Key Points
Tac/Sir prophylaxis provides equivalent GVHD-free survival when compared with Tac/Mtx in MRD transplantation.
Tac/Sir is associated with more rapid engraftment and reduced oropharyngeal mucositis after MRD transplantation.
Abstract
Grades 2-4 acute graft-versus-host disease (GVHD) occurs in approximately 35% of matched, related donor (MRD) allogeneic hematopoietic cell transplantation (HCT) recipients. We sought to determine if the combination of tacrolimus and sirolimus (Tac/Sir) was more effective than tacrolimus and methotrexate (Tac/Mtx) in preventing acute GVHD and early mortality after allogeneic MRD HCT in a phase 3, multicenter trial. The primary end point of the trial was to compare 114-day grades 2-4 acute GVHD-free survival using an intention-to-treat analysis of 304 randomized subjects. There was no difference in the probability of day 114 grades 2-4 acute GVHD-free survival (67% vs 62%, P = .38). Grades 2-4 GVHD was similar in the Tac/Sir and Tac/Mtx arms (26% vs 34%, P = .48). Neutrophil and platelet engraftment were more rapid in the Tac/Sir arm (14 vs 16 days, P < .001; 16 vs 19 days, P = .03). Oropharyngeal mucositis was less severe in the Tac/Sir arm (peak Oral Mucositis Assessment Scale score 0.70 vs 0.96, P < .001), but otherwise toxicity was similar. Chronic GVHD, relapse-free survival, and overall survival at 2 years were no different between study arms (53% vs 45%, P = .06; 53% vs 54%, P = .77; and 59% vs 63%, P = .36). Based on similar long-term outcomes, more rapid engraftment, and less oropharyngeal mucositis, the combination of Tac/Sir is an acceptable alternative to Tac/Mtx after MRD HCT. This study was funded by the National Heart, Lung, and Blood Institute and the National Cancer Institute; and the trial was registered at www.clinicaltrials.gov as #NCT00406393.
Introduction
Despite improvements in allogeneic hematopoietic cell transplantation (HCT) outcomes in recent years,1 acute graft-versus-host disease (GVHD) is an important cause of early treatment-related mortality (TRM). Acute GVHD occurs in approximately 35% of recipients of HLA-matched, related donor (MRD) transplants for myelodysplastic disorders and acute leukemia, and contributes significantly to 1-year TRM estimates of approximately 20%.2,3 The standard regimen for acute GVHD prophylaxis, established in the mid-1980s, consists of a calcineurin inhibitor given in combination with a short course of methotrexate.4,5 With the exception of strategies that manipulate the graft content, randomized trials attempting to substitute,6 or add additional pharmacologic agents7,8 to this combination, have not improved GVHD outcomes.
Sirolimus is an immunosuppressive mammalian target of the rapamycin inhibitor whose immunomodulatory properties extend beyond T-cell inhibition to include effects on antigen-presenting cells, the thymus, and preservation of regulatory T-cell subsets after transplantation.9 Initial studies suggested a decreased incidence of acute GVHD and treatment-related toxicity after HLA-MRD and unrelated donor transplantation10-13 at the expense of higher rates of endothelial injury syndromes.14-16 Sirolimus has also demonstrated promising activity in reduced-intensity conditioning17,18 and umbilical cord blood transplantation.19 Methotrexate is associated with delayed neutrophil and platelet engraftment, severe oropharyngeal mucositis,20 and diffuse alveolar hemorrhage21 after transplantation. As an agent that causes tissue injury, there is a theoretical concern that methotrexate may paradoxically be implicated in GVHD initiation by augmenting the cytokine cascade associated with GVHD.22 We, therefore, sought to determine if substitution of methotrexate with sirolimus, when given in combination with the calcineurin inhibitor, tacrolimus, would lead to improved GVHD and TRM outcomes.
Methods
Study design
This was an open-label, phase 3, multicenter, randomized trial conducted by the Blood and Marrow Transplant Clinical Trials Network designed to test 2 GVHD prophylaxis strategies, tacrolimus with sirolimus (Tac/Sir) and tacrolimus with methotrexate (Tac/Mtx), after MRD peripheral blood stem cell (PBSC) transplantation. Randomization occurred within 7 days of initiation of pretransplantation conditioning therapy, and was performed in a 1:1 ratio with the use of random block sizes, stratified by transplantation center. The target enrollment was 312 subjects. The primary end point was grades 2-4 acute GVHD-free survival, assessed 114 days from the time of randomization, using an intention-to-treat analysis. This time-point was chosen to correspond approximately with the 100-day time-point from transplantation, taking into account the time from randomization to initiation and completion of conditioning therapy. Prespecified secondary end points included the times to neutrophil and platelet engraftment and first hospital discharge, the incidence of grades 3-4 acute GVHD and chronic GVHD, the incidence and severity of oropharyngeal mucositis, the incidence of endothelial-related toxicities, infections, malignant relapse, and relapse-free and overall survival. Enrollment began in November 2006 and ended in October 2011, and all subjects were followed for 2 years. The analysis includes data collected as of November 2013. The trial was registered at www.clinicaltrials.gov (#NCT00406393), as previously noted. It was approved by the Protocol Review Committee and the Data Safety Review Committee of the National Heart, Lung, and Blood Institute, and Institutional Review Board approval was obtained at each study center. All subjects gave written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki. All authors vouch for the accuracy and completeness of the reported data, analyses, and the adherence of the study to the protocol.
Subjects
Eligible subjects were <60 years of age and were undergoing transplantation for acute leukemia in remission, myelodysplastic disorder, or chronic myeloid leukemia in chronic or accelerated phase. All subjects had an HLA-matched sibling donor, defined by HLA-A and -B serologic typing (or higher resolution) and HLA-DRβ1 molecular typing, who was willing to donate PBSCs, and who met institutional guidelines for this donation. Exclusion criteria were: prior allogeneic or autologous transplantation, HIV infection or another uncontrolled active infection, pregnancy or breast-feeding, known allergy to sirolimus, or the continued requirement for voriconazole administration at the time of registration. Laboratory exclusion criteria included a calculated creatinine clearance <50 mL/minute/1.72 m2, a direct bilirubin, alanine aminotransferase or aspartate aminotransferase greater than two times the upper limit of normal, a forced vital capacity or forced expiratory volume in 1 second less than 60% predicted when corrected for hemoglobin, a cardiac ejection fraction <45% in adults or <26% shortening fraction in children, a cholesterol level >500 mg/dL, or a triglycerides level >500 mg/dL.
Treatment
Subjects received pretransplantation myeloablative conditioning with total body irradiation (TBI) (at least 1200 cGy of fractionated TBI) in combination with either cyclophosphamide ([CY]-TBI) or etoposide (VP16-TBI). Initially, a conditioning regimen comprised of myeloablative doses of busulfan with CY was permitted, however, due to excess toxicity and veno-occlusive disease (VOD) of the liver, the Data and Safety Monitoring Board recommended removal of the busulfan-based conditioning regimen. With the approval of the Data and Safety Monitoring Board, we report here, the analysis of patients receiving TBI-based conditioning only. Patients who received busulfan-based conditioning prior to the exclusion of the busulfan as a conditioning regimen option (n = 10), have already been reported separately.15 PBSC donors received filgrastim and underwent large volume apheresis according to institutional standards, with a goal of collecting 2 to 10 × 106/kg CD34+ stem cells. No graft manipulation was allowed prior to the infusion of the stem-cell product.
GVHD prophylaxis consisted of Tac/Sir or Tac/Mtx. Tacrolimus was begun on day −3 at a dose of 0.02 mg/kg/day by continuous IV infusion, adjusted to maintain a serum concentration of 5 to 10 ng/mL. Sirolimus was started on day −3 with a 12 mg oral loading dose, followed by daily oral doses of 4 mg, adjusted to maintain a serum trough concentration of 3 to 12 ng/mL as measured by high-performance liquid chromatography. Methotrexate was IV administered on day +1 (15 mg/m2), +3, +6, and +11 (10 mg/m2 each day). No blinding was attempted. Posttransplantation supportive care was provided according to institutional standards. Routine use of colony stimulating factors was not recommended. GVHD prophylaxis was to be tapered at the discretion of the treating physician starting at day 100, or earlier, in the context of disease relapse.
Outcome assessment
The primary end point of this trial was day 114 grades 2-4 acute GVHD-free survival. Acute GVHD was graded according to the Consensus Criteria.23 Neutrophil engraftment was defined as the first of 3 consecutive measurements with an absolute neutrophil count of 500 cells/µL or greater. Platelet engraftment was defined as the first of 3 consecutive measurements with a platelet count of 20 000/µL or greater without transfusion of platelets in the preceding 72 hours. Chronic GVHD was graded using the Shulman criteria.24 Oropharyngeal mucositis was scored thrice weekly by treating clinicians, using the modified Oral Mucositis Assessment Scale (OMAS) system through day +21 or discharge from hospital.25 A committee of investigators who were blinded to GVHD prophylaxis assignment reviewed all case records, focusing on the incidence of acute and chronic GVHD, relapse, toxicity, and causes of death.
Statistical analysis
The primary analysis was a point-wise comparison of grades 2-4 acute GVHD-free survival at day 114 from randomization, based on intention-to-treat. The study had 80% power to detect a 15% difference in the proportion surviving without grades 2-4 acute GVHD at 114 days between the 2 prophylaxis strategies (from 60% to 75%), after accounting for interim analyses using an O’Brien-Fleming boundary, based on the standardized difference in Kaplan-Meier estimates with a 2-sided α of 5%. Analyses of secondary end points were on patients who underwent transplantation and event times were calculated from the date of transplantation. Results are presented according to the randomization arm. The rates of neutrophil and platelet engraftment, acute and chronic GVHD, endothelial injury syndromes, nonrelapse mortality, and relapse were compared between the two groups using a log-rank test treating relapse as a competing event for nonrelapse mortality, death or relapse as competing events for CGVHD, and death as a competing risk for all other end points. Cumulative incidence curves were estimated for each group. Overall survival and disease-free survival were described using Kaplan-Meier estimates and compared between patients according to their randomized treatment assignment using a log-rank test. Mean and peak mucositis severity were described using descriptive statistics, and compared using the Mann-Whitney U test. Time to first hospital discharge was described using the cumulative incidence curve, treating death prior to discharge as a competing event, and compared using the log-rank test. Adjustment for multiple testing was not performed, so secondary end point results are considered exploratory. A planned secondary analysis of outcomes was conducted using Cox regression to adjust for patient characteristics. Covariates considered in the model building process were year of transplant, recipient characteristics (age, performance status, diagnosis and disease stage, time from diagnosis to transplantation, and cytomegalovirus [CMV] status), conditioning regimen, and donor-recipient sex match. No preplanned subgroup analyses were conducted. All statistical analyses were performed using SAS (version 9.2).
Results
Patient characteristics
The characteristics of the patients are shown in Table 1. Subjects were treated at 23 centers in the United States and at 1 center in France. The median age of participants was 45 (range, 19 to 59) and 43 (range, 13 to 58) in the Tac/Sir and Tac/Mtx groups, respectively (P = .4), and only 8 subjects were <20 years of age. The study groups were well balanced for gender stage of malignancy at transplantation, CMV serostatus and performance status at transplantation, and conditioning regimen selection. There was a slight excess of patients in acute lymphoblastic leukemia in the Tac/Mtx arm, but otherwise, the distribution of malignancies was similar. Treatment compliance was excellent, with 99% of subjects undergoing transplantation and 99% receiving their assigned GVHD prophylaxis regimen.
Patient characteristics
| Variable . | Sirolimus/Tacrolimus . | Tacrolimus/Methotrexate . | P . |
|---|---|---|---|
| Number of patients | 151 | 153 | — |
| Underwent transplantation | 149 (99) | 152 (99) | .55 |
| Age, median (range) | 45 (19-59) | 43 (13-58) | .40 |
| Male gender | 77 (51) | 85 (56) | .43 |
| Primary malignancy | .05 | ||
| Acute myelogenous leukemia | 71 (47) | 63 (41) | |
| Acute lymphoblastic leukemia | 51 (34) | 68 (44) | |
| Chronic myelogenous leukemia | 9 (6) | 14 (9) | |
| Myelodysplastic syndrome | 19 (13) | 7 (5) | |
| Acute biphenotypic leukemia | 1 (<1) | 1 (<1) | |
| Disease status at transplantation | |||
| Acute myelogenous leukemia | .55 | ||
| 1st complete remission | 60 (85) | 56 (89) | |
| 2nd complete remission | 11 (15) | 7 (11) | |
| Acute lymphoblastic leukemia | .90 | ||
| 1st complete remission | 41 (80) | 55 (81) | |
| 2nd complete remission | 10 (20) | 13 (19) | |
| Chronic myelogenous leukemia | .74 | ||
| Chronic phase | 7 (78) | 10 (71) | |
| Accelerated phase | 2 (22) | 4 (29) | |
| Acute biphenotypic leukemia | |||
| 1st complete remission | 1 | 1 | |
| Karnofsky score | .09 | ||
| 90% to 100% | 101 (67) | 116 (76) | |
| <90% | 50 (33) | 37 (24) | |
| Recipient-donor CMV status | .16 | ||
| +/+ | 59 (39) | 47 (31) | |
| +/− | 14 (9) | 24 (16) | |
| −/+ | 30 (20) | 44 (29) | |
| −/− | 38 (25) | 30 (20) | |
| Missing | 10 (7) | 8 (5) | |
| Donor-recipient gender match | .63 | ||
| Female-male | 33 (27) | 30 (20) | |
| Conditioning regimen | |||
| CY/TBI | 124 (82) | 122 (80) | .60 |
| Etoposide/TBI | 27 (18) | 31 (20) |
| Variable . | Sirolimus/Tacrolimus . | Tacrolimus/Methotrexate . | P . |
|---|---|---|---|
| Number of patients | 151 | 153 | — |
| Underwent transplantation | 149 (99) | 152 (99) | .55 |
| Age, median (range) | 45 (19-59) | 43 (13-58) | .40 |
| Male gender | 77 (51) | 85 (56) | .43 |
| Primary malignancy | .05 | ||
| Acute myelogenous leukemia | 71 (47) | 63 (41) | |
| Acute lymphoblastic leukemia | 51 (34) | 68 (44) | |
| Chronic myelogenous leukemia | 9 (6) | 14 (9) | |
| Myelodysplastic syndrome | 19 (13) | 7 (5) | |
| Acute biphenotypic leukemia | 1 (<1) | 1 (<1) | |
| Disease status at transplantation | |||
| Acute myelogenous leukemia | .55 | ||
| 1st complete remission | 60 (85) | 56 (89) | |
| 2nd complete remission | 11 (15) | 7 (11) | |
| Acute lymphoblastic leukemia | .90 | ||
| 1st complete remission | 41 (80) | 55 (81) | |
| 2nd complete remission | 10 (20) | 13 (19) | |
| Chronic myelogenous leukemia | .74 | ||
| Chronic phase | 7 (78) | 10 (71) | |
| Accelerated phase | 2 (22) | 4 (29) | |
| Acute biphenotypic leukemia | |||
| 1st complete remission | 1 | 1 | |
| Karnofsky score | .09 | ||
| 90% to 100% | 101 (67) | 116 (76) | |
| <90% | 50 (33) | 37 (24) | |
| Recipient-donor CMV status | .16 | ||
| +/+ | 59 (39) | 47 (31) | |
| +/− | 14 (9) | 24 (16) | |
| −/+ | 30 (20) | 44 (29) | |
| −/− | 38 (25) | 30 (20) | |
| Missing | 10 (7) | 8 (5) | |
| Donor-recipient gender match | .63 | ||
| Female-male | 33 (27) | 30 (20) | |
| Conditioning regimen | |||
| CY/TBI | 124 (82) | 122 (80) | .60 |
| Etoposide/TBI | 27 (18) | 31 (20) |
Day 114 acute GVHD-free survival and other GVHD outcomes
The primary end point was day +114 grades 2-4 acute GVHD-free survival in an intention-to-treat analysis. The probability of day 114 grades 2-4 acute GVHD-free survival in the Tac/Sir arm was 67% (95% CI, 59-74) vs 62% (54-70) in the Tac/Mtx arm (P = .38; Figure 1A). Outcomes were unchanged when analyzed only in transplanted subjects, or when adjusted for patient characteristics in a Cox model. The cumulative incidence of acute GVHD (grades 2-4) occurring 100 days from transplantation was similar in both treatment arms (26% [19-34] vs 34% [26-41], P = .48; Figure 1B). A post hoc point-wise comparison of severe, grades 3-4 acute GVHD suggested a reduction in the Tac/Sir arm a 100 days from transplantation (8% [4-13] vs 15% [10-21], P = .05; Figure 1C); however, in multivariable modeling, the hazard ratio of 0.7 (0.38-1.29) was not significant (P = .25). Among subjects who developed grades 2-4 acute GVHD, there was less skin involvement in the Tac/Sir arm (22.2% vs 43.4%; P = .005), but no differences in the involvement of the upper or lower gastrointestinal tract or the liver. There was a trend for an increased cumulative incidence of chronic GVHD at 2 years from transplantation in the Tac/Sir arm (53% [47-67] vs 45% [39-58], P = .06; Figure 1D), but in a multivariate Cox model adjusting for patient characteristics including donor-recipient sex match (P = .02), the relative risk of chronic GVHD remained statistically not significant (hazard ratio 1.27 [0.91-1.76], P = .16).
GVHD outcomes. (A) Grades 2-4 acute GVHD-free survival from randomization. (B) Cumulative incidence of grades 2-4 acute GVHD. (C) Cumulative incidence of grades 3-4 acute GVHD. (D) Cumulative incidence of chronic GVHD with death and relapse as competing risks.
GVHD outcomes. (A) Grades 2-4 acute GVHD-free survival from randomization. (B) Cumulative incidence of grades 2-4 acute GVHD. (C) Cumulative incidence of grades 3-4 acute GVHD. (D) Cumulative incidence of chronic GVHD with death and relapse as competing risks.
Engraftment
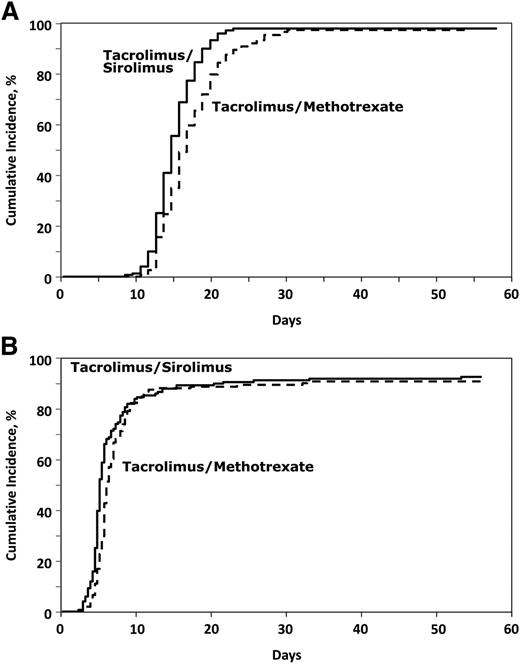
The median time to neutrophil engraftment was 2 days shorter in the Tac/Sir arm compared with the Tac/Mtx arm (14 [8-27] vs 16 [10-68] days, P < .001; Figure 2A), and the median time to platelet engraftment was 3 days shorter in the Tac/Sir arm (16 [7-782] vs 19 [9-730] days, P = .03; Figure 2B). Despite a more rapid time to engraftment, there was no significant difference in hospitalization time (20 vs 21 days, P = .37).
Engraftment outcomes. (A) Cumulative incidence of neutrophil engraftment. (B) Cumulative incidence of platelet engraftment.
Engraftment outcomes. (A) Cumulative incidence of neutrophil engraftment. (B) Cumulative incidence of platelet engraftment.
Oropharyngeal mucositis and treatment-related toxicity
Oropharyngeal mucositis was less severe in subjects who received Tac/Sir in comparison with Tac/Mtx. The peak OMAS score in Tac/Sir subjects was 0.70 (standard deviation [SD] = 0.51) compared with a peak OMAS score of 0.96 (SD = 0.63) in the Tac/Mtx subjects (P < .001). Similarly, the mean OMAS score was lower in the Tac/Sir subjects at 0.31 (SD = 0.28) vs 0.47 (SD = 0.40), P < .001; Figure 3). By 21 days from transplantation, the mean OMAS score had returned to baseline in the Tac/Sir subjects, but not in Tac/Mtx subjects.
Oral mucositis outcomes. Mean oral mucositis assessment scores after HSCT.
There was a trend toward increased rates of the endothelial injury syndromes, VOD, and thrombotic microangiopathy within 100 days of transplantation in the Tac/Sir arm compared with the Tac/Mtx arm (11% [6-16] vs 5% [2-9] at 100 days, P = .06 and 5% [3-10] vs 1% [0-4], P = .09). However, for both of these syndromes, there was no difference in the attributable mortality within 60 days of diagnosis (33% vs 56%, P = .2).
Other major toxicities of transplantation were balanced between study arms, with the exception of elevation of creatinine within the first 100 days from transplantation, which was more common in the Tac/Sir arm. Notably, grades 3-4 elevations in serum cholesterol or triglycerides were not more common in the Tac/Sir arm (2 vs 4% and 8 vs 6%, respectively). Reactivation of CMV infection was similar in both treatment arms (13% [9-19] vs 15% [10-21]). Treatment-related mortality in the 2 study arms was no different (7% [3-11) vs 7% [4-12] at 100 days, 20% [13-26] vs 16% [11-23] at 2 years, P = .44).
Disease relapse and survival
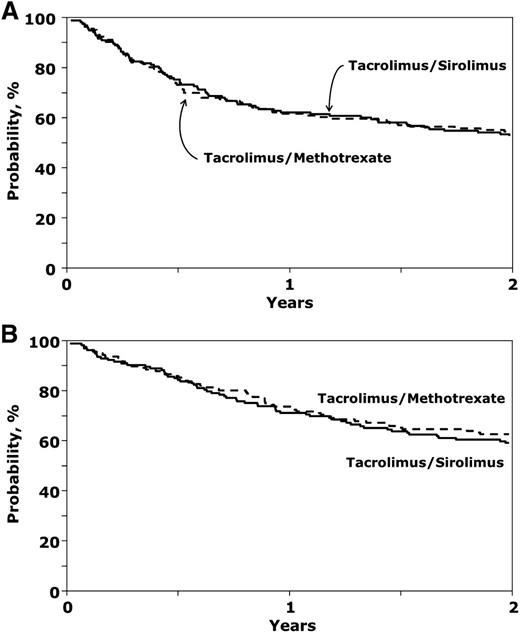
There was no difference in the incidence of malignant disease relapse in the 2 study arms (28% [20-35] vs 29% [22-37], P = .80). The 2-year disease-free and overall survival rates from the time of HCT were similar between study arms (53% [44-60] vs 54% [46-62], P = .77 and 59% [51-67] vs 63% [55-70], P = .36) (Figure 4A-B). In multivariable analysis, advanced disease status at transplantation predicted relapse (P = .026), disease-free survival (P = .017), and overall survival (P = .034). An analysis of causes of death revealed no difference in the causes of death (P = .58), with a trend toward more deaths related to acute and chronic GVHD in the Tac/Mtx arm (18% and 14%, respectively), compared with the Tac/Sir arm (8% and 11%, respectively), but fewer deaths related to organ failure (19% vs 9%).
Discussion
In this phase 3, multicenter, randomized trial comparing Tac/Sir with Tac/Mtx for the prevention of GVHD after MRD allogeneic HCT, we did not demonstrate a statistically significant increase in grades 2-4 acute GVHD-free survival, 114 days from the time of randomization in the experimental Tac/Sir arm. Despite this, subjects who received Tac/Sir engrafted significantly earlier and had significantly less oropharyngeal mucositis after transplantation. These improvements did not result in long-term advantages in the Tac/Sir arm.
The results of our preliminary studies suggested as much as a 50% relative risk reduction in GVHD when sirolimus was used.10,11 In 2 recent randomized trials, the addition of sirolimus to Tac/Mtx in adults and children was associated with grades 2-4 acute GVHD risk reduction from 25% to 9% and 31% to 18%, respectively.26,27 However, both of these trials included subjects with unrelated donors, and it is possible that the inclusion of these higher risk subjects is required to demonstrate a benefit. Although we were unable to demonstrate a reduction of the magnitude demonstrated in other randomized trials, we demonstrated a trend toward a reduction in the incidence of severe acute GVHD. The rate of GVHD noted in the Tac/Mtx group in this trial was lower than in historical controls, where the rate of grades 2-4 acute GVHD after MRD allogeneic HCT was generally higher than 35%.28 Similarly, early mortality in the Tac/Mtx arm was lower than historical data, and taken together, the lower rates of acute GVHD and early mortality were likely responsible for the negative primary composite outcome measure of GVHD-free survival. One reason to explain these differences is the inclusion of a relatively favorable patient group, with over two-thirds of enrolled patients having acute leukemia in first complete remission.
Despite the lack of improvement in GVHD-free survival early after HCT, there are reasons to consider the Tac/Sir regimen as an alternative for GVHD prophylaxis in MRD allogeneic HCT. First, both neutrophil and platelet engraftment were more rapid with the omission of methotrexate in the Tac/Sir arm. Relating to both the speed of engraftment and the mucotoxic effects of the antiproliferative agent methotrexate, the incidence and severity of oropharyngeal mucositis was markedly reduced in the Tac/Sir arm. Oropharyngeal mucositis has been reported as the worst complication of transplantation according to patients,29 and a reduction in oropharyngeal mucositis can be associated with less IV narcotic use, less total parenteral nutrition use, and shorter hospital stays.30 However, the costs of sirolimus and its monitoring, as well as the trend toward more endothelial injury syndromes, may counterbalance these advantages. Other GVHD regimens that either lower methotrexate dose31 or replace methotrexate with other agents,32,33 have either not yet been tested in prospective trials or are associated with increased risks of GVHD.
The mechanisms involved in endothelial injury syndromes after HCT have not been fully elucidated, and there are no standard management approaches. High serum sirolimus levels have been associated with post-HCT thrombotic microangiopathy,16 and von Willebrand factor, thrombomodulin, and soluble intercellular adhesion molecule-1 have been identified as biomarkers for VOD.34 With careful monitoring of sirolimus levels and these biomarkers, the risk of these endothelial injury syndromes may be mitigated. Although we cannot recommend the use of the busulfan-CY conditioning regimen with sirolimus-based prophylaxis due to excess VOD, others have used sirolimus following busulfan in combination with other conditioning agents, without noting an excess of VOD.13
In summary, we observed no difference in the primary end point of GVHD-free survival between the 2 treatment arms, but there were advantages to Tac/Sir as measured in the secondary end points of the trial. As an alternative to Tac/Mtx, the Tac/Sir regimen can be considered in patients undergoing TBI-based transplantation who are at higher risk for oropharyngeal mucositis (ie, intensive prior chemotherapy or prior head-and-neck radiotherapy), and in patients in whom timely engraftment is required (due to ongoing infection or risk of infection) after appropriate screening for risks of excessive hepatotoxicity are excluded (alcohol abuse, chronic hepatitis, or concomitant hepatotoxic medication use). Given the trend toward a reduction in reducing severe GVHD noted, it is possible that the benefit of the Tac/Sir regimen might be more prominent in higher-risk transplantations, such as mismatched or unrelated donor HCT. Further studies to test novel GVHD prophylaxis regimens with a goal of improving HCT outcomes are required.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
C.C. was supported by the Stem Cell Cyclists of the Pan-Mass Challenge. J.H.A. was supported by the Jock and Bunny Adams Research and Education Endowment. This study was supported in part by the National Heart, Lung, and Blood Institute and the National Cancer Institute (#U01HL069294).
Authorship
Contribution: C.C., B.L., M.M.H., and J.H.A. were responsible for study concept and design; C.C., B.L., R.N., L.J., S.C., D.P., M.P., M.L.M., S.G., P.M., S.L.C., M.M.H., and J.H.A. comprised the Study Protocol Writing Committee; C.C., R.N., L.J., S.C., D.P., W.J.H., J.H., E.K.W., P.M., and J.H.A. were responsible for patient accrual and care; B.L., J.W., Z.-H.H., and S.L.C. performed the data management; C.C., R.N., L.J., S.C., D.P., W.J.H., M.P., and J.H.A. performed the blinded data review; C.C. drafted the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Corey Cutler, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: corey_cutler@dfci.harvard.edu.