Key Points
HIV-1 Nef reprograms human macrophage migration favoring the mesenchymal mode, which translates in vivo to macrophage tissue accumulation.
Nef enhances mesenchymal migration by influencing podosome organization and function via the phagocyte-specific kinase Hck and WASP.
Abstract
Macrophages are motile leukocytes, targeted by HIV-1, thought to play a critical role in host dissemination of the virus. However, whether infection impacts their migration capacity remains unknown. We show that 2-dimensional migration and the 3-dimensional (3D) amoeboid migration mode of HIV-1–infected human monocyte-derived macrophages were inhibited, whereas the 3D mesenchymal migration was enhanced. The viral protein Nef was necessary and sufficient for all HIV-1–mediated effects on migration. In Nef transgenic mice, tissue infiltration of macrophages was increased in a tumor model and in several tissues at steady state, suggesting a dominant role for mesenchymal migration in vivo. The mesenchymal motility involves matrix proteolysis and podosomes, cell structures constitutive of monocyte-derived cells. Focusing on the mechanisms used by HIV-1 Nef to control the mesenchymal migration, we show that the stability, size, and proteolytic function of podosomes are increased via the phagocyte-specific kinase Hck and Wiskott-Aldrich syndrome protein (WASP), 2 major regulators of podosomes. In conclusion, HIV-1 reprograms macrophage migration, which likely explains macrophage accumulation in several patient tissues, which is a key step for virus spreading and pathogenesis. Moreover, Nef points out podosomes and the Hck/WASP signaling pathway as good candidates to control tissue infiltration of macrophages, a detrimental phenomenon in several diseases.
Introduction
Together with CD4+ T lymphocytes, macrophages are the main targets of HIV and key effectors in AIDS pathogenesis. In contrast to T cells, macrophages are not subjected to virus-induced cytotoxicity and can survive for months, even in patients undergoing antiretroviral therapy.1-5 Based on their ability to migrate through tissues, macrophages are thought to participate in virus dissemination.1 Once infected, many functions of macrophages are affected (eg, phagocytosis and cytokine production),1,6 and they gain the ability to fuse and form multinucleated giant cells (MGCs) found in several tissues of patients.7,8 Macrophages and MGCs are considered as reservoirs that represent a substantial obstacle for virus eradication from patient tissues, and they play a key role in HIV-1 pathogenesis. Their accumulation in the brain participates in neurologic disorders.7 In the gastrointestinal tract, infected macrophages induce severe damages to mucosal barriers, resulting in translocation of others pathogens, a hallmark of HIV-1 infection.9-11 Therefore, control of tissue infiltration of HIV-infected macrophages represents a therapeutic challenge.7,9,12
Although there are no studies investigating whether macrophage migration is modified on HIV-1 infection, several reports have addressed this issue in infected CD4+ T lymphocytes. Infected T cells are less motile than uninfected cells, and the HIV-1 protein Nef controls this process both in vitro and in vivo via actin remodeling.13-18 Nef is involved in AIDS pathogenesis, and it interacts with a plethora of host proteins and manipulates many signal transduction processes.19-24
With the exception of the blood vessel endothelium and lumen of organs, where migration takes place in 2 dimensions (2D), cell migration in vivo mostly occurs in 3-dimensional (3D) environments.25,26 All types of leukocytes migrate in 3D by using the amoeboid migration mode, which strongly depends on the rho-associated protein kinase (ROCK) pathway and actomyosin contractility, but not on proteolytic activity.27-30 We compiled evidence demonstrating that macrophages are the only leukocytes able to also migrate using the mesenchymal mode. This mode involves adhesion cell structures called podosomes and proteases to create paths in matrices with low porosity.30,31 Targeting functional factors of this migration mode translates into defective migration in vivo32-35 and emerges as a therapy task in several diseases including cancer, chronic inflammation, and obesity, in which macrophage tissue infiltration has deleterious effects.
As macrophages and T lymphocytes can use distinct modes of migration,27,36 results obtained with HIV-1–infected T cells will not predict the behavior of HIV-1–infected macrophages. Therefore, we examined the consequences of HIV-1 infection on macrophage migration, using in vitro and in vivo approaches. We also identified the cellular and molecular mechanisms involved in HIV-1–induced enhancement of mesenchymal migration and proposed a therapeutic strategy for diseases associated with deleterious tissue infiltration of macrophages.
Methods
Mice
Cell culture, transduction, and transfection
Human monocytes were isolated from blood of healthy donors and differentiated as previously described.8 Human monocyte-derived macrophages (hMDMs) were used at days 5 to 8 of differentiation. hMDMs were transduced with lentiviral vector (multiplicity of infection [MOI] 1)39 (50% of transduction efficiency). The Neon MP5000 electroporation system (Invitrogen) was used for transient expression of proteins in hMDMs (1000 V, 40 ms, 2 pulses, 1 μg of DNA for 2 × 105 cells). Cells were used within 24 hours. hMDMs were transfected with a control small interfering RNA (siRNA) and Hck siRNA (100 nM) using the Hiperfect system.40 Bone marrow-derived macrophages (BMDMs) were isolated from femurs and tibias of CD4C/HIVNef mice.32
Infection of hMDMs
hMDMs were infected at a high cell density (1800 cells/mm2) with the macrophage-tropic HIV-1 isolate ADA41 or HIV-1Δnef8 at MOI 0.1. Virus stocks were generated by transfection of 293T cells,8,42 supernatants were harvested 2 days after transfection, and their infectivity was assessed using TZM-bl indicator cells, as previously described.43,44 HIV-1 p24 Ag concentration was also determined by enzyme-linked immunosorbent assay, and 35ng p24 of ADA or ADA-1Δnef was used to infect 2 × 106 hMDMs. In some experiments, hMDMs were infected with HIV-1 Ba-L strains (gift from J. Izopet, Toulouse, France) at MOI 0.3 to obtain a similar infection rate (percentage of p24-positive cells) as ADA. Experiments were assessed 8 days after infection. Infectivity was assessed by measuring extracellular p24 levels by enzyme-linked immunosorbent assay8 and p24-positive macrophages by immunostaining (∼60%).
Analysis of tumor infiltrating myeloid cells
The aggressive mouse C3L5 mammary tumor cells (2 x 106) were inoculated subcutaneously into non-Tg and CD4C/HIVNef-Tg littermates. Mice were euthanized 8 to 10 days later. Tumors were collected, and cells were dispersed as described before.45 CD45+ cells were analyzed by flow cytometry analysis as previously published.46 Acquisition was performed on BD-LSR (BD Biosciences). Data were analyzed using CellQuest Pro software (BD Biosciences). Cell sorting was performed on a Mo-Flo cell sorter (Cytomation).
For more information on chemicals and antibodies, DNA constructs and lentiviral vectors, immunoblotting, immunofluorescence microscopy and matrix degradation assays, adhesion, 2D and 3D migration assays, immunohistology, podosome lifespan and fluorescence recovery after photobleaching (FRAP) analysis, scanning electron microscopy, and statistical analysis, see the supplemental Methods available on the Blood Web site.
Results
HIV-1 infection modifies migration modes of macrophages in 2D and 3D environments
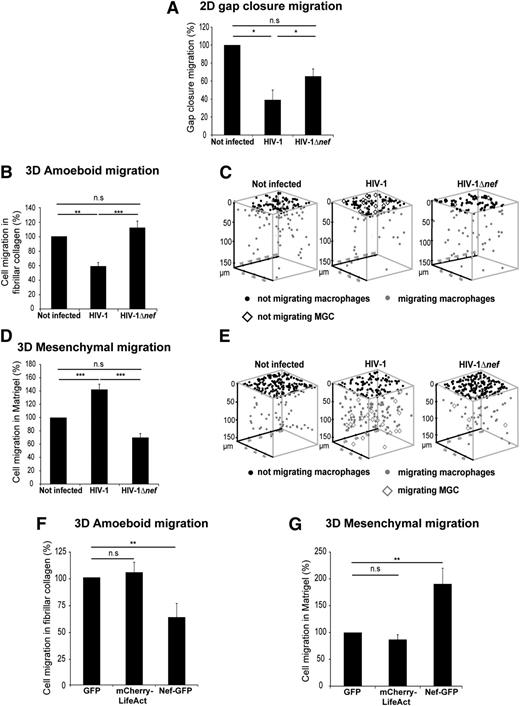
We examined the consequences of HIV-1 infection on 2D migration of hMDMs. Using an insert removal assay,26 we observed that infected macrophages had a significantly reduced gap closure migration capacity compared with uninfected macrophages (Figure 1A). Next, to study the migration of macrophages in 3D environments, macrophages were seeded on matrices of different porosity polymerized as thick layers inside transwells.30 In fibrillar collagen I, a matrix that triggers amoeboid migration, the number of HIV-1–infected cells able to infiltrate the matrix was markedly decreased (Figure 1B-C). None of the infected MGCs, formed by HIV-1 infection of hMDMs,8 could infiltrate the matrix (Figure 1C), probably because of their large size. In Matrigel, a nonporous matrix that triggers mesenchymal migration,30 the percentage of infected macrophages infiltrating the matrix was increased by 40% compared with noninfected cells (Figure 1D-E). Despite their large size, HIV-1–infected MGCs efficiently infiltrated Matrigel and covered a longer migration distance than mononucleated macrophages (Figure 1E). Moreover, we observed that the migration distance covered by all cells was decreased in fibrillar collagen matrices and increased in Matrigel on infection (supplemental Figure 1A-B). A signature of mesenchymal migration is the requirement of proteases and a dispensable ROCK signaling. Consistently, migration of HIV-1–infected macrophages in Matrigel was inhibited by protease inhibitors but not by the ROCK inhibitor Y27632 (supplemental Figure 1C). Finally, we showed that the effect of HIV-1 on mesenchymal migration was increased (by 70%) when the MOI was multiplied by 10 (MOI 1), and the effects of the strain ADA used above on 2D and 3D migration can be duplicated with another macrophage-tropic virus strain, Ba-L (data not shown; supplemental Figure 1D-E). Altogether, these results show that HIV-1 inhibits 2D and 3D amoeboid migrations of macrophages and enhances the 3D mesenchymal mode.
HIV-1 infection modifies migration modes of macrophages in 2D and 3D environments via Nef. (A-E) hMDMs were infected or not with HIV-1 or HIV-1Δnef and seeded (A) in Ibidi culture inserts or (B-C) on thick layers of fibrillar collagen I (D-E) or of Matrigel polymerized in transwell chambers. (A) The percentage of cells closing the gap after 24 hours was measured and reported as 100% for controls. Mean ± standard deviation (SD), n = 4. *P ≤ .05; **P ≤ .01; and ***P ≤ .001. (B,D) The percentage of migrating cells after 72 hours was measured and reported as 100% for controls. Mean ± standard error of the mean (SEM), n = 7 in B and n = 11 in D. (C,E) 3D positions of macrophages and MGCs for representative experiments using TopCat Software. (F-G) hMDMs were transduced with GFP (control), mCherry-LifeAct, or NefSF2-GFP lentivirus and layered (F) on fibrillar collagen I or (G) on Matrigel. The percentage of migrating cells after 72 hours was measured. Mean ± SEM, n = 4.
HIV-1 infection modifies migration modes of macrophages in 2D and 3D environments via Nef. (A-E) hMDMs were infected or not with HIV-1 or HIV-1Δnef and seeded (A) in Ibidi culture inserts or (B-C) on thick layers of fibrillar collagen I (D-E) or of Matrigel polymerized in transwell chambers. (A) The percentage of cells closing the gap after 24 hours was measured and reported as 100% for controls. Mean ± standard deviation (SD), n = 4. *P ≤ .05; **P ≤ .01; and ***P ≤ .001. (B,D) The percentage of migrating cells after 72 hours was measured and reported as 100% for controls. Mean ± standard error of the mean (SEM), n = 7 in B and n = 11 in D. (C,E) 3D positions of macrophages and MGCs for representative experiments using TopCat Software. (F-G) hMDMs were transduced with GFP (control), mCherry-LifeAct, or NefSF2-GFP lentivirus and layered (F) on fibrillar collagen I or (G) on Matrigel. The percentage of migrating cells after 72 hours was measured. Mean ± SEM, n = 4.
Modulation of macrophage migration by HIV-1 is mediated by Nef
Considering the effect of the viral factor Nef on the motility of infected CD4+ T cells,14-17 we investigated its role on macrophage migration. We used a nef-deleted HIV-1 ADA strain (HIV-1Δnef) that exhibits the same level of infectivity in hMDMs than wt-HIV-18 (at day 8 after infection: wt virus, 16.6 ± 5.9 ng/mL of the viral protein p24 released in the extracellular medium vs 18.3 ± 8.7 with the Δnef mutant; n = 3 donors). When macrophages were infected with HIV-1Δnef, gap closure migration and 3D amoeboid and mesenchymal migrations of macrophages were not significantly different from uninfected cells (Figure 1A-E). Next, we examined whether Nef expression in macrophages can duplicate the effects of HIV-1 on 3D migration. When the HIV-1 NefSF2 protein was expressed in hMDMs by lentiviral transduction,47 the amoeboid migration was inhibited (Figure 1F), and the mesenchymal migration was increased by almost twofold compared with control cells (Figure 1G). The same effects were observed with another Nef allele, NefNL4-3 (data not shown). The migration of NefSF2-expressing macrophages in Matrigel was inhibited by protease inhibitors but not by Y27632 (supplemental Figure 1F), demonstrating the use of the mesenchymal mode. Using BMDMs expressing Nef from Tg mice (CD4C/HIVNef mice),37 we also observed that the amoeboid migration mode was inhibited and the mesenchymal migration significantly enhanced compared with BMDMs from non-Tg mice (supplemental Figure 2A-B). We conclude that Nef is necessary and sufficient to reprogram the migration of macrophages.
Nef expression increases the number of tissue- and tumor-infiltrated macrophages in vivo
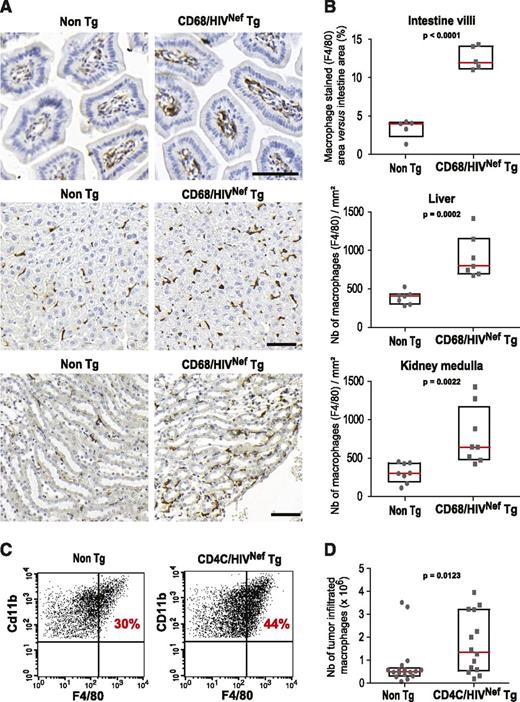
Having shown that HIV-1 Nef modifies the macrophage motility in vitro, we investigated whether it could have consequences in vivo. In HIV+ patients and simian immunodeficiency virus (SIV)+ macaques, increased macrophage content has been reported in several tissues including intestine, kidney, and liver.7,9,48,49 We examined by immunohistochemistry whether macrophage tissue accumulation was also a characteristic of Nef-Tg mice that only express Nef in macrophages and dendritic cells (CD68/HIVNef mice).38 As shown in Figure 2A-B, the number of F4/80-positive macrophages in the intestine mucosa was increased by 2.5-fold in Nef-Tg mice compared with non-Tg littermates. The number of macrophages was also increased in liver and kidney medulla of these mice to a lesser extent. Solid tumors are made of dense tissues that are rapidly occupied by newly recruited macrophages derived from blood monocytes,50 thus constituting an appropriate model to compare macrophage tissue recruitment in Nef-Tg37 and non-Tg mice. One week after subcutaneous injection of C3L5 cells, the number of tumor-infiltrated macrophages, defined as CD45+F4/80+CD11b+ cells,50 was increased by twofold in Nef-Tg mice (Figure 2C-D), whereas the total number of recruited leukocytes was not modified, indicating that infiltration of monocytes/macrophages in tumors was selectively enhanced. In agreement with the described positive correlation between the number of tumor-associated macrophages and tumor growth,50 in mice expressing Nef, the weight of tumors was increased (0.25 ± 0.24g in non-Tg compared with 0.45 ± 0.15g in Nef-Tg mice; P = .013, n = 14).
Nef increases macrophage infiltration in tumors and promotes macrophage tissue accumulation. (A) Representative histological sections of (top) intestine mucosa villi, (middle) liver, and (bottom) medulla area of kidney of non-Tg and CD68/HIVNef-Tg mice stained with F4/80 antibodies (macrophages) and counterstained with hematoxylin (nuclei). Scale bars, 50 μm. (B) Intestine: percentage of the F4/80-stained surface relative to the villi surface, n = 5, 3 sections per mouse. Liver and kidney: quantification of the number of F4/80-positive cells, n = 7 and 8, respectively, 3 sections per mouse. Box without whisker plots (interquartile range) and all dot plots (gray) are shown with mean value (red line). (C) Representative flow cytometry analysis of C3L5 tumor-infiltrated hematopoietic cells stained for CD45.2, CD11b, F4/80, and Gr1. CD11b- and F4/80-positive cells are illustrated, after gating on CD45.2+/Gr1low cells. (D) Number of F4/80-positive cells inside tumors: n = 14 for CD4C/HIVNef-Tg and n = 16 for non-Tg mice.
Nef increases macrophage infiltration in tumors and promotes macrophage tissue accumulation. (A) Representative histological sections of (top) intestine mucosa villi, (middle) liver, and (bottom) medulla area of kidney of non-Tg and CD68/HIVNef-Tg mice stained with F4/80 antibodies (macrophages) and counterstained with hematoxylin (nuclei). Scale bars, 50 μm. (B) Intestine: percentage of the F4/80-stained surface relative to the villi surface, n = 5, 3 sections per mouse. Liver and kidney: quantification of the number of F4/80-positive cells, n = 7 and 8, respectively, 3 sections per mouse. Box without whisker plots (interquartile range) and all dot plots (gray) are shown with mean value (red line). (C) Representative flow cytometry analysis of C3L5 tumor-infiltrated hematopoietic cells stained for CD45.2, CD11b, F4/80, and Gr1. CD11b- and F4/80-positive cells are illustrated, after gating on CD45.2+/Gr1low cells. (D) Number of F4/80-positive cells inside tumors: n = 14 for CD4C/HIVNef-Tg and n = 16 for non-Tg mice.
In conclusion, the Nef-dependent reprogrammed migration of macrophages observed in vitro (ie, inhibition of 2D migration and 3D amoeboid migration and increase of 3D mesenchymal migration) results in tissue accumulation of macrophages in Nef-Tg mice at the steady state and enhanced recruitment of tumor-associated macrophages. Thus, in these tissues, macrophage accumulation might be due to activation of the mesenchymal migration by Nef.
HIV-1 modifies podosome organization and function via Nef
Thus, we focused on the cellular mechanisms used by HIV-1 Nef to increase the 3D mesenchymal migration in hMDMs. This migration mode requires specialized structures named podosomes30,32,34,36,51,52 formed constitutively in macrophages and cells of the monocyte lineage.26,53 We investigated whether HIV-1 infection has an effect on podosome organization and function. Podosomes are mechano-sensing and adhesion structures with a proteolytic activity toward the extracellular matrix (ECM), which have been mostly studied in cells cultured in 2D.26,53-55 On 2D surfaces, podosomes are scattered at the ventral face of the cell and characterized by F-actin dots surrounded by a ring of adhesion proteins and actin-binding proteins, including vinculin, talin, and paxillin.56 In activated macrophages, podosomes organize as superstructures called belts or rosettes with enhanced ECM proteolysis.32,57 First, we noticed that podosomes in HIV-1–infected macrophages display higher F-actin fluorescence intensity compared with control cells and that ∼40% of p24-positive macrophages organized their podosomes into rosettes or belts compared with <10% in controls (Figure 3A; data not shown). Second, we examined podosome functions. Adhesion of macrophages (Figure 3B) and their capacity to degrade matrices (Matrigel and gelatin fluorescein isothiocyanate) (Figure 3C-D; supplemental Figure S3) were enhanced after infection. In particular, infected MGCs are more efficient at forming podosome rosettes and degrading the matrix than their infected mononucleated counterpart (Figure 3A,C,D). In contrast, HIV-1Δnef–infected macrophages formed less podosome rosettes/belts than HIV-1–infected macrophages (Figure 3A) and had a reduced capacity to adhere to substrates and to degrade gelatin (Figure 3B-D). As shown in Figure 3E-F, ectopic expression of NefSF2 was sufficient to trigger the formation of podosome rosettes and to enhance ECM degradation.
HIV-1 Nef triggers the formation of podosome rosettes, cell adhesion, and matrix degradation. (A) Immunofluorescence (IF) images of HIV-1–infected hMDMs on glass. HIV-1 p24 (red) and Alexa Fluor 488-coupled phalloidin (F-actin in green). (Upper) Arrowheads show individual podosomes in a noninfected cell and arrows show a podosome rosette in a mononucleated infected cells (p24 positive). (Lower) Arrows show a podosome rosette/belt in an infected MGC. (Right) Percentage of cells forming podosome rosettes is shown. Mean ± SD, n = 4. Only p24-positive cells were counted for HIV-1, HIV-1Δnef, and MGCs. (B) hMDMs were infected or not with HIV-1 or HIV-1Δnef and seeded on glass or on fibronectin. The percentage of adhesive cells after 2 hours was measured and reported as 100% for controls. Mean ± SD, n = 4. (C) (Upper) Scanning electron microscopy images of a noninfected macrophage and an infected MGC on top of Matrigel matrices after 24 hours of culture. Arrowhead: hole in Matrigel reflecting the matrix proteolytic activity of infected cells. (Lower) hMDMs were infected with HIV-1 or HIV-1Δnef and seeded on glass coverslips coated with gelatin fluorescein isothiocyanate (blue) for 24 hours. p24 (red) and F-actin (green). White arrowhead: noninfected macrophage; red arrowhead: infected macrophage/MGC degrading the ECM. (D) Quantification of matrix degradation is shown: number of cells degrading the matrix and degraded area/cell area. Mean ± SD, n = 4 (10 fields [×40] per condition). (E-F) hMDMs were transduced with a lentiviral vector expressing NefSF2-GFP or GFP (control). (E) F-actin staining (red) of a NefSF2-GFP expressing cell (green) on glass with podosome rosettes (arrow) and quantification of the number of cells forming podosome rosettes. Mean ± SD, n = 5. (F) Quantification of the number of cells degrading Alexa Fluor 594-conjugated fibrinogen. Mean ± SD, n = 3. Scale bars, 10 μm.
HIV-1 Nef triggers the formation of podosome rosettes, cell adhesion, and matrix degradation. (A) Immunofluorescence (IF) images of HIV-1–infected hMDMs on glass. HIV-1 p24 (red) and Alexa Fluor 488-coupled phalloidin (F-actin in green). (Upper) Arrowheads show individual podosomes in a noninfected cell and arrows show a podosome rosette in a mononucleated infected cells (p24 positive). (Lower) Arrows show a podosome rosette/belt in an infected MGC. (Right) Percentage of cells forming podosome rosettes is shown. Mean ± SD, n = 4. Only p24-positive cells were counted for HIV-1, HIV-1Δnef, and MGCs. (B) hMDMs were infected or not with HIV-1 or HIV-1Δnef and seeded on glass or on fibronectin. The percentage of adhesive cells after 2 hours was measured and reported as 100% for controls. Mean ± SD, n = 4. (C) (Upper) Scanning electron microscopy images of a noninfected macrophage and an infected MGC on top of Matrigel matrices after 24 hours of culture. Arrowhead: hole in Matrigel reflecting the matrix proteolytic activity of infected cells. (Lower) hMDMs were infected with HIV-1 or HIV-1Δnef and seeded on glass coverslips coated with gelatin fluorescein isothiocyanate (blue) for 24 hours. p24 (red) and F-actin (green). White arrowhead: noninfected macrophage; red arrowhead: infected macrophage/MGC degrading the ECM. (D) Quantification of matrix degradation is shown: number of cells degrading the matrix and degraded area/cell area. Mean ± SD, n = 4 (10 fields [×40] per condition). (E-F) hMDMs were transduced with a lentiviral vector expressing NefSF2-GFP or GFP (control). (E) F-actin staining (red) of a NefSF2-GFP expressing cell (green) on glass with podosome rosettes (arrow) and quantification of the number of cells forming podosome rosettes. Mean ± SD, n = 5. (F) Quantification of the number of cells degrading Alexa Fluor 594-conjugated fibrinogen. Mean ± SD, n = 3. Scale bars, 10 μm.
Finally, we examined whether HIV-1 impacts 3D podosomes that are involved in mesenchymal migration.26,32,33,53,58-60 Even though the analysis of podosomes is a challenging task in 3D Matrigel,26,31 we showed that HIV-1–infected and Nef-expressing cells formed F-actin- and vinculin-positive protrusions (Figure 4A-B; data not shown), as defined for 3D podosomes.26,31,61 The number of these protrusions per cell was increased on HIV-1 infection (8.6 vs 2.7, n > 84, P < .0001) and in Nef-expressing macrophages (Figure 4C). Similarly, Nef-expressing BMDMs from CD4C/HIVNef-Tg mice formed more podosome rosettes in 2D and cell protrusions in 3D than controls (supplemental Figure 2C-D; data not shown). To conclude, HIV-1 modifies podosome function and their organization as rosettes via Nef.
HIV-1–infected and Nef-expressing cells form cell protrusions in Matrigel defined as 3D podosomes. (A-B) Confocal images of (A) HIV-1–infected and (B) NefSF2-expressing cells migrating inside Matrigel. (A) (Upper) HIV-1 p24 (red), F-actin (green), and 4,6 diamidino-2-phenylindole (blue); inset is ×2 zoom. (Lower) Vinculin (red), F-actin (green), and 4,6 diamidino-2-phenylindole (blue). (B) Nef (green), F-actin (blue), and vinculin (red). Inset, ×3.5 zoom. Arrowheads: 3D podosomes. (C) Brightfield images of control and NefSF2-expressing macrophages inside Matrigel and quantification of the number of protrusions per cell. Mean ± SD, n = 4 (>20 cells per condition per donor). Arrowheads: 3D protrusions. Scale bars, 10 μm.
HIV-1–infected and Nef-expressing cells form cell protrusions in Matrigel defined as 3D podosomes. (A-B) Confocal images of (A) HIV-1–infected and (B) NefSF2-expressing cells migrating inside Matrigel. (A) (Upper) HIV-1 p24 (red), F-actin (green), and 4,6 diamidino-2-phenylindole (blue); inset is ×2 zoom. (Lower) Vinculin (red), F-actin (green), and 4,6 diamidino-2-phenylindole (blue). (B) Nef (green), F-actin (blue), and vinculin (red). Inset, ×3.5 zoom. Arrowheads: 3D podosomes. (C) Brightfield images of control and NefSF2-expressing macrophages inside Matrigel and quantification of the number of protrusions per cell. Mean ± SD, n = 4 (>20 cells per condition per donor). Arrowheads: 3D protrusions. Scale bars, 10 μm.
Nef controls the size and stability of podosomes
As podosome function and collective patterning are dependent on intrinsic podosome structure and stability,26,62 we investigated the subcellular localization of Nef and its effects on individual podosomes. In addition to the perinuclear zone,8 we show here that a fraction of NefNL4-3-green fluorescent protein (GFP); or NefSF2, (data not shown) localized to podosome ring areas (Figure 5A-B; supplemental Figure 4) and flowed around podosomes (supplemental Video 1). In Matrigel, we also observed that Nef colocalized with F-actin at 3D podosomes (Figure 5C). The presence of Nef did not affect the localization of markers of the podosome core (F-actin, cortactin, and phospho-tyrosine) or the podosome ring (vinculin and paxillin) (supplemental Figure 4). Next, to get information about the actin polymerization dynamics at podosomes, we performed FRAP experiments. In hMDMs coexpressing NefNL4-3-GFP and β-actin-red fluorescent protein (RFP), a slight decrease in the rate of actin polymerization was observed, with a half-time (t1/2) of recovery of 58 seconds compared with 44 seconds in GFP-expressing cells (Figure 5D). Furthermore, we showed that the podosome life span was strongly enhanced in cells expressing NefNL4-3 (Figure 5E-F; supplemental Videos 2 and 3). Finally, we observed that podosomes were significantly larger in the presence of Nef (Figure 5E,G; supplemental Videos 2 and 3). A closer look into the relative amount of F-actin inside podosomes revealed that the fluorescence intensity of F-actin and the area covered by F-actin signal were significantly increased, whereas F-actin density inside podosomes (normalization of F-actin intensity by podosome area) was not modified by Nef (Figure 5G). When Nef proteins from HIV-1SF2, 2 distinct HIV-2 strains, or SIV strains were tested, a similar increase of podosome intensity and area was observed (Figure 5G). Together, these results indicate that Nef strongly impacts podosomes by increasing their diameter and stability, without modifying their composition.
Nef stabilizes podosomes and increases the size of the F-actin core. (A) Confocal images of NefNL4-3-expressing hMDMs on glass. Inset, 2× zoom; arrow, large podosomes where NefNL4-3 localizes. (B) Normalized fluorescence intensity profiles of F-actin and NefNL4-3 along the pink dotted line in A. (C) Confocal images of hMDMs expressing NefSF2-GFP inside Matrigel. Inset, 3.5× zoom; arrowheads, colocalization of NefSF2 with F-actin at 3D podosomes. (D) FRAP analyses in hMDMs cotransfected with mRFP-β-actin, and NefNL4-3-GFP or GFP (control). Mean ± SEM, representative of 6 independent experiments (donors) (>20 podosomes per condition for each donor). (E-F) Time-lapse analysis of podosomes. hMDMs were cotransfected with LifeAct-mCherry (F-actin) and NefNL4-3-GFP or GFP. (E) Images from supplemental Videos 2 and 3. (F) Quantification of podosome life span. n = 4 (>100 podosomes per condition). Red dotted lines: median. (G) Automatic quantification of F-actin intensity, fluorescence area, and F-actin density inside podosomes in hMDMs expressing the indicated Nef mutants. Nef from different strains were used: 2 HIV-1 (NL4-3 and SF2), 2 HIV-2 (rod and nep from Nepal84), and 1 SIV. Mean ± SEM, n = 3 (>1000 podosomes from ≥10 cells per donor). Scale bars, 10 μm.
Nef stabilizes podosomes and increases the size of the F-actin core. (A) Confocal images of NefNL4-3-expressing hMDMs on glass. Inset, 2× zoom; arrow, large podosomes where NefNL4-3 localizes. (B) Normalized fluorescence intensity profiles of F-actin and NefNL4-3 along the pink dotted line in A. (C) Confocal images of hMDMs expressing NefSF2-GFP inside Matrigel. Inset, 3.5× zoom; arrowheads, colocalization of NefSF2 with F-actin at 3D podosomes. (D) FRAP analyses in hMDMs cotransfected with mRFP-β-actin, and NefNL4-3-GFP or GFP (control). Mean ± SEM, representative of 6 independent experiments (donors) (>20 podosomes per condition for each donor). (E-F) Time-lapse analysis of podosomes. hMDMs were cotransfected with LifeAct-mCherry (F-actin) and NefNL4-3-GFP or GFP. (E) Images from supplemental Videos 2 and 3. (F) Quantification of podosome life span. n = 4 (>100 podosomes per condition). Red dotted lines: median. (G) Automatic quantification of F-actin intensity, fluorescence area, and F-actin density inside podosomes in hMDMs expressing the indicated Nef mutants. Nef from different strains were used: 2 HIV-1 (NL4-3 and SF2), 2 HIV-2 (rod and nep from Nepal84), and 1 SIV. Mean ± SEM, n = 3 (>1000 podosomes from ≥10 cells per donor). Scale bars, 10 μm.
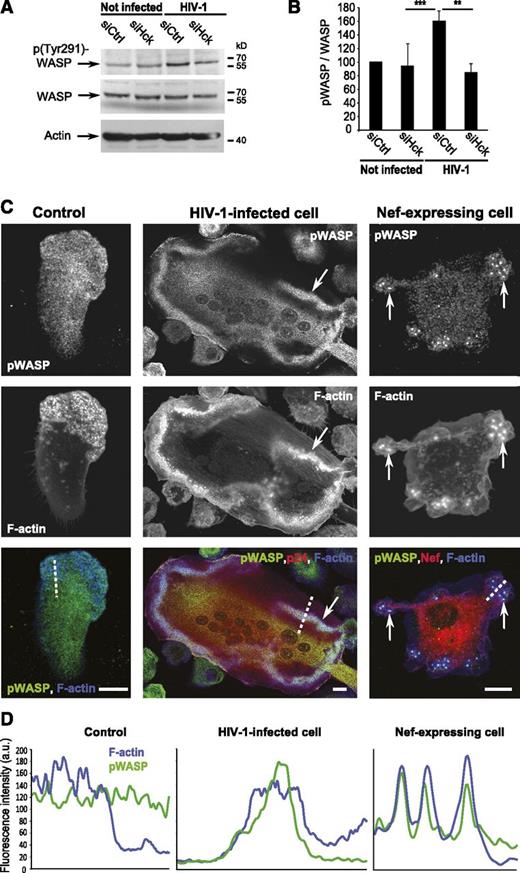
HIV-1 effects on podosomes and macrophage migration are mediated by Hck and Wiskott-Aldrich syndrome protein (WASP) phosphorylation
To define the molecular determinants in Nef that govern the observed modifications of podosomes, we screened a panel of well-characterized NefSF2 mutants.17 Only the E4A4 NefSF2 mutant (unable to interact with phosphofurin acidic cluster sorting proteins and clathrin-associated activator protein-1 complex) had the same behavior as wt NefSF2 as it partially accumulated at podosomes (supplemental Figure 5A) and enhanced the F-actin content (supplemental Figure 5B). All other NefSF2 variants, including 2 Nef variants (AxxA and VGF→AAA) that impact the interaction of Nef with SH3-binding domains, were not found around podosomes and were unable to modify their size (supplemental Figure 5A-B). When wt-NefSF2 was coexpressed with anti-Nef single-domain antibodies (sdAbs) and Neffins C1 and B6,63,64 we observed that NefSF2 was delocalized from podosomes (data not shown), and F-actin content in podosomes was significantly decreased (supplemental Figure 5C). Neffins C1 and B6 are anti-Nef sdAbs linked to the SH3 domain of the Src family member Hck, which inhibit most Nef biological activities.63,64 The strong inhibitory effect of Neffins on podosome size, together with the effects of Nef mutants AxxA and VGF→AAA, suggested a pivotal role of the Nef-SH3 interaction in podosome modulation. In addition, NefSF2 AxxA failed to increase the life span of podosomes compared with wt-NefSF2 (supplemental Figure 5D). Thus, podosome modulations rely on multiple molecular determinants of Nef, including the SH3-binding domain.
Nef is a well-known activator of Hck by high affinity binding to its SH3 domain.65-67 Hck regulates the stability and spatial organization of podosomes and specifically controls the mesenchymal migration of macrophages.32,34,58 We examined the contribution of Hck to HIV-1 effects on podosomes and cell migration using an siRNA approach. Because Hck is involved in HIV-1 entry and replication in macrophages,68-71 macrophages were first infected and then subjected to siRNA-mediated silencing of Hck (Figure 6A). Under these conditions, the release of HIV-1 particles, as measured by the level of p24 in supernatants, was not affected (Figure 6B). The efficiency of Hck depletion was 75 ± 15% (n = 7) at day 15 (Figure 6C) and day 18 (not shown). In infected macrophages, silencing of Hck abolished the enhanced mesenchymal migration, the formation of podosome rosettes that instead preferentially organized as clusters, and the increase of podosome fluorescence intensity (Figure 6D-G).
Hck is necessary for HIV-1 effect on podosomes and 3D mesenchymal migration of macrophages. (A) Representation of the siRNA experimental design. (B) Dosage of p24 from day 15 supernatants of HIV-1ADA–infected hMDMs. Mean ± SEM, n = 5. (C) (Upper) Western blot analysis of Hck depletion in infected hMDMs. (Lower) Quantification of Hck normalized to tubulin. Mean ± SD, n = 7. (D) The percentage of hMDMs migrating in Matrigel after 72 hours was measured and reported as 100% for controls. Mean ± SD, n = 5. (E) IF microscopy of HIV-1ADA–infected macrophages on glass subjected to control siRNA or Hck siRNA. Arrowhead: podosome rosettes/belts; arrow: podosome clusters. Scale bar, 10 μm. (F) Quantification of the number of cells forming podosome rosettes/belts. Mean ± SD, n = 3. (G) Automatic quantification of F-actin intensity inside podosomes. Mean ± SEM, n = 3 (>1000 podosomes from ≥10 cells per donor). NI, not infected.
Hck is necessary for HIV-1 effect on podosomes and 3D mesenchymal migration of macrophages. (A) Representation of the siRNA experimental design. (B) Dosage of p24 from day 15 supernatants of HIV-1ADA–infected hMDMs. Mean ± SEM, n = 5. (C) (Upper) Western blot analysis of Hck depletion in infected hMDMs. (Lower) Quantification of Hck normalized to tubulin. Mean ± SD, n = 7. (D) The percentage of hMDMs migrating in Matrigel after 72 hours was measured and reported as 100% for controls. Mean ± SD, n = 5. (E) IF microscopy of HIV-1ADA–infected macrophages on glass subjected to control siRNA or Hck siRNA. Arrowhead: podosome rosettes/belts; arrow: podosome clusters. Scale bar, 10 μm. (F) Quantification of the number of cells forming podosome rosettes/belts. Mean ± SD, n = 3. (G) Automatic quantification of F-actin intensity inside podosomes. Mean ± SEM, n = 3 (>1000 podosomes from ≥10 cells per donor). NI, not infected.
Finally, we looked for downstream effectors of Hck. WASP is a substrate of Hck involved in mesenchymal migration,33 and its phosphorylation on Tyr291 has been involved in regulation of podosomes.35,72 In HIV-1–infected macrophages, we observed an increase in p(Tyr291)-WASP (Figure 7A-B) that was abolished in Hck-depleted cells. Also, we noticed that p(Tyr291)-WASP accumulated at podosome rosettes/belts of HIV-1–infected macrophages in contrast to podosome areas in noninfected cells (Figure 7C-D) and massively colocalized with large podosomes induced by NefNL4-3 expression (Figure 7C-D). Although HIV-1– and Nef-mediated effects on F-actin organization and cell migration in T cells are associated to hyperphosphorylation of cofilin,17 we did not find any change in cofilin phosphorylation in infected macrophages (supplemental Figure 6). Altogether, these results provide evidence that HIV-1–mediated effects on podosomes and migration involve the Nef-Hck interaction and Hck-mediated phosphorylation of WASP at podosomes.
HIV-1 infection of human macrophages increases phosphorylation of WASP by Hck at podosomes. (A) hMDM whole-cell lysates western blot using antibodies against phosphoTyr291-WASP, WASP, and actin. (B) Quantification of the phosphoTyr291-WASP on total WASP ratio. Mean ± SD, n = 6. (C) Confocal images of hMDMs on glass. Phospho-WASP (green), p24, or Nef (red) and F-actin (blue). Arrows: accumulation of phospho-WASP at podosomes. Scale bars, 10 μm. (D) Normalized fluorescence intensity profiles of F-actin and phospho-WASP along the dotted lines in C.
HIV-1 infection of human macrophages increases phosphorylation of WASP by Hck at podosomes. (A) hMDM whole-cell lysates western blot using antibodies against phosphoTyr291-WASP, WASP, and actin. (B) Quantification of the phosphoTyr291-WASP on total WASP ratio. Mean ± SD, n = 6. (C) Confocal images of hMDMs on glass. Phospho-WASP (green), p24, or Nef (red) and F-actin (blue). Arrows: accumulation of phospho-WASP at podosomes. Scale bars, 10 μm. (D) Normalized fluorescence intensity profiles of F-actin and phospho-WASP along the dotted lines in C.
Discussion
This study reveals for the first time that HIV-1 infection dramatically modifies all macrophage migration modes via Nef. In vivo, in Nef-Tg mice, we provide evidence for macrophage tissue accumulation, which may represent both a strategy of HIV-1 dissemination and a cause of pathogenesis.
HIV-1 infection modifies many functions of macrophages in vitro.1,6,8,42 For the first time, we show here that HIV-1 reprograms the migration of hMDMs by inhibiting the 2 migration modes that are shared by all leukocytes, namely 2D and 3D amoeboid motilities, and enhancing the 3D mesenchymal migration, a migration mode restricted to macrophages.30,36 Migration of HIV-1–infected MGCs, spontaneously formed by fusion of infected hMDMs,8,73 is also strongly reprogrammed. All effects of HIV-1 on macrophage migration are under the control of Nef as shown by using nef-deleted HIV-1 and Nef transfection. This reprogramed motility observed in vitro correlates with increased tissue infiltration of macrophages in Nef-Tg mice at the steady state and in tumors, suggesting that activation of the mesenchymal migration by Nef exerts a predominant effect in vivo. Actually, in HIV+ patients and SIV-infected macaques, an accumulation of macrophages in several tissues that participate in pathogenesis has been described.9,48,49 In contrast, no accumulation of T cells in patient tissues has been reported, probably because they are poorly resistant to the cytotoxic effect of HIV-1, and their migration capacity is inhibited after infection.14,16-18,23,74 Interestingly, also in T cells, Nef is responsible for migration defects. It inhibits their 2D migration and reduces their velocity in 3D collagen matrices,16,17 which is mediated by amoeboid motility.25,36 Similarly, 2D and 3D amoeboid migrations are inhibited by HIV-1 in macrophages, the major difference being the enhancement of the protease-dependent mesenchymal migration, a migration mode that is not used by T lymphocytes.36 Thus, Nef is a master regulator of several forms of cell motility used by HIV-1 host cells.
The F-actin cytoskeleton is one of the major actors of cell migration in 2D and 3D environments,26 and HIV-1 Nef is considered an essential determinant for actin remodeling.19,75,76 Macrophages concentrate their F-actin in podosomes, which are cell structures involved in cell adhesion and protease-dependent mesenchymal migration.26,30,32,36,51,55 We show here that HIV-1 infection or Nef expression is able to stabilize podosomes and increase their size, effects that we found conserved among Nef proteins from various virus strains. In addition, podosomes preferentially organize into superstructures, and their proteolytic function is exacerbated, resulting in enhanced 3D mesenchymal migration. The involvement of other viral proteins in controlling the structure/function of podosomes is likely because HIV-1Δnef infection does not fully reproduce noninfected conditions. Finally, the increased adhesion is certainly the result of bigger and more stable podosomes, as they constitute the unique adhesion structures of macrophages.26 Increased cell adhesion correlates with decreased 2D migration,77 suggesting that, by acting on podosomes, HIV-1 affects both 2D motility and 3D mesenchymal migration. To our knowledge, HIV-1 is the first pathogen able to modify the structure and function of constitutive podosomes in macrophages.
We previously reported that Hck is necessary for the stability of podosomes, their organization into rosettes, and mesenchymal migration in macrophages and osteoclast precursors, whereas it is dispensable for amoeboid migration.32,34,58 It is therefore consistent that Nef, a potent activator of Hck that binds to its SH3 domain,65-67 has profound effects on podosome functions and mesenchymal migration of infected macrophages. These effects are abolished by Hck knockdown. It is interesting that Nef targets a phagocyte-specific kinase in macrophages given that this viral factor uses ubiquitous proteins to inhibit the migration of T lymphocytes, such as cofilin.13-17 We also show that WASP, a regulator of podosomes and mesenchymal migration,33,35,72 is a downstream effector of the Nef/Hck signaling pathway that we found highly phosphorylated by Hck in infected macrophages. Furthermore, phosphorylated WASP is present at podosomes. We propose that Hck activation by Nef and the subsequent phosphorylation of WASP could promote the targeting of phospho-WASP at podosomes and the observed modifications in the structure/function of podosomes. In addition to Hck and WASP, other Nef effectors are likely involved in regulating podosome structure/function. Our study on Nef variants revealed that, in addition to the Nef domains involved in interaction with SH3 domains, a domain involved in intracellular traffic of vesicles21,78 is also active. Actually, this is not surprising because vesicular trafficking is essential for podosome function.61,79,80 Thus, multiple partners could mediate Nef effects on podosomes, but strikingly, results obtained with Neffin antibodies64 and with siRNA-mediated gene silencing demonstrated that Hck is a main effector. Consequently, we propose that pharmacological disruption of the Nef/Hck interaction70 may specifically reduce mesenchymal migration of infected macrophages and reduce HIV-1 dissemination and pathogenesis.
Investigating macrophage tissue infiltration in Nef-Tg mice was possible because the number of circulating monocytes is not modified in Nef-Tg mice.37 Tumors are generally denser and stiffer than healthy tissues.81,82 Thus, the increased recruitment of Nef-expressing macrophages in tumors makes sense because the migration mode used to infiltrate dense environments, namely the mesenchymal mode, is enhanced by Nef in vitro. We also observed accumulation of macrophages in the intestine, liver, and the kidney medulla at the steady state in Nef-Tg mice, mimicking the unexplained macrophage accumulation in HIV-1–infected patient tissues that contributes to local tissue inflammation.7,9,48,49 In conclusion, our results suggest that HIV-1 reprograms macrophage migration to disseminate in the host.
Several diseases including cancer, chronic inflammation, and obesity are associated with deleterious tissue infiltration of macrophages. Consequently, the concept that control of macrophage migration would be a new therapeutic approach has emerged.53,83 HIV-1 has developed a strategy to reprogram macrophage migration by targeting podosomes via Hck and WASP. These observations point out podosomes and this signaling pathway as targets to inhibit tissue infiltration of macrophages in several pathologies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Al Saati and F. Capilla from US006/CREFRE and F.-X. Frenois of the Imag’In platform for histology, G. C. Lugo-Villarino and A. Proag for critical reading of the manuscript, C. Lastrucci and K. Pingris for in vivo experiments, G. Masse, E. Priceputu, and Z. Hanna for excellent assistance with Tg mice experiments, L. van den Bergh, M. Cazabat, M. Requena, and J. Izopet from the BiVic facility and the Toulouse Reseau Imagerie platform.
O.T.F., a member of the Cluster of Excellence EXO81, received funding from the Deutsche Forschungsgemeinschaft (grants SFB1129 project 8 and FA 378/10-2). This work was supported in part by grants from the Agence Nationale de la Recherche contre le Cancer (ARC 8505), the Agence Nationale de la Recherche (ANR 2010-01301), the Agence Nationale de la Recherche contre le SIDA et les Hépatites (ANRS 2010-061), Sidaction AI-22-1-01892, the Fondation pour la Recherche Médicale en France (FRM DEQ 2011 0421312), and the European Community’s Seventh frame-work program (HEALTH-F4-2011-282095-Tarkinaid).
Authorship
Contribution: C.V. designed and performed experiments, analyzed data, and wrote the paper; S.S. and R.P. designed and performed experiments, analyzed data, and helped with manuscript preparation; E.B. and C.K. performed experiments; P.J. and B.R.-M. designed experiments and analyzed data; I.F. contributed analytical tools; S.B. provided reagents; A.I. and O.T.F. produced reagents; and I.M.-P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle Maridonneau-Parini, Institut de Pharmacologie et de Biologie Structurale, 205 Route de Narbonne, 31 077 Toulouse Cedex 4; e-mail: isabelle.maridonneau-parini@ipbs.fr.

![Figure 3. HIV-1 Nef triggers the formation of podosome rosettes, cell adhesion, and matrix degradation. (A) Immunofluorescence (IF) images of HIV-1–infected hMDMs on glass. HIV-1 p24 (red) and Alexa Fluor 488-coupled phalloidin (F-actin in green). (Upper) Arrowheads show individual podosomes in a noninfected cell and arrows show a podosome rosette in a mononucleated infected cells (p24 positive). (Lower) Arrows show a podosome rosette/belt in an infected MGC. (Right) Percentage of cells forming podosome rosettes is shown. Mean ± SD, n = 4. Only p24-positive cells were counted for HIV-1, HIV-1Δnef, and MGCs. (B) hMDMs were infected or not with HIV-1 or HIV-1Δnef and seeded on glass or on fibronectin. The percentage of adhesive cells after 2 hours was measured and reported as 100% for controls. Mean ± SD, n = 4. (C) (Upper) Scanning electron microscopy images of a noninfected macrophage and an infected MGC on top of Matrigel matrices after 24 hours of culture. Arrowhead: hole in Matrigel reflecting the matrix proteolytic activity of infected cells. (Lower) hMDMs were infected with HIV-1 or HIV-1Δnef and seeded on glass coverslips coated with gelatin fluorescein isothiocyanate (blue) for 24 hours. p24 (red) and F-actin (green). White arrowhead: noninfected macrophage; red arrowhead: infected macrophage/MGC degrading the ECM. (D) Quantification of matrix degradation is shown: number of cells degrading the matrix and degraded area/cell area. Mean ± SD, n = 4 (10 fields [×40] per condition). (E-F) hMDMs were transduced with a lentiviral vector expressing NefSF2-GFP or GFP (control). (E) F-actin staining (red) of a NefSF2-GFP expressing cell (green) on glass with podosome rosettes (arrow) and quantification of the number of cells forming podosome rosettes. Mean ± SD, n = 5. (F) Quantification of the number of cells degrading Alexa Fluor 594-conjugated fibrinogen. Mean ± SD, n = 3. Scale bars, 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/10/10.1182_blood-2014-08-596775/4/m_1611f3.jpeg?Expires=1765938486&Signature=bTjOo0T8mQTQYGS4HGKE~SHHCLsNSFZQI4LLe5VRxO~AV9TmXSWi0RxBnNfOvXTg7wdqc0aAS~U7t2JVOFnmo1Uqn-w02~uON2-GGP8lbN80YQsQcK3E35xwD6z8RtfEhYBB0cBHtyNykrpy5nI-fWTafPaxUXa3B-HizKPXVMe1BcR5-3-lwkg0Lhrf68W9P6HREDv3vtD1QSY3ATry9WYQdEydUVGki9Sp7a1i2fAWmDLwJ8KW8Ng4DIf0GzkzSGAyqfURSwdkQUsr-cTfvsI0xyYtM~BNF2edWWUAc4PRlam0SLe9rMQsQN9s5XOyh9TOdqkEOVWNzd2hLBE6Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)