In this issue of Blood, Iacovelli et al provide the first in vivo experimental evidence on the proleukemogenic relevance of autonomous (exo-antigen–independent) B-cell receptor (BCR) stimulation in conjunction with ligand (autoantigen)- mediated BCR signaling in chronic lymphocytic leukemia (CLL).1
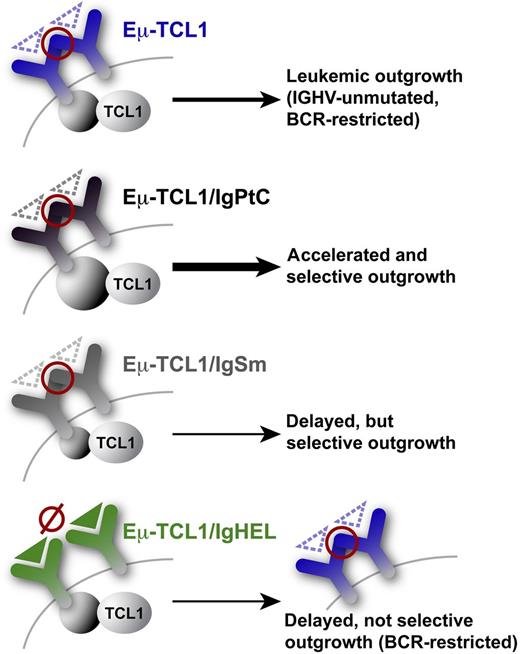
The 4 basal tg BCR systems employed by Iacovelli et al are shown.1 Red circles: exo-antigen–independent inter- (or intra-) smIG autorecognition via HCDR3–FR2 (VRQ)/FR3 (YYC) engagements leading to autonomous BCR signals. Such inter-BCR self-engagement (intra-BCR less likely due to missing crosslinking) potentially occurs at the single-cell and intercellular level. Triangles: cognate antigens (dashed for low-affinity vs solid for high-affinity). Autonomous BCR interactions appear as prerequisites for leukemic outgrowth from the Eµ-TCL1 tg backbone, as there are no leukemias arising from the high-affinity IgHEL receptor, which lacked antigen-independent autonomous signaling in vitro. Additional influence of ligand affinity: low-affinity autoantigens like PtC or Sm drive leukemia-associated selection for their BCRs. However, a protumorigenic synergism was only observed for the PtC receptors, which in contrast to the low-responsive leukemic IgSm, elicited robust intracellular signals upon ligand engagement.
The 4 basal tg BCR systems employed by Iacovelli et al are shown.1 Red circles: exo-antigen–independent inter- (or intra-) smIG autorecognition via HCDR3–FR2 (VRQ)/FR3 (YYC) engagements leading to autonomous BCR signals. Such inter-BCR self-engagement (intra-BCR less likely due to missing crosslinking) potentially occurs at the single-cell and intercellular level. Triangles: cognate antigens (dashed for low-affinity vs solid for high-affinity). Autonomous BCR interactions appear as prerequisites for leukemic outgrowth from the Eµ-TCL1 tg backbone, as there are no leukemias arising from the high-affinity IgHEL receptor, which lacked antigen-independent autonomous signaling in vitro. Additional influence of ligand affinity: low-affinity autoantigens like PtC or Sm drive leukemia-associated selection for their BCRs. However, a protumorigenic synergism was only observed for the PtC receptors, which in contrast to the low-responsive leukemic IgSm, elicited robust intracellular signals upon ligand engagement.
The BCR is a central growth-promoting factor in the pathogenesis of CLL. Prevailing concepts of CLL histogenesis and immunobiology favor particular subsets of normal B lymphocytes2 predestined by reactivity of their BCRs to a restricted set of (auto)antigens for affinity maturation and selection during precursor cell initiation and clonal progression.3 Although mechanistically insufficiently understood, there is mounting data on correlations of disease aggressiveness in CLL with immunoglobulin (IG) genetics,3,4 BCR signaling capacity,3,5 and receptor reactivity.3,6 BCR pathway components are at the very focus of novel interventional strategies in CLL with great clinical success.
Corroborating the theories of antigen-based selection, the surface membrane IGs (smIGs) (the antigen recognition units) and the third complementary determining region (HCDR3) of the IG heavy chain variable (IGHV) domain in CLL exhibit remarkable stereotypy.4 Underlying this is a “preferred” constitution of the leukemic BCRs of specific IGHV genes, including their association with certain IGHD-J genes and particular IGLVκ/λ’s. When superimposing these repertoire biases onto a disease categorization based on IGHV somatic mutations (unmutated-CLL [U-CLL] vs mutated-CLL [M-CLL]), CLLs can be clustered into those with sets of stereotyped smIGs (collectively ∼1/3 of cases and mostly U-CLL), those using specific IGHVs (U- and M-CLL), and those with heterogeneous or no obvious IG characteristics (primarily M-CLL). With respect to their cognate antigens, U-CLLs typically carry polyreactive smIGs of low affinity toward processed autologous neo-antigens (eg, myosin chains, vimentin, oxidized lipoproteins, dsDNA, and the lupus-associated ribonucleoprotein Smith [Sm]-antigen), or to microbial (foreign) antigens (eg, pneumococcal polysaccharides and pUL32 of cytomegalovirus).3 The structurally less restricted smIGs of M-CLL react with foreign antigens (eg, yeast-derived β-(1,6)-glucans and Fc-tails of rheumatoid factors) in high-affinity and high-specificity engagements.3 The void of data on the actual leukemogenic contribution of such (auto)antigen-based BCR signaling was one of the issues addressed by Iacovelli et al1 (see below).
At the level of BCR responsiveness, U-CLLs show a higher signaling capacity than M-CLLs, which is in part explained by higher sIgM densities, differential expression of signaling modulators (eg, CD38, ZAP70, and TCL1),5 and the kinetics of BCR membrane microdomain formation. Nonmanipulated CLL cells (U- and M-CLL), however, commonly show higher-than-normal ostensibly basal levels of signaling activity (“anergy phenotype”). Yet, recurrent constitutively activating mutations in BCR pathway components are, in contrast to other B-cell lymphomas, not detected in CLL. Therefore, the existence of a genuine receptor-mediated “tonic” BCR signaling in both CLL subsets was concluded. This second form of BCR-input kinetics, besides the (auto)antigen-based repeated engagements, had lacked a plausible molecular correlate until recent seminal studies.
First, Binder et al6 emphasized that a functional overlap across CLL cases, according to binding of their BCRs to phage-display isolated epitope mimics, supersedes IGHV genetics (ie, stereotypy). They further discovered sequences within the IGHV framework region (FR) 2, and later in FR3,7 as epitopes recognized by virtually all CLLs and implicated a unique cell-autonomous antigen-independent mechanism involving an HCDR3, namely, internal epitope interaction. Such exemplary autoreactivity might be functionally grouped alongside other smIG–Ig interactions (eg, as seen in autoimmunity) in a class of BCR–super-antigen interactions. Expanding on this, Dühren-von Minden et al8 reveal, as a precursor for this paper,1 compelling functional data on the robust signal induction of leukemic BCRs in the absence of their smIG ligand. This cell-autonomous signaling was not observed in normal B cells, was exclusive to CLL as compared with other B-cell lymphomas, and was irrespective of IG constellations (eg, somatic mutations, stereotyped/IGHV restricted, or nondescript).8
Against this complex backdrop of evidence for the contribution of at least two kinds of BCR signals (autonomous and antigen-dependent) toward CLL, Iacovelli et al1 provide a valuable integrative set of data on the individual or combined leukemogenic relevance of both categories of signal generation. They take advantage of the Eµ-TCL1 transgenic (tg) mouse model and its access to stages of an evolving CLL that carries restricted (autoantigenic or microbial glycero-phospholipids, lipoproteins, and polysaccharides) and IGHV-unmutated BCRs.9 Introduction of tg BCRs against defined cognate low-affinity autoepitopes (phosphatidylcholine [PtC] and Sm) to mimic U-CLL antigens, or against foreign high-affinity antigens (hen egg lysozyme [HEL]), into that TCL1-tg backbone markedly changed the signaling and clinical behavior of the resulting leukemias (see figure). After insertion of their smIGs into receptorless BCR adapter-inducible cell lines, exo-antigen–independent signaling was an exclusive feature of all leukemic BCRs. This emphasizes the prerequisite nature of autonomous BCR signaling, at least in CLL initiation, not necessarily drive. Furthermore, there was intriguing selection for the low-affinity BCRs against PtC or Sm in the double-tg leukemias against the Eµ-TCL1 inherent bias, but surprisingly not for anti-HEL BCRs.1 This argues for the additional importance of autoantigen-mediated interactions. However, increased disease aggressiveness was only conferred by the Eµ-TCL1/IgPtC pool, which was characterized by a much more robust intracellular signaling upon specific ligand engagement in vitro than the more indolent Eμ-TCL1/IgSm clones.
Some of the results by Iacovelli et al,1 such as why IgHELs are outcompeted, must also be interpreted at the level of co-operations between BCR interactions and the impact of signal modulating oncogenes. The oncogenic adapter molecule TCL1 acts as a BCR-response sensitizer5 and might confer an apoptotic net effect in the presence of high-affinity ligands (ie, HEL in these models). Alternatively, TCL1, as a subtle oncogene, might not be able to perturb the robust anergy phenotype dictated by high-affinity IgHELs, whereas in fact, MYC has shown to do so by creating a CLL-like disease.10
Collectively, these data implicate that the most advantageous CLL-permissive context requires the presence of cell-autonomous “tonic” BCR signaling (eg, through BCR autorecognition) in conjunction with (auto)antigenic input alongside the “appropriate dose” of oncogenes. Here, Iacovelli et al1 have set the stage to tackle the next level of issues, such as (1) modeling gradual antigen affinities; (2) facultative roles of co-receptors, including IgD; (3) niche-specific hierarchies of BCR signals; (4) impact of receptor turnover (internalization kinetics) and their posttranslational modifications (glycosylations); and (5) differential pharmacotherapeutic amenabilities of both signal categories at the overt leukemic stage.
Conflict-of-interest disclosure: The authors declare no competing financial interests.