 cell extracts was performed under (C) native or (D) reduced conditions for αCD21 antibodies, including epitopes from the EBNA3C (5H11 and 3H10) and the EBNA3B (B9) proteins. An antibody specific to mouse immunoglobulin HC and LC was used to visualize the antibody proteins. In panel C, Ab represents the complete antibody molecule containing 2 HC and 2 LC and * represents other variations of the HC and LC complexes. In panel D, * represents a nonspecific band present in all samples.
List of antigens incorporated into AgAbs and of the T-cell clones used
| . | T-cell clone . | Name and the AA position of the T-cell epitope* . | HLA restriction . |
|---|---|---|---|
| CD8 | EBNA1-HPV | HPV AA407-417 −HPVGEADYFEY− | HLA-B*3501 |
| CD4 | EBNA1-3G2 | 3G2 AA514-528 −KTSLYNLRRGTALAI− | HLA-DRB1*1101 |
| EBNA2-5A11 | 5A11 AA138-150 −MANYIVRQSRGDRGL− | HLA-DRB1*0801 | |
| EBNA3B-B9 | B9 AA139-153 −EFIWMCMTVRHRCQA− | HLA-DRB1*1301 | |
| EBNA3C-3H10 | 3H10 AA627-640 −VVRMFMRERQLPQS− | HLA-DRB1*1101 | |
| EBNA3C-5H11 | 5H11 AA325-339 −ENPYHARRGIKEHVI− | HLA-DRB1*0801 |
| . | T-cell clone . | Name and the AA position of the T-cell epitope* . | HLA restriction . |
|---|---|---|---|
| CD8 | EBNA1-HPV | HPV AA407-417 −HPVGEADYFEY− | HLA-B*3501 |
| CD4 | EBNA1-3G2 | 3G2 AA514-528 −KTSLYNLRRGTALAI− | HLA-DRB1*1101 |
| EBNA2-5A11 | 5A11 AA138-150 −MANYIVRQSRGDRGL− | HLA-DRB1*0801 | |
| EBNA3B-B9 | B9 AA139-153 −EFIWMCMTVRHRCQA− | HLA-DRB1*1301 | |
| EBNA3C-3H10 | 3H10 AA627-640 −VVRMFMRERQLPQS− | HLA-DRB1*1101 | |
| EBNA3C-5H11 | 5H11 AA325-339 −ENPYHARRGIKEHVI− | HLA-DRB1*0801 |
AA, amino acid.
Epitope position based on the sequence of the B95-8 EBV strain.
LCLs efficiently present antigen targeted by AgAbs to CD4+ T cells
To determine the ability of LCLs to present antigen targeted by AgAbs, T-cell response assays were performed using CD4+ T-cell clones specific to epitopes from various EBV proteins (Figure 2; Table 1).23,26,29 We investigated the 5H11 and 3H10 epitopes of EBNA3C, the B9 epitope of EBNA3B, and the 5A11 epitope of EBNA2 (Table 1).23,26,29 T cells were mixed with HLA-matched LCLs (E:T ratio of 2:1) loaded with peptide controls; AgAbs specific to CD21, 20, 19, and 22, or the corresponding native antibodies; or with untreated LCLs (LCLs only). We also included an IgG2a isotype control AgAb that we constructed by cloning the 5H11 epitope within the heavy chain of an antibody that recognizes mouse CD22 (supplemental Figure 2). This control AgAb is well expressed in 293 cells and does not bind to human B cells (supplemental Figure 2). T-cell responses were determined by measuring IFN-γ production. Peptide controls stimulated strong responses in all CD4+ T-cell clones at amounts ranging from 1 μg to 1 ng peptide (per 5 × 104 LCLs). Untreated LCLs showed no T-cell activation, and, similarly, LCLs loaded with antibodies containing no epitope or with the isotype control AgAb induced no IFN-γ production. LCLs treated with AgAbs loaded with EBV epitopes activated very strong T-cell responses at amounts ranging from 10 ng to 10 pg (per 5 × 104 LCLs). This was observed for LCLs presenting antigen to T-cell clones of varying peptide specificities and HLA restrictions. Specifically, antibodies targeted to all 4 receptors induced EBNA3C 5H11 epitope presentation very efficiently. In 1 example, 1 ng αCD21-5H11 stimulated T-cell responses roughly equivalent to those stimulated by 1 µg 5H11 peptide (Figure 2A). Considering that the epitope comprises approximately 2.3% of the total weight of the antibody molecule, this corresponds to a >4300-fold difference in the amount of 5H11 peptide required to stimulate an equivalent T-cell response by peptide alone vs antibody-targeted peptide treatment. AgAbs targeted the EBNA3C 3H10 epitope to LCLs at a similar efficiency (Figure 2B). For example, 100 pg of the αCD21-3H10 activated similar levels of T-cell responses as 10 ng of 3H10 peptide, demonstrating that approximately 4300-fold less 3H10 peptide is required to stimulate a comparable response with antibody-targeted peptide treatment vs peptide alone. AgAbs targeting the EBNA3B B9 and EBNA2 5A11 epitopes to LCLs were also efficient at stimulating T-cell responses, but at a lower level (Figure 2C-D). For example, in both cases, 10 ng of the αCD21-AgAbs and 100 ng of peptide alone activated comparable T-cell responses, indicating that approximately 430-fold less B9 and 5A11 peptides are required with antibody-targeted peptide treatment vs treatment with peptide alone (Figure 2Ci-D). However, this response was highly specific: matched T cells that recognize the M1 influenza protein did not react with LCLs treated with the αCD21-B9 AgAb (Figure 2Cii). AgAbs directed against CD19, CD20, and CD22 demonstrated similar responses for EBNA3C 3H10–, EBNA3C 5H11–, and EBNA3B B9–specific T-cell clones (these antibodies were not studied for EBNA2-specific T-cell clones).
LCLs present antigen targeted by AgAb treatment to CD4+ T cells. Multiple T-cell recognition assays were performed using LCLs pulsed with various amounts of αCD-21, -20, -19, or -22 antibodies fused with different EBV epitopes, including (A) EBNA3C 5H11, (B) EBNA3C 3H10, (Ci-Cii) EBNA3B B9, and (D) EBNA2 5A11 (αCD21 antibodies only for EBNA2 epitope). LCLs, HLA-matched with EBV-specific CD4+ T-cell clones, were incubated for 24 hours either with appropriate peptide controls (100 pg, 1 ng, 10 ng, 100 ng, and 1 μg, corresponding to 500 pg/mL, 5 ng/mL, 50 ng/mL, 500 ng/mL, and 5 μg/mL, respectively; gray bars in A,B,Ci,D), medium only, or with antibodies containing epitopes (10 pg, 100 pg, 1 ng, and 10 ng, corresponding to 50 pg/mL, 500 pg/mL, 5 ng/mL, and 50 ng/mL, respectively), or containing no epitopes (10 ng, corresponding to 50 ng/mL). Panel A also includes the results of a T-cell assay performed with the anti–CD21-5H11 AgAb isotype control, whereas panel Cii shows an experiment performed with a matched T-cell clone that recognizes the influenza epitope M1 (gray bars in Cii). EBV epitope–specific CD4+ T cells were incubated with these target cells at an E:T cell ratio of 2:1. T-cell activity was determined after 18 hours by measuring IFN-γ secretion by ELISA. Results are given in picograms/milliliter. For each chart, data represent triplicate values and error bars indicate standard deviations. One representative experiment of at least 3 is shown.
LCLs present antigen targeted by AgAb treatment to CD4+ T cells. Multiple T-cell recognition assays were performed using LCLs pulsed with various amounts of αCD-21, -20, -19, or -22 antibodies fused with different EBV epitopes, including (A) EBNA3C 5H11, (B) EBNA3C 3H10, (Ci-Cii) EBNA3B B9, and (D) EBNA2 5A11 (αCD21 antibodies only for EBNA2 epitope). LCLs, HLA-matched with EBV-specific CD4+ T-cell clones, were incubated for 24 hours either with appropriate peptide controls (100 pg, 1 ng, 10 ng, 100 ng, and 1 μg, corresponding to 500 pg/mL, 5 ng/mL, 50 ng/mL, 500 ng/mL, and 5 μg/mL, respectively; gray bars in A,B,Ci,D), medium only, or with antibodies containing epitopes (10 pg, 100 pg, 1 ng, and 10 ng, corresponding to 50 pg/mL, 500 pg/mL, 5 ng/mL, and 50 ng/mL, respectively), or containing no epitopes (10 ng, corresponding to 50 ng/mL). Panel A also includes the results of a T-cell assay performed with the anti–CD21-5H11 AgAb isotype control, whereas panel Cii shows an experiment performed with a matched T-cell clone that recognizes the influenza epitope M1 (gray bars in Cii). EBV epitope–specific CD4+ T cells were incubated with these target cells at an E:T cell ratio of 2:1. T-cell activity was determined after 18 hours by measuring IFN-γ secretion by ELISA. Results are given in picograms/milliliter. For each chart, data represent triplicate values and error bars indicate standard deviations. One representative experiment of at least 3 is shown.
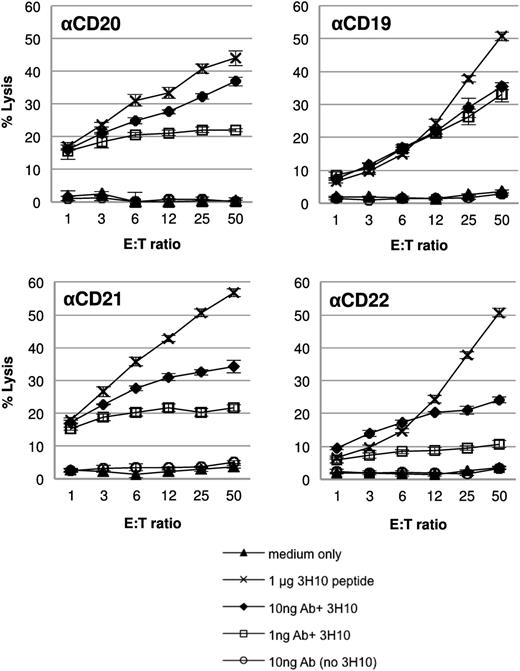
Next, we investigated whether CD4+ T cells activated by AgAbs containing the EBNA3C 3H10 epitope were capable of killing the LCL target cells (Figure 3). We used 51Cr-release killing assays to determine cytotoxic activity. Target LCLs (1.5 × 105) were loaded with 1 μg 3H10 peptide, 10 ng or 1 ng 3H10 AgAbs, or 10 ng antibodies without epitope, or left untreated (medium only) and mixed with effector T cells. The percentage of total cell lysis was determined as an indicator of the ability of the T cells to recognize and kill their targets. All AgAbs (αCD20-, αCD19-, αCD21-, and αCD22-3H10) induced target cell killing at amounts of both 10 and 1 ng (Figure 3). Furthermore, peptide alone induced target cell killing, whereas treatment with antibody containing no epitope produced cell lysis at levels equivalent to the media only control. Taken together, these data demonstrate that AgAb treatment of LCLs leads to antigen presentation to CD4+ T cells and T-cell activation that results in directed T-cell killing of the target LCLs.
CD4+ T cells activated by AgAb treatment can kill target LCLs. A 4-hour 51Cr-release cytotoxicity assay was performed. LCLs were either untreated (medium only) or treated with 1 μg EBNA3C 3H10 peptide (corresponding to 5 μg/mL), 10 ng or 1 ng antibodies (corresponding to 50 ng/mL or 5 ng/mL of αCD20, αCD19, αCD21, and αCD22) containing the EBNA3C 3H10 epitope, or 10 ng antibody without epitope (corresponding to 50 ng/mL) for 24 hours. LCLs were then incubated with CD4+ T cells specific to the EBNA3C 3H10 epitope for 4 hours at varying E:T ratios. Cell lysis was determined by 51Cr release. All assays were performed in triplicate and means and standard deviations are shown.
CD4+ T cells activated by AgAb treatment can kill target LCLs. A 4-hour 51Cr-release cytotoxicity assay was performed. LCLs were either untreated (medium only) or treated with 1 μg EBNA3C 3H10 peptide (corresponding to 5 μg/mL), 10 ng or 1 ng antibodies (corresponding to 50 ng/mL or 5 ng/mL of αCD20, αCD19, αCD21, and αCD22) containing the EBNA3C 3H10 epitope, or 10 ng antibody without epitope (corresponding to 50 ng/mL) for 24 hours. LCLs were then incubated with CD4+ T cells specific to the EBNA3C 3H10 epitope for 4 hours at varying E:T ratios. Cell lysis was determined by 51Cr release. All assays were performed in triplicate and means and standard deviations are shown.
Expression of B-cell markers and HLA molecules on B lymphoma cell lines
Because LCLs effectively present antigen, we next sought to investigate B lymphoma cell lines for their ability to present antigen to T cells. We obtained B lymphoma cell lines generated from BL and tested their HLA type. Cells matching the HLA restriction of the CD4+ T-cell clones were further investigated. This included the Awia, Yakobo, and AG876 cell lines for the EBNA3C 3H10–specific T cells and the DG75 cell line for the EBNA3B B9–specific T cells. First, we determined the level of expression of the cell surface markers including CD21 (supplemental Figure 3), CD19, CD20, and CD22 (supplemental Figure 4) by flow cytometry analysis. The CD21 receptor was expressed on the surface of all BL cell lines with the exception of DG75. In addition, expression of the CD19, CD20, and CD22 receptors was also detected on the surface of all of the BL cell lines (supplemental Figure 4). We also performed flow cytometry analysis to determine the level of HLA I and II antigen expression on the surface of the BL cell lines to investigate their potential to present AgAb-targeted antigens to T cells (supplemental Figure 3). All BL cells demonstrated expression of HLA I and II antigens on their surface.
B lymphoma cell lines efficiently present antigen targeted by AgAbs to CD4+ T cells
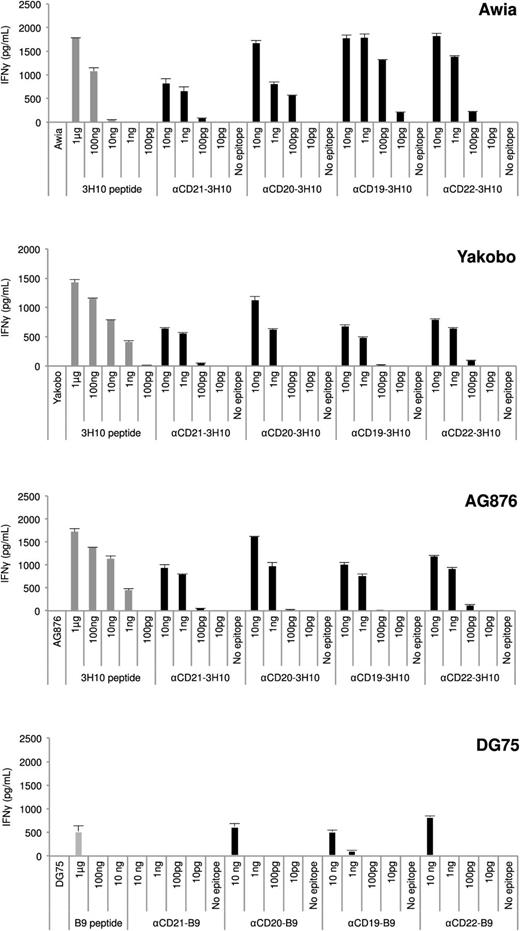
To investigate the propensity for BL cells to present antigen targeted by AgAbs to T cells, T-cell response assays were performed using CD4+ T-cell clones specific to epitopes from various EBV proteins and that matched the haplotypes of the BL cells (Figure 4). T cells were mixed with HLA-matched BL cells (E:T ratio of 2:1) loaded with peptide controls; AgAbs specific to CD21, 20, 19, and 22, or the corresponding antibodies without epitopes; or with untreated LCLs (LCLs only). T-cell responses were determined by measuring IFN-γ production. After treatment with 1 μg to 1 ng peptide only (per 5 × 104 BL cells), Awia, Yakobo, and AG876 cells presented antigen to T cells, resulting in a strong level of activation (Figure 4). The DG75 cells showed a much lower propensity to activate T cells after peptide treatment, with 1 μg peptide resulting in a low level of T-cell activation and no T-cell activation with lesser amounts of peptide. Furthermore, untreated BL cells and BL cells loaded with antibodies containing no epitope did not activate T cells. The T-cell responses to AgAbs in the BL cells reflected the peptide controls. In the Awia, Yakobo, and AG876 cells, AgAbs induced strong T-cell responses (Figure 4). However, in DG75 cells, only very low responses to AgAbs were observed. AgAbs were not as efficient in mediating antigen presentation to CD4+ T cells in BL cells vs LCLs. For example, the αCD21-3H10 AgAb was 430-, 43-, and 43-fold more effective than peptide alone in Awia, Yakobo, and AG876 cells, respectively, compared with 4300-fold more effective than peptide in LCLs. However, the αCD21-AgAb was the least efficient of the AgAbs with regard to BL-cell activation of T cells. In another example, the αCD22-AgAbs were 4300-, 430-, 430-, and 4300-fold more effective than peptide alone in Awia, Yakobo, AG876, and DG75 cells, respectively. In the DG75 BL cells, we observed only low levels of T-cell activation, but for the αCD21-B9 AgAb, we did not see any T-cell responses, which is not surprising given the lack of CD21 on the surface of these cells (supplemental Figure 3). Interestingly, DG75 cells that were induced to express CD21 after stable transfection with a CD21 expression plasmid could present antigen targeted via CD21 to CD4+ T cells (supplemental Figure 5).
B lymphoma cells can present antigen targeted by AgAbs to CD4+ T cells. T-cell recognition assays were performed using B lymphoma cell lines HLA-matched for CD4+ T cells specific to either the EBNA3C 3H10 or EBNA3B B9 epitope. B lymphoma cells including Awia, Yakobo, AG876, and DG75 were incubated with various amounts of αCD-21, -20, -19, or -22 antibodies. B lymphoma cells were incubated for 1 hour with appropriate peptide controls (100 pg, 1 ng, 10 ng, 100 ng, and 1 μg, corresponding to 500 pg/mL, 5 ng/mL, 50 ng/mL, 500 ng/mL, and 5 μg/mL, respectively; gray bars) or for 24 hours with either medium only, or with antibodies containing epitopes (10 pg, 100 pg, 1 ng, and 10 ng, corresponding to 50 pg/mL, 500 pg/mL, 5 ng/mL, and 50 ng/mL, respectively) or containing no epitopes (10 ng, corresponding to 50 ng/mL). EBV epitope–specific CD4+ T cells were incubated with these target cells at an E:T cell ratio of 2:1. T-cell activity was determined after 18 hours by measuring IFN-γ secretion by ELISA. Results are given in picograms/milliliter. For each chart, data represent triplicate values and error bars indicate standard deviations. One representative experiment of at least 3 is shown.
B lymphoma cells can present antigen targeted by AgAbs to CD4+ T cells. T-cell recognition assays were performed using B lymphoma cell lines HLA-matched for CD4+ T cells specific to either the EBNA3C 3H10 or EBNA3B B9 epitope. B lymphoma cells including Awia, Yakobo, AG876, and DG75 were incubated with various amounts of αCD-21, -20, -19, or -22 antibodies. B lymphoma cells were incubated for 1 hour with appropriate peptide controls (100 pg, 1 ng, 10 ng, 100 ng, and 1 μg, corresponding to 500 pg/mL, 5 ng/mL, 50 ng/mL, 500 ng/mL, and 5 μg/mL, respectively; gray bars) or for 24 hours with either medium only, or with antibodies containing epitopes (10 pg, 100 pg, 1 ng, and 10 ng, corresponding to 50 pg/mL, 500 pg/mL, 5 ng/mL, and 50 ng/mL, respectively) or containing no epitopes (10 ng, corresponding to 50 ng/mL). EBV epitope–specific CD4+ T cells were incubated with these target cells at an E:T cell ratio of 2:1. T-cell activity was determined after 18 hours by measuring IFN-γ secretion by ELISA. Results are given in picograms/milliliter. For each chart, data represent triplicate values and error bars indicate standard deviations. One representative experiment of at least 3 is shown.
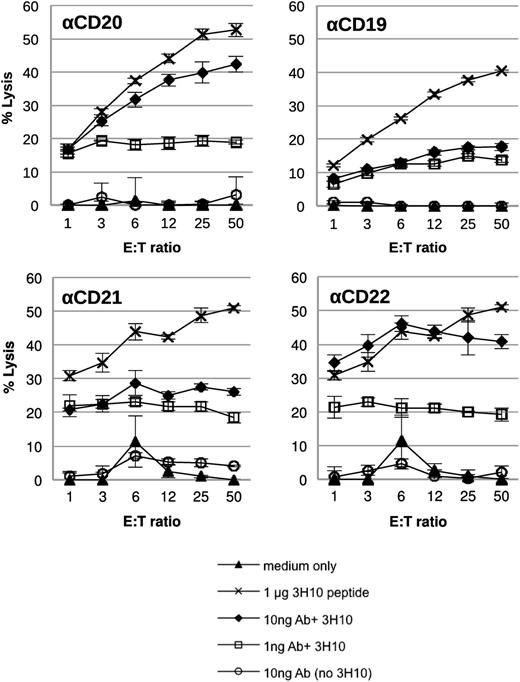
Next we investigated whether CD4+ T cells activated by AgAbs containing the EBNA3C 3H10 epitope were capable of killing AG876 BL target cells (Figure 5). In an assay similar to the one performed using LCLs as the target cells (Figure 3), we used 51Cr-release killing assays to determine cytotoxic activity. All AgAbs (αCD20-, αCD19-, αCD21-, and αCD22-3H10) induced target cell killing at amounts of both 10 ng and 1 ng (Figure 5).
CD4+ T cells activated by AgAb treatment can kill target B lymphoma cells. A 4-hour 51Cr-release cytotoxicity assay was performed, in which the BL cells (AG876) targeted by AgAb treatment were used as target cells. BL cells were either untreated (medium only) or treated with 1 μg EBNA3C 3H10 peptide (corresponding to 5 μg/mL), 10 ng, or 1 ng antibodies (corresponding to 50 ng/mL and 5 ng/mL; αCD20, αCD19, αCD21, and αCD22) containing the EBNA3C 3H10 epitope, or 10 ng antibody without epitope (corresponding to 50 ng/mL) for 24 hours. BL cells were then incubated with CD4+ T cells specific to the EBNA3C 3H10 epitope for 4 hours at varying E:T ratios. Cell lysis was determined by 51Cr release. All assays were performed in triplicate wells and means and standard deviations are shown.
CD4+ T cells activated by AgAb treatment can kill target B lymphoma cells. A 4-hour 51Cr-release cytotoxicity assay was performed, in which the BL cells (AG876) targeted by AgAb treatment were used as target cells. BL cells were either untreated (medium only) or treated with 1 μg EBNA3C 3H10 peptide (corresponding to 5 μg/mL), 10 ng, or 1 ng antibodies (corresponding to 50 ng/mL and 5 ng/mL; αCD20, αCD19, αCD21, and αCD22) containing the EBNA3C 3H10 epitope, or 10 ng antibody without epitope (corresponding to 50 ng/mL) for 24 hours. BL cells were then incubated with CD4+ T cells specific to the EBNA3C 3H10 epitope for 4 hours at varying E:T ratios. Cell lysis was determined by 51Cr release. All assays were performed in triplicate wells and means and standard deviations are shown.
These results suggest that treatment of BL cells with AgAbs can lead to the induction of a specific CD4+ T cell response, which results in a cytotoxic response against the BL cells. These data highlight the potential of AgAb treatment as a therapy against B-cell lymphoma, provided that these molecules can stimulate memory T cells specific for the antigen included in the AgAb.
AgAb can efficiently vehicle large protein segments into B cells to induce their presentation
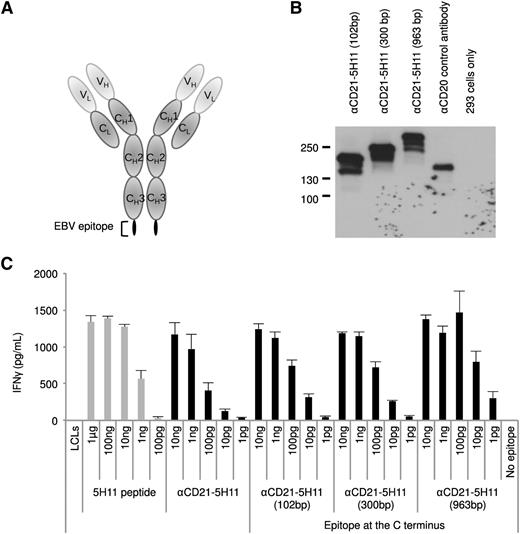
The results obtained so far unequivocally demonstrated that EBV-immortalized B cells or B-cell lymphomas can efficiently present a well-characterized epitope cloned into an AgAb. However, only cells that express the suitable MHC class II haplotype would present this epitope. Therefore, we cloned increasingly larger portions (102 bp, 300 bp, 963 bp) of the EBNA3C gene that includes the 5H11 peptide into the CD21-specific antibody to enlarge the panel of epitopes included in the AgAb and expand the size of the population that would recognize them. We then assessed the ability of these fusion proteins to generate a potent immune response using LCLs as antigen-presenting cells and a T-cell clone specific for 5H11. To avoid steric hindrance within the carrier antibody, we introduced the viral antigens at the C-terminal end of the Ig protein. These AgAbs are very well expressed after transfection in 293 cells, as shown in Figure 6. We successfully used 3 of the ex vivo–expanded specific T-cell clones to perform an IFN-γ release assay after pulsing the LCLs with the enlarged AgAbs. The result of a representative experiment with 1 of these clones is shown in Figure 6. We wished to extend these experiments to another large AgAb that comprises 600 bp from EBNA1. This EBNA1 fragment contains a CD4-restricted epitope (3G2) and a CD8-restricted epitope (HPV) (Table 1). This AgAb was expressed in 293 cells and used as an antigen source in an IFN-γ release assay with specific CD4- and CD8-restricted T-cell clones. Although the CD4-specific response was convincing, the signals generated by the CD8 epitope were at the limit of detection and difficult to interpret. Representative T-cell assays interrogating both epitopes are shown in supplemental Figure 6. These results confirm that the AgAb approach could be successfully adopted for the treatment of a large number of patients with diverse MHC haplotypes.
AgAb can accommodate and efficiently elicit presentation of large viral antigens. (A) We fused fragments of EBNA3C with the C terminus of the Ig heavy chain that recognizes human CD21 and assessed their ability to induce specific T-cell recognition. Three AgAbs were thus generated with a 102-, 300-, or 963-bp fragment from the EBNA3C open reading frame, each of which include the 5H11 epitope. (B) The AgAbs were expressed in 293 cells and submitted to a western blot analysis using antibodies specific for mouse IgG proteins. Nontransfected 293 cells provided negative controls and anti-human CD20 antibody was used as a control for transfection. (C) The results of a T-cell assay performed in triplicate with 1 of the ex vivo–expanded 5H11-specific T-cell clones and LCLs loaded with the larger AgAbs. All assays were performed in triplicate and means and standard deviations are shown.
AgAb can accommodate and efficiently elicit presentation of large viral antigens. (A) We fused fragments of EBNA3C with the C terminus of the Ig heavy chain that recognizes human CD21 and assessed their ability to induce specific T-cell recognition. Three AgAbs were thus generated with a 102-, 300-, or 963-bp fragment from the EBNA3C open reading frame, each of which include the 5H11 epitope. (B) The AgAbs were expressed in 293 cells and submitted to a western blot analysis using antibodies specific for mouse IgG proteins. Nontransfected 293 cells provided negative controls and anti-human CD20 antibody was used as a control for transfection. (C) The results of a T-cell assay performed in triplicate with 1 of the ex vivo–expanded 5H11-specific T-cell clones and LCLs loaded with the larger AgAbs. All assays were performed in triplicate and means and standard deviations are shown.
Ex vivo stimulation of CD4+ memory T cell clones
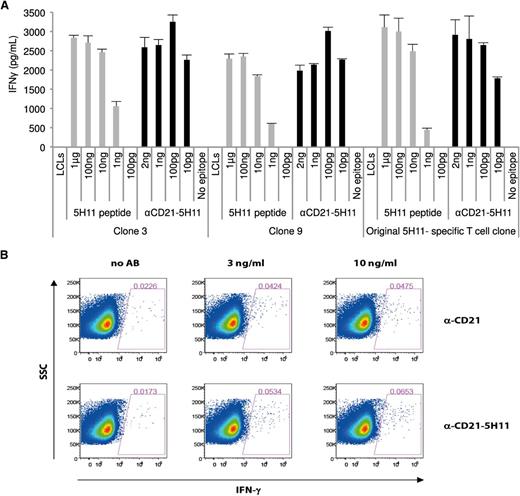
We then set out to determine the ability of the AgAbs to induce expansion and stimulate antigen-specific memory T cells. To that end, we incubated PBMCs from an EBV-positive male donor with the 5H11-coupled CD21-specific AgAb. After 7 rounds of successive stimulation, we obtained 10 clones, 4 of which were tested in IFN-γ release assay using the αCD21 antibody armed with the 5H11 epitope. All T-cell clones responded efficiently to this AgAb, whereas there was no response against LCLs loaded with αCD21 native antibody or against untreated LCLs (Figure 7 shows results for 2 clones). IFN-γ production by the T-cell clones was still significant when LCLs were loaded with 10 pg of the AgAb only. All tested newly generated T-cell clones showed an activation level comparable to the initially established 5H11 T-cell line (Figure 7). We then attempted to directly reactivate peripheral blood memory CD4+ T cells with AgAbs by performing an intracellular IFN-γ cytokine staining in fresh PBMCs from the same donor. Here we used the CD21-specific AgAbs that contained the 963-bp EBNA3C fragment. This led to a linear increase in the percentage of IFN-γ positive CD4+ cells from 0.0173% to 0.0653% that was higher than the background levels seen after incubation of PBMCs with control antibodies. The low frequency of EBNA3C-specific CD4+ T cells is consistent with studies performed in EBV-positive individuals with specific tetramers.30 These results provide solid evidence that AgAbs can reactivate circulating resting EBV-specific memory T cells and strongly increase the probability that this approach will elicit a potent T-cell response in vivo.
AgAb can stimulate the ex vivo activation and expansion of EBV-specific memory T-cell clones from an EBV-positive donor. (A) PBMCs were obtained from an EBV-positive donor who expressed the restricting HLA allele of the T-cell clone EBNA3C 5H11. These cells were subjected to 7 consecutive rounds of stimulation with 1.3 ng of αCD21 fused with 5H11. We obtained 10 T-cell clones by limiting dilution. A T-cell recognition assay was then performed with 4 of these clones using LCLs pulsed with the αCD21-5H11 AgAb. The results of assays performed in triplicate with 2 independent clones are shown here with obtained means and standard deviations. (B) Intracellular IFN-γ staining of PBMCs from the same donor. Freshly isolated PBMCs were incubated with the indicated concentrations of the 963-bp CD21-EBNA3C AgAb or α-CD21 control antibody. The percentage of IFN-γ positive CD3+CD4+ T cells was determined by intracellular cytokine staining 1 day later.
AgAb can stimulate the ex vivo activation and expansion of EBV-specific memory T-cell clones from an EBV-positive donor. (A) PBMCs were obtained from an EBV-positive donor who expressed the restricting HLA allele of the T-cell clone EBNA3C 5H11. These cells were subjected to 7 consecutive rounds of stimulation with 1.3 ng of αCD21 fused with 5H11. We obtained 10 T-cell clones by limiting dilution. A T-cell recognition assay was then performed with 4 of these clones using LCLs pulsed with the αCD21-5H11 AgAb. The results of assays performed in triplicate with 2 independent clones are shown here with obtained means and standard deviations. (B) Intracellular IFN-γ staining of PBMCs from the same donor. Freshly isolated PBMCs were incubated with the indicated concentrations of the 963-bp CD21-EBNA3C AgAb or α-CD21 control antibody. The percentage of IFN-γ positive CD3+CD4+ T cells was determined by intracellular cytokine staining 1 day later.
Discussion
Although the concept of AgAb treatment was originally intended as a vaccination strategy, in this article, we suggest an additional, alternative application. We have investigated this strategy as a method of activating circulating antigen–specific T cells to induce a cytotoxic response directed against proliferating B cells expressing the antigen on their surface. Indeed, we showed that treatment of LCLs and BL cells with AgAbs targeting B-cell surface receptors and containing various EBV epitopes resulted in specific activation of cytotoxic CD4+ T cells that were able to kill the target B cells. Despite multiple molecular abnormalities, including a downregulation of costimulatory molecules that impede the ability of BL cells to activate CD4+ T cells, AgAbs-treated lymphoma cells were efficiently recognized by antigen-specific CD4+ T cells.31 This underscores the power of these fusion proteins and suggests that they might even be more active in other lymphoma types. Moreover, we were able to reactivate EBV-specific memory T cells ex vivo using PBMCs from an EBV-positive donor loaded with AgAbs fused with EBV epitopes. These results suggest that the AgAb will elicit an immune response against targeted tumor cells in vivo and highlight the potential of the AgAb strategy as a therapeutic option to stimulate B lymphoma–specific T-cell responses in EBV-seropositive patients. We have also shown that it is possible to incorporate large fragments of the viral proteins into AgAbs. These contain multiple epitopes that will be recognized by a large number of MHC molecules. Therefore, it should be feasible to efficiently treat all individuals with a B-cell lymphoma with AgAbs independently of their MHC genotype.
CD19, CD20, CD21, and CD22 were efficiently targeted by AgAb treatment in our study. Indeed, the cell surface expression of these molecules can vary between cell lines and between lymphoma subtypes. In addition, relapse in lymphoma patients after treatment with monoclonal antibodies such as rituximab has been associated with downregulation of CD20 receptors.9,32 For these reasons, it may be advantageous to use a combination of antibodies targeting various cell surface receptors for treating lymphoma. This would provide alternative targets in the case that 1 molecule is not expressed or is downregulated.
One of the concerns of antibody-based therapies is the downregulation of normal immune cell populations. The B-cell receptors investigated here are expressed on B cells at varying stages of development. CD19 is present from the pro–B-cell until the early plasma B cell, CD22 and CD20 are expressed from the pre–B-cell stage through to the germinal center stage, and CD21 is expressed on both immature and mature B cells.33 Therefore, treatment with antibodies targeting these receptors will not result in permanent depletion of normal B cells. However, it is likely that there will be depletion of both normal and malignant B cells expressing these receptors, although it is unlikely that this will occur at a greater extent than what is already observed with current monoclonal antibody-based therapies.
Most therapeutic approaches against cancer based on cytotoxic cells have used CD8+ cells. However, cytotoxic CD4+ T cells are also extremely efficient at eliminating cells that present a specific antigen on MHC class II.34 This fits with the observation that EBNA1-specific cytotoxic CD4+ cells are able to kill EBV+ BL cells.35 Our work reports evidence that the therapeutic potential of CD4+ cytotoxic cells warrants further investigation.
In summary, this study demonstrates that the potential of the AgAb strategy extends further than vaccination and that AgAbs could be used to stimulate CD4+ T-cell responses specifically directed against B lymphoma cells; as a result, we propose this approach as a novel therapeutic strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Gerhard Moldenhauer for providing us with the 1F5 mouse anti-human CD20 IgG2a hybridoma cells, Professor Alan Rickinson for the gift of many of the Burkitt’s lymphoma cell lines, and Ms Carmen Hofmann for excellent technical support.
This work was funded by the German Cancer Aid (Deutsche Krebshilfe contract 110621).
Authorship
Contribution: J.M., R.F., E.J.B., and H.-J.D. designed the experiments; X.Y., E.J.B., M.I., V.S., J.M., and R.F. performed the experiments; X.Y., M.I., R.F., J.M., E.J.B., and H.-J.D. analyzed the data; R.B. provided essential technical support and advice; M.I., J.M., R.F., and H.-J.D. wrote the article; and all authors approved the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for R.F. is Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Molecular Immunology, Marchioninistrasse 25, 81377 Munich, Germany. The current affiliation for X.Y. is Department of Hepato-Pancreato-Biliary Surgery, The First Affiliated Hospital, Anhui Medical University, Meishan Rd 81, Hefei 230000, Anhui, China.
Correspondence: Henri-Jacques Delecluse, Pathogenesis of Virus-Associated Tumors (F100), DKFZ, Im Neuenheimer Feld 242, 69120 Heidelberg, Germany; e-mail: h.delecluse@dkfz.de.
References
Author notes
X.Y. and M.I. contributed equally.
J.M., R.F., and H.-J.D. contributed equally.