In this issue of Blood, Crudele et al describe a novel study of adeno-associated virus (AAV) vector-mediated gene therapy that induced immune tolerance to factor IX (FIX) in a hemophilia B (HB) dog with previously formed anti-FIX inhibitor antibodies (IAs).1
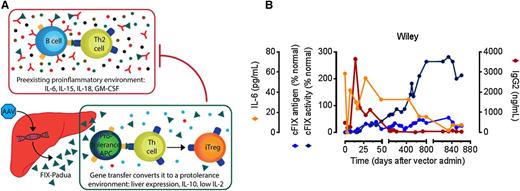
AAV liver gene therapy eradicates FIX inhibitors in a hemophilia B dog. (A) Expression of hepatocyte-restricted FIX Padua (green triangle) leads to a protolerance cytokine profile including IL-10 secretion (blue circles), which may modify the preexisting inflammatory environment including high IL-6 levels, facilitating tolerance induction of anti-FIX antibodies (red). (B) Time course of preexisting inhibitor eradication following AAV liver gene therapy in a hemophilia B dog (Wiley). The increased specific activity of FIX Padua results in high levels of FIX activity, with an activity-to-antigen ratio of ∼8. Adapted from Figures 2 and 3 in the article by Crudele et al beginning on page 1553.
AAV liver gene therapy eradicates FIX inhibitors in a hemophilia B dog. (A) Expression of hepatocyte-restricted FIX Padua (green triangle) leads to a protolerance cytokine profile including IL-10 secretion (blue circles), which may modify the preexisting inflammatory environment including high IL-6 levels, facilitating tolerance induction of anti-FIX antibodies (red). (B) Time course of preexisting inhibitor eradication following AAV liver gene therapy in a hemophilia B dog (Wiley). The increased specific activity of FIX Padua results in high levels of FIX activity, with an activity-to-antigen ratio of ∼8. Adapted from Figures 2 and 3 in the article by Crudele et al beginning on page 1553.
The discovery of a naturally occurring gain-of-function in FIX has prompted the development of highly effective, immune-tolerizing gene therapy for HB. The mutation in question was described in an Italian family with a unique form of X-linked thrombophilia, caused by the substitution of leucine for arginine at position 338 (R338L; R384L including the activation peptide) in FIX, which increased the specific activity of FIX by 5- to 10-fold and was termed FIX-Padua.2
IAs complicate replacement therapy in hemophilia generally, although IA formation is much more frequent in hemophilia A than in HB. The fundamental issue remains that no effective treatment is available to eliminate IAs, especially in HB where immune tolerance induction frequently fails and has been complicated by allergic reactions and nephrotic syndrome.3 A novel development, led by Finn et al, has been the administration of gene therapy to overexpress coagulation factor VIII in dogs with hemophilia A, thereby overwhelming the IA response and establishing immune tolerance.4 Subsequently, 2 groups showed that overexpression of FIX could similarly induce immune tolerance to FIX through a mechanism dependent on regulatory T-cell activation in HB mice.5,6 Given that an AAV vector-mediated FIX-Padua gene therapy was highly effective and nonimmungenic in HB mice7 and dogs,8 it remained for Crudele et al to demonstrate that it would induce immune tolerance in an HB dog with preformed IA.
The AAV vector encoding FIX-Padua was administered at dosages approximately equivalent to a recent successful clinical trial involving patients with HB.9 Notably, the specific activity of FIX-Padua was 8- to 12-fold higher, in comparison with wild-type FIX, reaching 25% to 40% of normal without provoking anti-FIX antibodies in 2 naïve HB dogs. The third HB dog treated (Wiley) had previously formed antibodies that increased after vector administration, signifying a transient anamnestic response, followed by disappearance of anti-FIX by day 70. Remarkably, FIX activity continued to rise in Wiley’s blood for 800 days while the proinflammatory cytokine interleukin (IL)-6 and anti-FIX antibodies decreased concurrently (see figure). Cytokine profiling suggested that the induction of regulatory T cells occurred both following the initial vector administration and following an immune challenge after day 800, given the rise in both interferon-γ and IL-10 at these times. Importantly, no evidence for thrombosis or nephrotic syndrome was observed during the monitoring of treated HB dogs. An accompanying dose escalation study in mice revealed evidence for thrombosis at the highest levels of FIX activity, a known complication of supraphysiologic FIX activity, which occurred at equivalent FIX activities for either FIX-Padua or wild-type FIX.2-6
Thus, gene therapy with FIX-Padua features safety and efficacy promises success in future clinical trials, with the caveat that supraphysiologic FIX activity presents a risk for thrombosis necessitating careful monitoring.2,7,8 Importantly, FIX-Padua gene therapy could lower the dose requirements for gene therapy in HB, given the high degree of efficacy in the HB dog model at clinically acceptable dosages, which will help to address the well-documented risk for dose-related anti-capsid T-cell responses to AAV vectors in HB clinical trials.9,10 Overall, it seems reasonable to predict a bright future for a “natural choice” for HB gene therapy in FIX-Padua.
Conflict-of-interest disclosure: D.D.K. is a paid member of the Data and Safety Monitoring Board for a clinical trial of AAV-FIX Padua funded by Baxter International, Inc.