Key Points
Fibrinogen, and particularly fibrinogen γ′, counteracts plasma APC resistance, the most common risk factor for venous thrombosis.
The C-terminal peptide of the fibrinogen γ′ chain inhibits protein C activation, but still improves the response of plasma to APC.
Abstract
Activated protein C (APC) resistance, often associated with the factor V (FV) Leiden mutation, is the most common risk factor for venous thrombosis. We observed increased APC resistance in carriers of fibrinogen γ gene (FGG) haplotype 2, which is associated with reduced levels of the alternatively spliced fibrinogen γ′ chain. This finding prompted us to study the effects of fibrinogen and its γ′ chain on APC resistance. Fibrinogen, and particularly the γA/γ′ isoform, improved the response of plasma to added APC in the thrombin generation-based assay. Similarly, a synthetic peptide mimicking the C-terminus of the fibrinogen γ′ chain, which binds thrombin and inhibits its activities, greatly increased the APC sensitivity of normal and FV Leiden plasma, likely due to its ability to inhibit thrombin-mediated activation of FV and FVIII. Although the fibrinogen γ′ peptide also inhibited protein C activation by the thrombin/thrombomodulin complex, it still increased the sensitivity of plasma to endogenously formed APC when thrombin generation was measured in the presence of soluble thrombomodulin. We conclude that fibrinogen, and particularly fibrinogen γ′, increases plasma APC sensitivity. The fibrinogen γ′ peptide might form the basis for pharmacologic interventions to counteract APC resistance.
Introduction
The protein C pathway1,2 is a pivotal anticoagulant mechanism, which is initiated by thrombin bound to the membrane protein thrombomodulin (TM). The thrombin/TM complex converts the zymogen protein C into the serine-protease activated protein C (APC), which inactivates coagulation factors Va (FVa) and VIIIa (FVIIIa) by limited proteolysis. The in vitro addition of APC to plasma produces an anticoagulant response, which can be quantified in an activated partial thromboplastin time (APTT)-based assay (as a prolongation of the clotting time) or in a thrombin generation-based assay (as a decrease of the area under the thrombin generation curve).3 A poor response of plasma to the anticoagulant action of APC, known as APC resistance,4 is the most prevalent risk factor for venous thrombosis in the Caucasian population. APC resistance is usually associated with carriership of the factor V (FV) Arg506→Gln (FV Leiden) mutation,5 which abolishes 1 of the APC-cleavage sites on FVa. However, altered levels of several procoagulant and anticoagulant factors, such as prothrombin, FV, FVIII, factor X, protein S, and tissue factor pathway inhibitor, also cause plasma APC resistance.6,7
Fibrinogen is a large glycoprotein that is produced in the liver and circulates in plasma at a concentration of ∼3 mg/mL. Structurally, it comprises 3 polypeptide chains (Aα, Bβ, and γ), each of which is present in 2 copies in the fibrinogen molecule. The human γ chain, encoded by the FGG gene, occurs in 2 structurally and functionally different isoforms that are generated by alternative splicing and polyadenylation of the FGG pre-mRNA.8 The more abundant γA isoform has a short C-terminal tail of 4 amino acids (γA408-411, AGDV) containing a platelet-binding site, whereas the less abundant γ′ isoform has an extended C-terminal tail of 20 amino acids (γ′408-427, VPREHPAETEYDSLYPEDDL) containing a high-affinity binding site for thrombin exosite II.9-11 Random pairing of the γ chain isoforms generates 3 types of fibrinogen molecules: γA/γA (85%-92%), γA/γ′ (8%-15%) and γ′/γ′ (<0.5%).
Being the precursor of fibrin and promoting platelet aggregation, fibrinogen plays a major role in hemostasis. However, it has been known for a long time that fibrin(ogen) also binds thrombin and inhibits its activity.12 Although this anticoagulant activity of fibrin(ogen), known as “antithrombin I,” is still poorly understood, it has been recently shown that the interaction of the C-terminus of the fibrinogen γ′ chain with thrombin exosite II inhibits thrombin-mediated activation of FVIII13 and FV,14 as well as platelets.15,16 The physiological relevance of this anticoagulant mechanism is underscored by the two- to threefold increased risk of venous thrombosis associated with the common FGG H2 haplotype, which is associated with reduced levels of fibrinogen γ′ without affecting total fibrinogen levels.17-21
Recently, we observed that carriership of the FGG H2 haplotype is associated with higher plasma APC resistance in FV Leiden carriers. This unexpected finding prompted us to investigate the effect of fibrinogen and its γ chain isoforms on APC resistance in normal and FV Leiden plasma.
Materials and methods
Population study
In the frame of a previous study, approved by the Ethical Committee of Padua Academic Hospital and conducted in accordance with the Declaration of Helsinki, we determined the effects of several hemostasis-related single nucleotide polymorphisms (SNPs) on thrombin generation initiated by 6.8 pM tissue factor (TF) in the absence and presence of ∼10 nM APC in 188 FV Leiden heterozygotes.22
In the present study, the same population was genotyped for the FGG 10034 C>T SNP (rs2066865), which tags the FGG H2 haplotype.17 Genotyping was performed by polymerase chain reaction (PCR)-mediated amplification of a 435 bp genomic DNA fragment followed by restriction analysis with Rsa I (Fermentas, Thermo Fischer Scientific, Landsmeer, The Netherlands).
The effect of FGG H2 on thrombin generation was determined by multiple regression analysis and expressed as unstandardized regression coefficient (B). Besides the FGG H2 genotype (0, 1, or 2), the regression model included age, gender, and the levels of prothrombin, factor X, antithrombin, protein S, and tissue factor pathway inhibitor as independent variables.
Plasma samples
A normal plasma pool was prepared by pooling citrated plasma from 34 healthy volunteers (20 males and 14 females; mean age, 34.5 years) free of medication. Pooled FV Leiden plasma was prepared from citrated plasma of 16 heterozygous carriers of FV Leiden not using any medication (referred to as FV Leiden heterozygous plasma throughout). In some experiments, these plasmas were defibrinated by incubation with 1.17 U/mL Ancrod (National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom) for 10 minutes at 37°C and subsequent clot removal with a spatula.
Congenitally fibrinogen-deficient plasma was purchased from George King Bio-Medical Inc. (Overland Park, KS) and reconstituted with either N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered saline (25 mM HEPES, 175 mM NaCl, pH 7.7 at room temperature) or purified γA/γA or γA/γ′ fibrinogen to a final concentration of 1.4 mg/mL.
Purification of γA/γA and γA/γ′ fibrinogen isoforms
Human fibrinogen was purchased from Enzyme Research Laboratories (Swansea, UK). The γA/γA (peak 1) and γA/γ′ (peak 2) fibrinogen isoforms were separated by ion-exchange chromatography, as described.23,24 To eliminate factor XIII, both peaks were subjected to a 2-step ammonium sulfate precipitation protocol.25 In step 1, the fibrinogen peaks were supplemented with CaCl2 to a final concentration of 7.9 mM and an ammonium sulfate solution was added up to 20% saturation under stirring at 4°C. After 90 minutes the solution was centrifuged at 3000g for 20 minutes. In step 2, the ammonium sulfate concentration in the supernatant of step 1 was increased to 33% saturation and after 90 minutes the solution was again centrifuged at 3000g for 20 minutes. The pellets, which contained γA/γA or γA/γ′ fibrinogen, were dialyzed against HEPES-buffered saline. Purity was examined by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and concentration was determined by absorbance at 280 nm, using an extinction coefficient (ε) of 1.51 mg−1 mL cm−1.
Fibrinogen γ′ peptides
Synthetic wild-type fibrinogen γ′ C-terminal peptide (WT-S12, Ac-VPREHPAETEY[SO3]DSLY[SO3]PEDDL-OH) and scrambled control peptide (SCR-S12, Ac-DESELDAY[SO3]TPLPRPVEDHY[SO3]E-OH) were obtained from Pepscan (Lelystad, The Netherlands). Both peptides were sulfated on both tyrosine residues. Synthesis and purification details have been previously reported.14 Lyophilized peptides were resuspended in HEPES-buffered saline and stored in aliquots at −80°C.
Thrombin generation-based APC resistance assay
Thrombin generation was measured using Calibrated Automated Thrombography,26 essentially as described.14 Briefly, plasma was mixed with corn trypsin inhibitor (Hematologic Technologies, Essex Junction, VT) to prevent contact activation, and incubated with TF (Dade Innovin, Marburg, Germany) and synthetic phospholipid vesicles (1,2-dioleoyl-sn-glycero-3-phosphoserine/1,2-dioleoyl-sn-glycero-3-phosphocholine/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 20 mol/60 mol/20 mol) for 10 minutes at 37°C. Subsequently, APC (Kordia, Leiden, The Netherlands) or soluble TM (sTM) (Asahi Kasei Pharma, Tokyo, Japan) was added and coagulation was immediately initiated with a mixture of CaCl2 and fluorogenic substrate for thrombin (Z-Gly-Gly-Arg-AMC, I-1140; Bachem, Bubendorf, Switzerland). Final concentrations in the wells were 44 µg/mL corn trypsin inhibitor, 10 pM TF (for experiments with APC) or 4 pM TF (for experiments with sTM), 30 μM phospholipids, 0 to 10 nM APC or 0 to 20 nM sTM, 12 mM added CaCl2 and 300 μM thrombin substrate. All thrombin generation curves were measured at least in duplicate. Fluorescence was read in a Fluoroskan Ascent reader (Thermo Labsystems, Helsinki, Finland) and thrombin generation parameters were calculated using the Thrombinoscope software (Thrombinoscope, Maastricht, The Netherlands). APC resistance was expressed as the ratio of the endogenous thrombin potentials (ETPs) obtained in the presence and absence of APC or sTM (residual ETP). The higher the residual ETP, the higher the APC resistance.
APTT-based APC resistance assay
The APTT-based APC resistance of normal and FV Leiden heterozygous plasma was measured using the Coatest APC resistance kit (Chromogenix, Milan, Italy), according to the manufacturer’s instructions. Clotting times were measured in triplicate. The APC sensitivity ratio (APCsr) was defined as the ratio of the clotting times determined in the presence and absence of APC. The lower the APCsr, the higher the APC resistance.
Protein C activation assay
Purified human protein C (500 nM; Hyphen BioMed, Neuville-sur-Oise, France) was activated at 37°C with 5 nM human thrombin (Hematologic Technologies) and 10 nM human sTM in 25 mM HEPES, 140 mM NaCl, 3 mM CaCl2, pH 7.7 at room temperature, and 5 mg/mL bovine serum albumin in the presence of increasing concentrations (0-500 µM) of fibrinogen γ′ WT-S12 or SCR-S12 peptide. At 0, 30, and 60 minutes of activation, subsamples were drawn from these mixtures into an EDTA buffer (50 mM Tris, 175 mM NaCl, 20 mM EDTA, pH 7.9 at room temperature, 0.5 mg/mL ovalbumin, and 5 nM hirudin to prevent further activation of protein C). APC activity was determined spectrophotometrically with chromogenic substrate Biophen CS-21(66) (Hyphen BioMed). The assay was calibrated using known amounts of APC. Rates of protein C activation were plotted as a function of peptide concentration and fitted to the equation of a rectangular hyperbola, from which half-maximal inhibitory concentrations (IC50) were calculated.
Results
Effect of FGG H2 haplotype on APC resistance in FV Leiden carriers
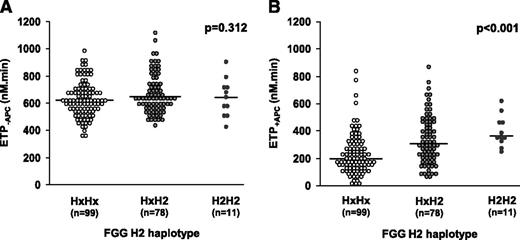
In the frame of a study aimed at determining the effect of common genetic variation on thrombin generation,22 we genotyped 188 FV Leiden heterozygotes for several SNPs in hemostasis-related genes. In the present study, this population was genotyped for FGG 10034 C>T (rs2066865), which tags the FGG H2 haplotype. Figure 1 shows the effect of the H2 haplotype on the ETP. The ETP−APC was 634.4 ± 129.8 nM⋅minute in noncarriers (N = 99), 673.5 ± 143.8 nM⋅minute in heterozygous carriers (N = 78) and 641.5 ± 139.0 nM⋅minute in homozygous carriers (N = 11) of the minor allele (Figure 1A), indicating that the FGG H2 genotype does not affect the ETP−APC (B =11.0 nM⋅min/haplotype copy; P = .312). In contrast, the ETP+APC was 230.5 ± 149.0 nM⋅minute in noncarriers, 337.6 ± 176.5 nM⋅minute in heterozygous carriers and 396.6 ± 113.1 nM⋅minute in homozygous carriers of the minor allele (Figure 1B), indicating that the FGG H2 haplotype considerably increases the ETP+APC (B = 62.4 nM⋅minute/haplotype copy; P < .001). This unexpected finding suggested a possible relationship between fibrinogen γ′ level and plasma APC resistance and prompted us to study the effect of fibrinogen and its γ chain isoforms on the response of normal and FV Leiden plasma to APC.
Effect of the FGG H2 genotype on plasma APC resistance.FGG H2 genotype and thrombin generation at 6.8 pM TF in the absence and presence of 10 nM APC were determined in 188 FV Leiden heterozygotes. The ETP−APC (A) and the ETP+APC (B) were plotted as a function of the FGG H2 genotype. Hx indicates any other FGG haplotype except H2. The P values refer to the regression coefficients of the ETP−APC and ETP+APC vs FGG H2 genotype.
Effect of the FGG H2 genotype on plasma APC resistance.FGG H2 genotype and thrombin generation at 6.8 pM TF in the absence and presence of 10 nM APC were determined in 188 FV Leiden heterozygotes. The ETP−APC (A) and the ETP+APC (B) were plotted as a function of the FGG H2 genotype. Hx indicates any other FGG haplotype except H2. The P values refer to the regression coefficients of the ETP−APC and ETP+APC vs FGG H2 genotype.
Effect of total fibrinogen and the purified γ chain fibrinogen isoforms on the APC response
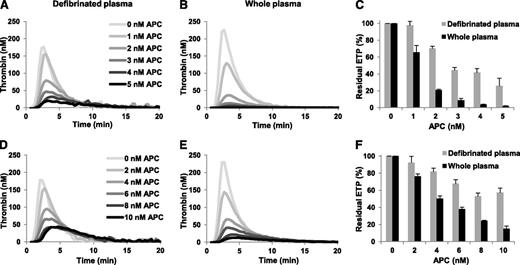
To study the effect of total fibrinogen on the plasma APC response, we compared thrombin generation in whole and defibrinated normal and FV Leiden heterozygous plasma in the absence and presence of increasing concentrations of APC. In the absence of APC, thrombin generation was higher in whole plasma than in defibrinated plasma (ETP 678.2 nM⋅minute and 479.6 nM⋅minute for whole and defibrinated normal plasma; 661.9 nM⋅minute and 503.2 nM⋅minute for whole and defibrinated FV Leiden heterozygous plasma) (Figure 2). This is in line with published data and is probably due to the ability of fibrinogen to protect thrombin from inhibition by antithrombin and α2-macroglobulin.27-30 However, the addition of APC decreased thrombin generation relatively more in whole plasma, resulting in lower ETPs in whole plasma than in defibrinated plasma at each APC concentration (Figure 2). At the highest APC concentration the ETP was almost 10 times (normal plasma) and >3 times (FV Leiden heterozygous plasma) lower in whole than in defibrinated plasma. Consequently, in both plasmas the residual ETP at each APC concentration was also lower in whole plasma than in defibrinated plasma (Figure 2C,F), suggesting that total fibrinogen makes plasma more sensitive to the anticoagulant action of APC. Whole plasma from a FV Leiden homozygous carrier was also more sensitive to APC than defibrinated plasma of the same individual (data not shown).
Effect of total fibrinogen on the ETP-based APC response in pooled normal plasma and in FV Leiden heterozygous plasma. Thrombin generation was initiated in defibrinated and whole normal plasma (A-B) with 10 pM TF in the presence of 0-5 nM APC and the residual ETP (ETP+APC/ETP−APC) was plotted as a function of the APC concentration (C). Thrombin generation was initiated in defibrinated and whole FV Leiden heterozygous plasma (D-E) with 10 pM TF in the presence of 0-10 nM APC and the residual ETP was plotted as a function of the APC concentration (F). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
Effect of total fibrinogen on the ETP-based APC response in pooled normal plasma and in FV Leiden heterozygous plasma. Thrombin generation was initiated in defibrinated and whole normal plasma (A-B) with 10 pM TF in the presence of 0-5 nM APC and the residual ETP (ETP+APC/ETP−APC) was plotted as a function of the APC concentration (C). Thrombin generation was initiated in defibrinated and whole FV Leiden heterozygous plasma (D-E) with 10 pM TF in the presence of 0-10 nM APC and the residual ETP was plotted as a function of the APC concentration (F). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
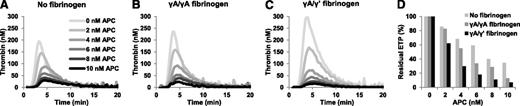
To check whether the 2 fibrinogen-γ chain isoforms differ in their effects on plasma APC response, we reconstituted congenitally fibrinogen-deficient plasma with 1.4 mg/mL purified γA/γA or γA/γ′ fibrinogen (corresponding to ∼50% of the total fibrinogen concentration in normal plasma), and we measured thrombin generation in the absence and presence of increasing concentrations of APC. In line with the results obtained with whole and defibrinated plasma, supplementation of fibrinogen-deficient plasma with fibrinogen resulted in higher thrombin generation in the absence of APC, but also increased the sensitivity of plasma to the anticoagulant action of APC (Figure 3). Both effects were more pronounced when fibrinogen-deficient plasma was reconstituted with γA/γ′ fibrinogen compared with reconstitution with γA/γA fibrinogen. The ETPs obtained in the absence of APC and at the highest APC concentration (10 nM) were 627 nM⋅minute and 217 nM⋅minute (residual ETP, 34.6%) in fibrinogen-deficient plasma, 852 nM⋅minute and 109 nM⋅minute (residual ETP, 12.8%) in plasma reconstituted with γA/γA fibrinogen, and 1310 nM⋅minute and 92 nM⋅minute (residual ETP, 7.0%) in plasma reconstituted with γA/γ′ fibrinogen. These results indicate that, although both fibrinogen isoforms increase the sensitivity of plasma for APC, the γA/γ′ isoform has a more pronounced effect.
Effect of γA/γA and γA/γ′ fibrinogen on the ETP-based APC response in reconstituted fibrinogen-deficient plasma. Thrombin generation was initiated with 10 pM TF in the presence of 0 to 10 nM APC in fibrinogen-deficient plasma supplemented with buffer (A), or with 1.4 mg/mL γA/γA fibrinogen (B), or with 1.4 mg/mL γA/γ′ fibrinogen (C). Residual ETPs (ETP+APC/ETP−APC) were plotted as a function of the APC concentration (D). Averages of duplicate experiments are shown.
Effect of γA/γA and γA/γ′ fibrinogen on the ETP-based APC response in reconstituted fibrinogen-deficient plasma. Thrombin generation was initiated with 10 pM TF in the presence of 0 to 10 nM APC in fibrinogen-deficient plasma supplemented with buffer (A), or with 1.4 mg/mL γA/γA fibrinogen (B), or with 1.4 mg/mL γA/γ′ fibrinogen (C). Residual ETPs (ETP+APC/ETP−APC) were plotted as a function of the APC concentration (D). Averages of duplicate experiments are shown.
Effects of the fibrinogen γ′ peptides on the APC response of normal and FV Leiden heterozygous plasma
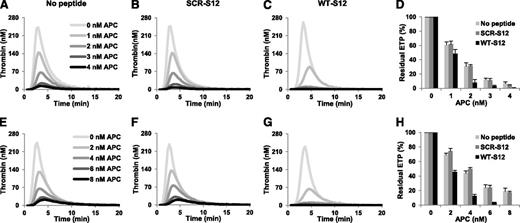
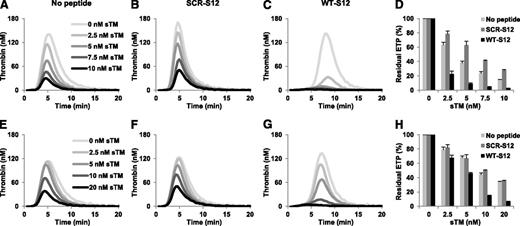
To investigate whether the effect of γA/γ′ fibrinogen on the APC response of plasma is mediated by thrombin inhibition via occupation of exosite II, we used a synthetic 20 amino acid peptide homologous to the C-terminus of this chain (WT-S12) and a scrambled control peptide (SCR-S12). Thrombin generation was measured in normal plasma at increasing concentrations of APC in the absence and presence of 250 µM peptide (Figure 4A-C) and residual ETPs were calculated (Figure 4D). Addition of the WT-S12 peptide only slightly affected the ETP−APC (which was 783.3 nM⋅minute and 677.3 nM⋅minute in the absence and presence of peptide, respectively), as previously observed at this TF concentration,14 but markedly decreased the ETP+APC and the residual ETP. At 3 nM APC, the ETP in the presence of the WT-S12 peptide was 19.7 nM⋅minute vs 94.7 nM⋅minute in the absence of peptide. Accordingly, the residual ETP in the presence of WT-S12 peptide was >75% lower than in its absence. In contrast, addition of the SCR-S12 did not appreciably influence the ETP in the absence or presence of APC or the residual ETP.
Effect of fibrinogen γ′ peptides on the ETP-based APC response in normal and FV Leiden heterozygous plasma. Thrombin generation was initiated in normal plasma (A-C) with 10 pM TF in the presence of 0-4 nM APC in the absence (A) and presence of 250 μM SCR-S12 peptide (B) or WT-S12 peptide (C). The residual ETP (ETP+APC/ETP−APC) was plotted as a function of the APC concentration (D). Thrombin generation was initiated in FV Leiden heterozygous plasma (E-G) with 10 pM TF in the presence of 0-8 nM APC in the absence (E) and presence of 250 μM SCR-S12 peptide (F) or WT-S12 peptide (G). The residual ETP was plotted as a function of the APC concentration (H). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
Effect of fibrinogen γ′ peptides on the ETP-based APC response in normal and FV Leiden heterozygous plasma. Thrombin generation was initiated in normal plasma (A-C) with 10 pM TF in the presence of 0-4 nM APC in the absence (A) and presence of 250 μM SCR-S12 peptide (B) or WT-S12 peptide (C). The residual ETP (ETP+APC/ETP−APC) was plotted as a function of the APC concentration (D). Thrombin generation was initiated in FV Leiden heterozygous plasma (E-G) with 10 pM TF in the presence of 0-8 nM APC in the absence (E) and presence of 250 μM SCR-S12 peptide (F) or WT-S12 peptide (G). The residual ETP was plotted as a function of the APC concentration (H). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
Similar experiments were performed in the FV Leiden heterozygous plasma (Figure 4E-H). As in normal plasma, the WT-S12 and SCR-S12 peptides hardly affected thrombin generation in the absence of APC, whereas the WT-S12 peptide (but not the SCR-S12 peptide) considerably reduced thrombin generation in the presence of APC. At 6 nM APC, the WT-S12 peptide decreased the ETP from 170.3 nM⋅minute to 22.7 nM⋅minute, whereas the SCR-S12 peptide had virtually no effect (163.3 nM⋅minute). This was reflected in the residual ETP, which was >80% lower in the presence of WT-S12 compared with the residual ETP in its absence. Similar effects of WT-S12 and SCR-S12 peptides were observed in plasma from a FV Leiden homozygous carrier (data not shown).
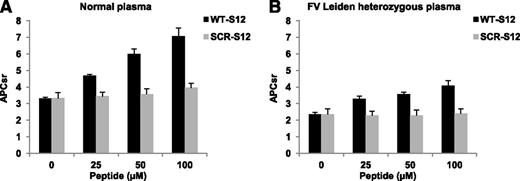
The effect of the fibrinogen γ′ peptide on plasma APC resistance was also tested with the commonly used APTT-based assay (Figure 5). To this end, clotting times in the absence and presence of APC were measured in plasma supplemented with 0-100 µM WT-S12 or SCR-S12 peptide. In normal plasma, increasing concentrations of the WT-S12 peptide caused a progressive prolongation of the APTT−APC (from 35.0 to 56.3 seconds), and even more of the APTT+APC (from 109.9 to 398.3 seconds), increasing the APCsr from 3.14 in the absence of peptide to 7.08 in the presence of 100 μM peptide (Figure 5A). In contrast, the SCR-S12 peptide had only a minor effect on the clotting times and on the APCsr, which increased from 3.22 in the absence of peptide to 3.71 in the presence of 100 μM peptide. In FV Leiden heterozygous plasma, the WT-S12 peptide prolonged the APTT−APC from 34.7 to 56.9 seconds and the APTT+APC from 82.2 to 232 seconds, causing the APCsr to increase from 2.37 in the absence of peptide to 4.07 in the presence of 100 μM peptide (Figure 5B). Interestingly, the addition of 25 µM WT-S12 peptide was already sufficient to normalize the APCsr of FV Leiden heterozygous plasma. In contrast, the SCR-S12 peptide was virtually ineffective, changing the APCsr of FV Leiden heterozygous plasma from 2.37 in the absence of peptide to 2.42 in the presence of 100 μM peptide.
Effect of fibrinogen γ′ peptides on the APTT-based APC response in normal and FV Leiden heterozygous plasma. APC resistance was measured with the APTT-based assay in normal (A) and FV Leiden heterozygous (B) plasma supplemented with 0 to 100 μM WT-S12 or SCR-S12 peptide. APCsr were plotted as a function of the peptide concentration. All clotting times were measured in triplicate. Error bars indicate the standard error of the mean.
Effect of fibrinogen γ′ peptides on the APTT-based APC response in normal and FV Leiden heterozygous plasma. APC resistance was measured with the APTT-based assay in normal (A) and FV Leiden heterozygous (B) plasma supplemented with 0 to 100 μM WT-S12 or SCR-S12 peptide. APCsr were plotted as a function of the peptide concentration. All clotting times were measured in triplicate. Error bars indicate the standard error of the mean.
Overall, these data show that the fibrinogen γ′ C-terminal peptide improves the anticoagulant response of normal and FV Leiden plasma to APC.
Effect of fibrinogen γ′ peptides on thrombin generation determined in the absence and presence of sTM in normal and FV Leiden heterozygous plasma
In vivo, protein C is activated by thrombin bound to TM. Because the C-terminus of the fibrinogen γ′ chain targets exosite II of thrombin, it might inhibit protein C activation by the thrombin/TM complex, thereby abolishing its own anticoagulant effect observed in the presence of pre-formed APC. Hence, we also determined the effects of the fibrinogen γ′ peptides on thrombin generation measured at 4 pM TF in the absence and presence of sTM. In the absence of peptides, increasing concentrations of sTM progressively decreased thrombin generation in normal plasma (Figure 6A) and, to a lesser extent, in FV Leiden heterozygous plasma (Figure 6E). Addition of the WT-S12 peptide (Figure 6C,G), but not of the SCR-S12 peptide (Figure 6B,F), dramatically decreased thrombin generation at each sTM concentration. At the highest sTM concentration, the WT-S12 peptide reduced the ETP+TM from 94.4 nM⋅minute to 16.5 nM⋅minute in normal plasma and from 132.5 nM⋅minute to 25.9 nM⋅minute in FV Leiden heterozygous plasma. As a result, the residual ETP was ∼80% lower it the presence of WT-S12 than in its absence in both normal and FV Leiden plasma (Figure 6D,H).
Effect of fibrinogen γ′ peptides on the ETP-based sTM response of normal and FV Leiden heterozygous plasma. Thrombin generation was initiated in normal plasma (A-C) with 4 pM TF in the presence of 0-10 nM sTM in the absence (A) or presence of 250 μM SCR-S12 peptide (B) or WT-S12 peptide (C). The residual ETP (ETP+TM/ETP−TM) was plotted as a function of the sTM concentration (D). Thrombin generation was initiated in FV Leiden heterozygous plasma (E-G) with 4 pM TF in the presence of 0-20 nM sTM in the absence (E) or presence of 250 μM SCR-S12 peptide (F) or WT-S12 peptide (G). The residual ETP was plotted as a function of the sTM concentration (H). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
Effect of fibrinogen γ′ peptides on the ETP-based sTM response of normal and FV Leiden heterozygous plasma. Thrombin generation was initiated in normal plasma (A-C) with 4 pM TF in the presence of 0-10 nM sTM in the absence (A) or presence of 250 μM SCR-S12 peptide (B) or WT-S12 peptide (C). The residual ETP (ETP+TM/ETP−TM) was plotted as a function of the sTM concentration (D). Thrombin generation was initiated in FV Leiden heterozygous plasma (E-G) with 4 pM TF in the presence of 0-20 nM sTM in the absence (E) or presence of 250 μM SCR-S12 peptide (F) or WT-S12 peptide (G). The residual ETP was plotted as a function of the sTM concentration (H). Averages of triplicate experiments are shown. Error bars indicate the standard error of the mean.
Effect of the fibrinogen γ′ peptides on protein C activation
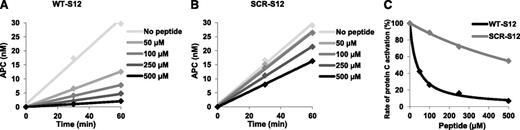
Finally, we also investigated the direct effect of the WT-S12 and SCR-S12 peptides on protein C activation by the thrombin/sTM complex in a model system. Both peptides inhibited protein C activation in a dose-dependent manner, but WT-S12 was clearly more potent than SCR-S12 (Figure 7A-B). At the highest peptide concentration (500 μM), protein C activation was inhibited ∼90% by the WT-S12 peptide vs ∼40% by the SCR-S12 peptide. The IC50 values were 36.5 µM for WT-S12 and 582.8 µM for SCR-S12 (Figure 7C).
Effect of fibrinogen γ′ peptides on protein C activation by the thrombin/sTM complex. Purified protein C (500 nM) was activated with thrombin (5 nM) and sTM (10 nM) in the presence of 0 to 500 µM WT-S12 (A) or SCR-S12 (B) peptide. Subsamples were taken from the activation mix after 30 and 60 minutes and were assayed for APC activity. Rates of protein C activation were plotted as a function of peptide concentration and fitted to the equation of a rectangular hyperbola (C).
Effect of fibrinogen γ′ peptides on protein C activation by the thrombin/sTM complex. Purified protein C (500 nM) was activated with thrombin (5 nM) and sTM (10 nM) in the presence of 0 to 500 µM WT-S12 (A) or SCR-S12 (B) peptide. Subsamples were taken from the activation mix after 30 and 60 minutes and were assayed for APC activity. Rates of protein C activation were plotted as a function of peptide concentration and fitted to the equation of a rectangular hyperbola (C).
Discussion
Beside its essential prohemostatic role, fibrin(ogen) is known to express anticoagulant activity as a thrombin inhibitor. In the present paper, we show that fibrinogen, and particularly fibrinogen γ′, increases the sensitivity of plasma to APC in the thrombin generation- and APTT-based assays. This phenomenon was not observed in previous studies that investigated the relationship between plasma factor levels and APC resistance,6,7 possibly because the variation in fibrinogen level in healthy individuals is not large enough for the effect to be picked up, and the effect is mainly (although not exclusively) due to fibrinogen γ′, which makes up only 8% to 15% of total fibrinogen in plasma. In our study design the effect of fibrinogen on APC sensitivity was magnified by comparing the complete absence of fibrinogen (defibrinated plasma or congenitally fibrinogen-deficient plasma) with 100% total fibrinogen (whole plasma) or with ∼50% γA/γA or γA/γ′ fibrinogen (reconstituted fibrinogen-deficient plasma). This was only possible in the ETP-based APC resistance assay, which can also be performed in plasma without fibrinogen. In the APTT-based assay, which relies on clotting and therefore cannot be performed in the absence of fibrinogen, the effect of fibrinogen γ′ on APC resistance could be reproduced by the addition of the synthetic γ′ chain C-terminal peptide.
Thrombin generation-based APC resistance was expressed as the residual ETP, which is the ratio of the ETPs determined in the presence and absence of APC (or sTM). Fibrinogen decreased the residual ETP, not only by decreasing the ETP+APC, but also by increasing the ETP−APC. The ability of fibrinogen to increase the ETP determined at high TF in the absence of APC is a well-known effect, which has been attributed to the fact that fibrin(ogen) binds thrombin and delays its inhibition by α2-macroglobulin and antithrombin,27-30 without affecting its ability to convert the fluorogenic substrate. The observation that γA/γ′ fibrinogen binds more thrombin with a higher affinity29 compared with γA/γA fibrinogen, can explain why this fibrinogen isoform is more effective than γA/γA fibrinogen in increasing the ETP−APC. On the other hand, the effect on the ETP+APC is likely due to the ability of fibrinogen, and particularly of the γ′ chain, to inhibit thrombin activity on FV14 and FVIII,13 as demonstrated by the fact that this effect could be reproduced by the fibrinogen γ′ C-terminal peptide alone. Therefore, in contrast to most determinants of APC resistance (such as FV Leiden, elevated levels of prothrombin and FVIII, as well as low protein S levels),6,7 which act by impairing FVa and/or FVIIIa inactivation, the fibrinogen γ′ chain improves the APC response by inhibiting FV and FVIII activation. This mechanism is similar to that of tissue factor pathway inhibitor, which also improves the APC response by decreasing the procoagulant drive.6,7
The thrombin generation-based APC resistance test used in our study was performed at 10 pM TF. Because it is well-established that the intrinsic FX-activating complex (ie, FVIIIa) does not contribute to thrombin generation measured at high TF concentrations,7 control experiments with anti-FVIII antibodies were performed to verify the FVIII-dependence of our thrombin generation-based APC resistance assay. These experiments showed that the addition of neutralizing anti-FVIII antibodies does not affect thrombin generation at 10 pM TF in the absence of APC, whereas in the presence of APC considerably less thrombin is formed in the presence than in the absence of anti-FVIII antibodies (data not shown). This indicates that FVIII is indeed a determinant of thrombin generation measured at high TF in the presence of APC and of the residual ETP.
Importantly, the APC-sensitizing action of fibrinogen was not confined to normal plasma, but also extended to plasma from heterozygous and homozygous carriers of FV Leiden. Although γA/γA and γA/γ′ fibrinogen could not be tested separately on a FV Leiden background, our population study showed a definite association between FGG H2 genotype (and hence reduced fibrinogen γ′ levels) and increased APC resistance in FV Leiden carriers. Moreover, the fibrinogen γ′ peptide effectively decreased APC resistance in FV Leiden plasma, both in the thrombin generation-based assay and in the APTT-based assay. These findings are in line with an in vivo study in which transgenic expression of the human γ′ chain substituting the murine γ′ chain reduced thrombus size in FV Leiden mice in an experimental model of venous thrombosis.31
Addition of pre-formed APC to plasma is not entirely physiological, because it bypasses the activation of protein C by the thrombin/TM complex. Because fibrinogen γ′ could interfere with this process by binding and inhibiting thrombin, we also studied the effect of the fibrinogen γ′ peptide on protein C activation and on thrombin generation measured in the presence of (soluble) TM. We observed that the peptide markedly decreased protein C activation by the thrombin/sTM complex in a model system, suggesting that the fibrinogen γ′ peptide affects both procoagulant and anticoagulant functions of thrombin. Interestingly, previous studies have shown that fibrinogen impairs APC generation by interfering with the formation of the thrombin/TM complex,32,33 which was originally attributed to the competition between fibrinogen and TM for thrombin exosite I. However, our data indicate that occupation of thrombin exosite II by the fibrinogen γ′ peptide may contribute to this effect, as also reported in a recent abstract.34 Nevertheless, addition of the fibrinogen γ′ peptide still decreased thrombin generation in the presence of sTM, both in normal and FV Leiden heterozygous plasma. In other words, the peptide increased the sensitivity of plasma to endogenously generated APC, suggesting that the anticoagulant effect of the peptide (inhibition of thrombin-mediated FV and FVIII activation) is stronger than its procoagulant effect (inhibition of protein C activation by the thrombin/sTM complex).
In summary, our study shows that fibrinogen and particularly its γA/γ′ isoform increases APC sensitivity in normal and FV Leiden plasma. This effect can be reproduced by the C-terminal peptide of the γ′ chain and is likely explained by the ability of the peptide to inhibit thrombin-mediated FV and FVIII activation. Although the fibrinogen γ′ peptide also inhibits protein C activation by the thrombin/TM complex, its net effect in plasma is still anticoagulant. Therefore, the fibrinogen γ′ peptide could form the basis for pharmacologic interventions to counteract APC resistance.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Olivier Segers for genotyping the FV Leiden population for the FGG 10034 C>T SNP. This work was supported by the Dutch Thrombosis Foundation (grant 2011-2).
Authorship
Contribution: F.O. designed and performed experiments; F.O., R.M.B., J.R., and E.C. analyzed data; S.U.d.W. and R.A.S.A. purified fibrinogen isoforms and provided helpful suggestions; P.S. collected the FV Leiden carriers for the population study; R.M.B. and J.R. jointly initiated and supervised the project, and provided intellectual input; E.C. supervised the study and participated in experimental design and execution; F.O. and E.C. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabetta Castoldi, Department of Biochemistry, Maastricht University, P.O. Box 616, 6200 MD Maastricht, The Netherlands; e-mail: e.castoldi@maastrichtuniversity.nl.