Key Points
Preadipocytes produce factor XIII-A, which acts as a negative regulator of adipogenesis by increasing plasma fibronectin matrix assembly.
Factor XIII-A and plasma fibronectin matrix promote preadipocyte proliferation and proproliferative effects of insulin.
Abstract
Factor XIII-A (FXIII-A) transglutaminase (TG) was recently identified as a potential causative obesity gene in human white adipose tissue (WAT). Here, we have examined the role of TG activity and the role of protein crosslinking in adipogenesis. Mouse WAT and preadipocytes showed abundant TG activity arising from FXIII-A. FXIII-A was localized to the cell surface and acted as a negative regulator of adipogenesis by promoting assembly of fibronectin (FN) from plasma into preadipocyte extracellular matrix. This modulated cytoskeletal dynamics and maintained the preadipocyte state. FXIII-A–assembled plasma FN (pFN) matrix promoted preadipocyte proliferation and potentiated the proproliferative effects of insulin (INS) while suppressing the prodifferentiating INS signaling. FXIII-A–deficient mouse embryonic fibroblasts showed increased lipid accumulation and decreased proliferation as well as decreased pFN assembly into extracellular matrix. Thus, FXIII-A serves as a preadipocyte-bound proliferation/differentiation switch that mediates effects of hepatocyte-produced circulating pFN.
Introduction
Obesity, which is characterized by abnormally high fat accumulation in adipose tissue and other organs, has a heritability range of 65% to 80%1,2 and is a risk factor for thrombosis and many severe chronic illnesses, including type 2 diabetes, coronary heart disease, arthritis, and cancer.3,4 Obesity is also associated with the hypercoagulable state caused by increased production of liver-derived clotting factors occurring as a reaction to increased circulating lipids and inflammatory cytokines caused by dysfunctioning adipose tissue.5-9 A recent genome-wide screen study examined gene expression changes linked to body mass index (BMI) from white adipose tissue (WAT) of monozygotic twin pairs discordant in BMI to seek potentially causative genes (vs reactive genes) for obesity. The study identified F13A1 (which encodes for factor XIII-A [FXIII-A] coagulation factor) as the top, potentially causative gene expressed in WAT with the high association to obesity.10 The significant association of F13A1 with obesity was further confirmed in a large European ENGAGE consortium study of more than 21 000 unrelated individuals as well as in the GenMets cohort study, which identified 7 single-nucleotide polymorphisms (SNPs) in F13A1 gene associated with BMI.10 These studies strongly suggest that the link between obesity and FXIII-A may differ from the link between obesity and other clotting factors in that FXIII-A may be produced by WAT and functioning in adipogenesis.
FXIII-A is a transglutaminase (TG) enzyme that stabilizes the fibrin network as the last step of the blood coagulation cascade.11,12 Circulating FXIII-A exists as a dimer that is bound to an inhibitory dimeric FXIII-B subunit. Together they form the heterotetrameric FXIII clotting factor.12 In contrast to most of the other clotting factors, the source of circulating FXIII-A is considered to be predominantly cells of bone marrow origin such as megakaryocytes.13,14 In addition to being found in plasma, FXIII-A is also found in tissues and is synthesized by various cells, including macrophages, chondrocytes, osteoblasts, and osteocytes, where it is found in the cytosol and nucleus, on the plasma membrane or cell surface, and in the extracellular matrix.12 Cellular FXIII-A activity has been shown to regulate fibroblast adhesion;15 megakaryocyte morphology; platelet maturation;16 proliferation and migration of monocytes, macrophages, and fibroblasts;17,18 and extracellular matrix synthesis and stabilization required for differentiation of cells of mesenchymal origin.19-22 FXIII-A, as a member of the TG family, catalyzes a Ca2+-dependent acyl-transfer reaction between polypeptide-bound glutamine residues and lysine residues, resulting in a covalent γ-(glutamyl)-ε-lysyl bond (isopeptide crosslink/bond) that can induce the formation of multimeric protein networks and change the conformation, structure, solubility, biochemical stability, and cell-adhesion properties of substrate proteins.23-29 In addition to fibrin, a major extracellular substrate for FXIII-A is fibronectin (FN).30 FN is an extracellular glycoprotein capable of regulating various cellular functions, including proliferation and differentiation.31-33 FN is found in human and mouse WAT and preadipocyte cultures,34-36 where its role is associated with inhibition of adipogenesis.35,36 In the physiological setting, FN exists as 2 pools: as cellular FN (cFN) synthesized by tissue-resident cells and as plasma FN (pFN) produced by the liver.31 pFN has recently been shown to accumulate from the circulation into several tissues (liver, brain, testis, heart, lungs, and bone),37,38 and to contribute to the majority of the FN extracellular matrix associated with several cell types.16,31-33 FXIII-A has been shown to increase FN matrix accumulation in fibroblasts.39
Given the association between the FXIII-A and obesity and its potential presence in WAT, our aim here was to explore the role of TG activity and FXIII-A in adipogenesis. Here, we provide the first report demonstrating that differentiating preadipocytes have abundant TG activity that derives from FXIII-A. Our studies using 3T3-L1 preadipocytes as well as normal and F13a1-deficient mouse embryonic fibroblasts (MEFs) show that FXIII-A is located on the cell surface, where it exerts its effects via promoting soluble pFN assembly into extracellular matrix of preadipocytes. This maintains focal adhesions, promotes proliferation, and potentiates proproliferative effects of insulin (INS) while acting as an antagonist for adipocyte differentiation and lipid accumulation. Our work suggests a novel function for FXIII-A and circulating pFN in energy metabolism.
Materials and methods
Animals
F13a1−/− mice were a generous gift from Dr Gerhard Dickneite (CSL Behring GmbH).40 Wild type mice (CBA/CaJ) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were kept under a normal diurnal cycle in a temperature-controlled room and fed with standard chow. Animal procedures (WAT extraction and MEFs isolation) and study protocols were approved by the McGill University Animal Care Committee.
Preadipocyte cell culture, differentiation, and Oil Red O staining
3T3-L1 cells (ATCC, Manassas, VA) were maintained in Dulbecco’s modified Eagle medium containing 10% calf serum, 100 U/mL penicillin G, and 100 µg/mL streptomycin. Differentiation into adipocytes was induced 2 days postconfluency with 10% fetal bovine serum (FBS), 1 µM dexamethasone (DEX), 0.5 mM isobutyl-1-methylzanthine (IBMX), and 1 µg/mL INS and is referred to as differentiation media (DM). After 2 days, the DM was replaced with maintenance medium, which includes 10% FBS and 1 µg/mL INS. After 2 days, the maintenance medium was replaced with medium containing 10% FBS, with the endpoint of the experiment being day 8. On day 8, intracellular triglycerides were stained by Oil Red O and quantified; cells were counterstained with hematoxylin and photographed with a light microscope as previously described.41 Treatments included NC9 and NC10 (20-40 µM), PI3-kinase inhibitor LY294002 (10 µM), biotin-F11 (DQMMLPWPAVAL) and biotin-F11QN (DNMMLPWPAVAL) peptides (50 µM), picropodophyllin (10 µM), and hydroxy-2-naphthalenylmethylphosphonic acid trisacetoxymethyl ester (10 µM).
Cell proliferation assay
Proliferation experiments were done in 96-well plates or in 60-mm plates. 3T3-L1 cells or MEFs were serum-starved for 20 hours, after which 3 × 105 cells/mL cells were plated and stimulated with the indicated media for 24 hours followed by analysis using the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide; 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay (thiazolyl blue tetrazolium bromide) as previously described.19
Complete “Materials and methods” are available in supplemental Material on the Blood Web site.
Results
TG activity is present in mouse WAT, differentiating 3T3-L1 preadipocytes, and arises from FXIII-A
To investigate the role of TG activity in adipogenesis, we first examined the presence of γ-glutamyl-ε-lysyl crosslinks (isopeptide bonds), indicative of TG activity, in mouse WAT. Immunohistochemistry demonstrated an abundance of isopeptide bonds at the periphery of adipocytes and adipose tissue stroma (Figure 1A). Assessment of TG activity using the 5-(biotinamido)pentylamine (BPA) incorporation assay in extracts of different mouse WAT deposits showed that TG activity was present in epididymal, mesenteric, perirenal/retroperitoneal, inguinal, and subcutaneous WAT deposits in vivo (Figure 1B). Messenger RNA (mRNA) analysis of TG family members TG1-TG7 and FXIII-A in mouse WAT and in the 3T3-L1 cell line showed only Tgm2 and F13a1 expression (Figure 1C). Both enzymes were also detected by whole-mount immunofluorescence microscopy of mouse WAT (Figure 1D). 3T3-L1 cell line is commonly used to study adipogenesis in vitro; this cell line is derived from MEF that can differentiate into lipid-storing adipocytes upon stimulation with DM (containing INS, IBMX, and DEX). Analyses of TG expression during adipocyte differentiation of 3T3-L1 cells showed that Tgm2 mRNA levels did not change during differentiation, whereas F13a1 mRNA responded to DM by an initial decrease at day 1, followed by an increase at day 2, and finally gradually decreasing as the cells began to accumulate lipids (Figure 1E).
Presence of TG activity, FXIII-A, and TG2 in mouse WAT and differentiating 3T3-L1 preadipocytes. (A) Immunohistochemical visualization of γ-glutamyl-ε-lysyl bonds (isopeptide bonds) in WAT showing abundant staining in the extracellular compartment. Epididymal fat pad tissue was obtained from 8-week-old mice. Specimens were counterstained with hematoxylin (H) (n = 2). Scale bar represents 100 µm. (B) In vitro TG activity of protein extracts of different WAT fat deposits of 6- to 8-week-old male mice. Protein extracts of epididymal (Ep), mesenteric (Me), perirenal/retroperitoneal (Re), inguinal (In), and subcutaneous (Sc) fat pads were assessed by microplate BPA incorporation assay (n = 2). (C) Reverse transcription-polymerase chain reaction analysis of TG enzyme family members in mouse WAT and 3T3-L1 preadipocytes. Only Tgm2 and F13a1 are expressed (n = 3). (D) Whole-mount immunofluorescence staining of mouse WAT showing the presence of TG2 (red) and FXIII-A (green) in the tissue. Epididymal fat pads of 2 mice were used. Scale bar represents 10 µm. (E) Reverse transcription-polymerase chain reaction analyses of Tgm2 and F13a1 during 3T3-L1 preadipocyte differentiation to adipocytes, showing different expression patterns for the 2 TGs during adipogenesis. Tgm2 levels remain constant while F13a1 levels are high at the early phase of adipogenesis, followed by a gradual decrease as the cells mature to adipocytes. Pparg2 and Cbpa are adipocyte differentiation markers. Gapdh was used as internal control. n = 3.
Presence of TG activity, FXIII-A, and TG2 in mouse WAT and differentiating 3T3-L1 preadipocytes. (A) Immunohistochemical visualization of γ-glutamyl-ε-lysyl bonds (isopeptide bonds) in WAT showing abundant staining in the extracellular compartment. Epididymal fat pad tissue was obtained from 8-week-old mice. Specimens were counterstained with hematoxylin (H) (n = 2). Scale bar represents 100 µm. (B) In vitro TG activity of protein extracts of different WAT fat deposits of 6- to 8-week-old male mice. Protein extracts of epididymal (Ep), mesenteric (Me), perirenal/retroperitoneal (Re), inguinal (In), and subcutaneous (Sc) fat pads were assessed by microplate BPA incorporation assay (n = 2). (C) Reverse transcription-polymerase chain reaction analysis of TG enzyme family members in mouse WAT and 3T3-L1 preadipocytes. Only Tgm2 and F13a1 are expressed (n = 3). (D) Whole-mount immunofluorescence staining of mouse WAT showing the presence of TG2 (red) and FXIII-A (green) in the tissue. Epididymal fat pads of 2 mice were used. Scale bar represents 10 µm. (E) Reverse transcription-polymerase chain reaction analyses of Tgm2 and F13a1 during 3T3-L1 preadipocyte differentiation to adipocytes, showing different expression patterns for the 2 TGs during adipogenesis. Tgm2 levels remain constant while F13a1 levels are high at the early phase of adipogenesis, followed by a gradual decrease as the cells mature to adipocytes. Pparg2 and Cbpa are adipocyte differentiation markers. Gapdh was used as internal control. n = 3.
To determine whether TG activity was present during adipocyte differentiation, we assessed TG activity in situ by using BPA incorporation assay in differentiating 3T3-L1 cells treated with DM. A dramatic and significant induction of TG activity on days 1 and 2 was observed (8-fold and 10-fold increases, respectively, compared with day 0), and this was followed by a gradual decrease in activity as the cells matured into adipocytes (Figure 2A). To examine which of the 2 TGs were active during cell differentiation, NC9, a TG inhibitor containing a dansyl probe, was used to detect activity. NC9 incorporates irreversibly into active TG enzymes, including TG242 and FXIII-A.43 Here, we further demonstrate that NC9 incorporates into thrombin activated FXIII-A in vitro (supplemental Figure 1A-B), but not into nonactivated FXIII-A as shown by dansyl detection after western blotting (WB) (supplemental Figure 1B). Immunofluorescence staining of cells treated with NC9 for dansyl shows colocalization with FXIII-A at the cell periphery (Figure 2B). No colocalization with TG2 was observed in the cells (supplemental Figure 2). This suggests that preadipocyte TG activity arises from FXIII-A and that TG2 may not be active as a TG or not in its open active conformation in these cells. WB detection of dansyl in cell-surface preparations of preadipocytes showed strong dansyl incorporation, mostly into a protein band >150 kDa and in lesser extend into a protein band between 50 and 75 kDa (Figure 2C). Immunoprecipitation of NC9 dansyl with dansyl antibody and detection with FXIII-A antibody demonstrated that the high-molecular weight (HMW) protein is FXIII-A (Figure 2D). HMW FXIII-A was also detected in 3T3-L1 extracts and MEFs, where it was induced upon differentiation treatment that also induced TG activity (Figure 2E). Because neither of the observed MWs correspond to the MW of circulating FXIII-A monomer (83 kDa), we used 2 anti-human FXIII-A antibodies (A-4 and ab976362) to detect mouse preadipocyte FXIII-A together with human FXIII-A as a positive control. Supplemental Figure 1A shows Coomassie Blue staining of the nonactivated and thrombin-activated human FXIII, which runs at ∼75 kDa. A HMW band was seen in the gels >150 kDa upon thrombin activation and in the WBs using the 2 anti-human FXIII-A antibodies (Figure 2F-G). This HMW FXIII-A band is likely a dimer. Comparing human FXIII-A to mouse platelet-rich plasma and 3T3-L1 extracts showed that the mouse preparations had a FXIII-A protein of smaller MW than human FXIII-A (Figure 2F). Another human FXIII-A antibody (Figure 2G) detected 2 FXIII-A bands in mouse platelet-rich plasma at 75 kDa and between 50 and 75 kDa, strongly suggesting that mouse platelets also have a smaller MW FXIII-A. Incubation of mouse platelet extracts and mouse plasma with NC9 in vitro showed its clear incorporation into a band between 50 and 75 kDa that corresponds to the smaller monomer FXIII-A found in preadipocytes (Figure 2H). The fact that ultimately 2 FXIII-A forms are detected in platelets suggests that the smaller form may be proteolytically cleaved from the full-length FXIII-A. Collectively, these results suggest that preadipocyte FXIII-A may be a cleaved form that complexes/dimerizes and gets activated at the cell surface of preadipocytes.
TG activity in preadipocytes arises from FXIII-A. (A) In situ assessment of TG activity during differentiation of 3T3-L1 preadipocytes shows a significant increase upon induction of differentiation until day 2, which is followed by a gradual decrease as cells begin to accumulate lipids. TG activity was assessed by BPA incorporation assay (n = 3). Statistical analysis was done using analysis of variance *P < .05; ***P < .001. Error bars represent SEM. (B) Immunofluorescence tracking of TG activity using NC9, which incorporates irreversibly into the active TG enzyme. Immunofluorescence microscopy shows colocalization (merge, yellow) of NC9-dansyl (green) and FXIII-A (red), identifying FXIII-A as the active TG enzyme in preadipocytes. Nuclei are visualized with 4,6 diamidino-2-phenylindole (DAPI) (blue). Color correlation distribution, constructed using the Color Inspector 3D plug-in of Image J, shows the extent correlation of colocalization (n = 2). Scale bar represents 10 µm. (C) TG activity is located on the cell surface. Cell-surface biotinylated samples were affinity purified using Neutravidin beads and subsequently detected with dansyl antibody, which showed a major band >150 kDa and a weaker band between 75 and 50 kDa (n = 3). (D) Immunoprecipitation of NC9-labeled material with anti-dansyl antibody and detection with anti-mouse FXIII-A antibody positively identified the active TG enzyme to be FXIII-A (running >150 kDa) (n = 5). (E) WB detection of FXIII-A in 3T3-L1 preadipocytes and MEFs before (D0) and after induction of differentiation (DM) (day 1) using anti-mouse FXIII-A antibody. HMW FXIII-A was observed at day 1 of differentiation (n = 3). (F) WB detection of nonactivated human FXIII (hFXIII), activated human FXIII (Act.hFXIII), mouse platelet-rich plasma (mPRP), and 3T3-L1 cell extract using anti-human FXIII-A antibody (A-4). WB shows human and mouse FXIII-A at different molecular weights, with mouse FXIII-A being smaller (n = 3). (G) WB detection of nonactivated human FXIII (hFXIII), activated human FXIII (Act.hFXIII), and mPRP using anti-human FXIII-A antibody (ab97636). WB shows detection of 2 FXIII-A bands in mPRP, one at 75 kDa and a smaller band between 50 and 75 kDa (n = 3). (H) A smaller FXIII-A band is active as a TG enzyme. Mouse platelet lysate (mPlts) and mouse plasma (mPlasma) were activated with thrombin and Ca2+ for 1 hour at 37°C and further incubated with NC9. Dansyl incorporation into the active enzyme was visualized by WB detection of dansyl. Dansyl was found to be integrated into a band between 50 and 75 kDa, which represents the smaller form of FXIII-A (n = 3).
TG activity in preadipocytes arises from FXIII-A. (A) In situ assessment of TG activity during differentiation of 3T3-L1 preadipocytes shows a significant increase upon induction of differentiation until day 2, which is followed by a gradual decrease as cells begin to accumulate lipids. TG activity was assessed by BPA incorporation assay (n = 3). Statistical analysis was done using analysis of variance *P < .05; ***P < .001. Error bars represent SEM. (B) Immunofluorescence tracking of TG activity using NC9, which incorporates irreversibly into the active TG enzyme. Immunofluorescence microscopy shows colocalization (merge, yellow) of NC9-dansyl (green) and FXIII-A (red), identifying FXIII-A as the active TG enzyme in preadipocytes. Nuclei are visualized with 4,6 diamidino-2-phenylindole (DAPI) (blue). Color correlation distribution, constructed using the Color Inspector 3D plug-in of Image J, shows the extent correlation of colocalization (n = 2). Scale bar represents 10 µm. (C) TG activity is located on the cell surface. Cell-surface biotinylated samples were affinity purified using Neutravidin beads and subsequently detected with dansyl antibody, which showed a major band >150 kDa and a weaker band between 75 and 50 kDa (n = 3). (D) Immunoprecipitation of NC9-labeled material with anti-dansyl antibody and detection with anti-mouse FXIII-A antibody positively identified the active TG enzyme to be FXIII-A (running >150 kDa) (n = 5). (E) WB detection of FXIII-A in 3T3-L1 preadipocytes and MEFs before (D0) and after induction of differentiation (DM) (day 1) using anti-mouse FXIII-A antibody. HMW FXIII-A was observed at day 1 of differentiation (n = 3). (F) WB detection of nonactivated human FXIII (hFXIII), activated human FXIII (Act.hFXIII), mouse platelet-rich plasma (mPRP), and 3T3-L1 cell extract using anti-human FXIII-A antibody (A-4). WB shows human and mouse FXIII-A at different molecular weights, with mouse FXIII-A being smaller (n = 3). (G) WB detection of nonactivated human FXIII (hFXIII), activated human FXIII (Act.hFXIII), and mPRP using anti-human FXIII-A antibody (ab97636). WB shows detection of 2 FXIII-A bands in mPRP, one at 75 kDa and a smaller band between 50 and 75 kDa (n = 3). (H) A smaller FXIII-A band is active as a TG enzyme. Mouse platelet lysate (mPlts) and mouse plasma (mPlasma) were activated with thrombin and Ca2+ for 1 hour at 37°C and further incubated with NC9. Dansyl incorporation into the active enzyme was visualized by WB detection of dansyl. Dansyl was found to be integrated into a band between 50 and 75 kDa, which represents the smaller form of FXIII-A (n = 3).
FXIII-A acts as an antagonist for adipogenesis
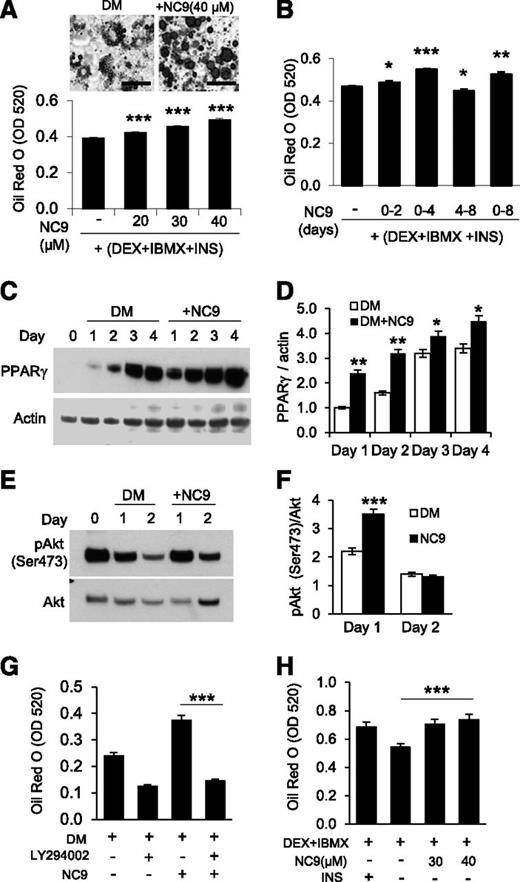
Given the high level of FXIII-A activity during adipocyte differentiation, we asked if the activity is required for cell differentiation. As shown in Figure 3A, NC9 significantly and in a concentration-dependent manner increased lipid accumulation and lipid droplet size in adipocytes. Control compound NC10, lacking the warhead acryloyl group, did not have an effect on adipogenesis (supplemental Figure 3). NC9 was most efficient in promoting lipid accumulation when given to 3T3-L1 cells between days 0 and 4, which enhanced lipid accumulation to the same extent as a full 8-day treatment. A significant increase in lipid accumulation was also seen when the inhibitor was given between days 0 and 2, and a decrease in lipid accumulation was observed in treatments occurring during days 4 to 8 (Figure 3B). Similar results were observed with MEFs, whose differentiation into adipocytes was promoted by NC9 in both a dose- and time-dependent manner (supplemental Figure 4). WB analysis and quantification of the adipogenic transcription factor peroxisome proliferator-activated receptor (PPARγ) showed a significant increase in NC9-treated cells (1.5-fold) compared with the control (Figure 3C-D). Analysis of phosphatidylinositol 3-kinase (PI3K)/Akt signaling, the main regulator of PPARγ expression,44,45 showed significant changes: (1) Akt phosphorylation was increased (1.5-fold) on day 1 following NC9 treatment (Figure 3E-F), and (2) LY294002, a PI3K inhibitor, reversed the increase in differentiation caused by NC9 (Figure 3G). Because inhibition of FXIII-A activity stimulated PI3K/Akt signaling, we differentiated cells with partial hormonal stimulation (DEX and IBMX only, no INS) in the presence and absence of NC9. Remarkably, inhibition of FXIII-A activity induced differentiation at a level similar to INS stimulation (Figure 3H). Collectively, these data strongly suggest that preadipocyte FXIII-A acts as an antagonist during the early phase of adipogenesis. No FXIII-A was detected in FBS used in the preadipocyte cultures (supplemental Figure 5), and thus its contribution to the observed effects can be excluded.
Inhibition of FXIII-A TG activity increases adipocyte differentiation and lipid accumulation. (A) Inhibition of TG activity with the irreversible TG inhibitor NC9 increases lipid accumulation in a concentration-dependent manner as assessed by quantification of Oil Red O staining of 3T3-L1 cultures on day 8 of differentiation. Images show the increased size of lipid droplets in Oil Red O-stained cells. (B) Inhibition of TG activity during different stages of 3T3-L1 culture shows that TG activity has its most prominent inhibitory effect on lipid accumulation when given during days 0 to 4. (C-D) WB analysis and quantification of PPARγ expression (normalized to actin) during adipocyte differentiation shows increased expression (and thus accelerated differentiation) of NC9-treated cells. (E-F) WB analysis and quantification of Akt phosphorylation at Ser473 show that inhibition of TG activity significantly increases Akt activation. (G) The PI3K pathway inhibitor LY294002 used from day 0 to 4 reversed the NC9-mediated increase in adipogenesis; the graph shows quantification of Oil Red staining of the cultures on day 8. (H) Inhibition of TG activity with NC9 between days 0 and 4 can function in a similar manner as insulin (INS) in DM to promote preadipocyte differentiation; the graph shows quantification of Oil Red O-stained cultures on day 8. All error bars represent SEM (n = 3). *P < .05; **P < .01; ***P < .001.
Inhibition of FXIII-A TG activity increases adipocyte differentiation and lipid accumulation. (A) Inhibition of TG activity with the irreversible TG inhibitor NC9 increases lipid accumulation in a concentration-dependent manner as assessed by quantification of Oil Red O staining of 3T3-L1 cultures on day 8 of differentiation. Images show the increased size of lipid droplets in Oil Red O-stained cells. (B) Inhibition of TG activity during different stages of 3T3-L1 culture shows that TG activity has its most prominent inhibitory effect on lipid accumulation when given during days 0 to 4. (C-D) WB analysis and quantification of PPARγ expression (normalized to actin) during adipocyte differentiation shows increased expression (and thus accelerated differentiation) of NC9-treated cells. (E-F) WB analysis and quantification of Akt phosphorylation at Ser473 show that inhibition of TG activity significantly increases Akt activation. (G) The PI3K pathway inhibitor LY294002 used from day 0 to 4 reversed the NC9-mediated increase in adipogenesis; the graph shows quantification of Oil Red staining of the cultures on day 8. (H) Inhibition of TG activity with NC9 between days 0 and 4 can function in a similar manner as insulin (INS) in DM to promote preadipocyte differentiation; the graph shows quantification of Oil Red O-stained cultures on day 8. All error bars represent SEM (n = 3). *P < .05; **P < .01; ***P < .001.
FXIII-A activity promotes actin dynamics and focal adhesion formation in preadipocytes via crosslinking FN
In the search of mechanisms for how preadipocyte FXIII-A inhibits adipogenesis and the P13K/Akt pathway, we considered that TG activity has been linked with cell-matrix interactions46 and that during adipocyte differentiation, preadipocytes undergo a major morphological change where the transition from fibroblast-like (preadipocytes) cells to rounded (adipocyte) cells is associated with extensive cytoskeletal and matrix remodeling.47-50 An examination of cytoskeletal dynamics in NC9-treated preadipocytes plated on FN under serum-free conditions showed that the inhibitor dramatically reduced actin stress fiber formation and increased cortical actin assembly compared with control cells. This was also associated with reduced focal adhesion assembly as seen by a lack of vinculin colocalization with actin stress fibers (Figure 4A). WB analysis of subcellular fractions of these cells showed redistribution of vinculin from the cytoskeleton to the cytosol upon NC9 treatment (supplemental Figure 6A). Furthermore, NC9 also reduced preadipocyte adhesion (supplemental Figure 6B) and ROCK kinase activity (supplemental Figure 6C), necessary for the maintenance of actin stress fibers and focal adhesions.51-53 The dansyl group of NC9 was detected on the cell periphery of preadipocytes by immunofluorescence microscopy, which was associated with a reduction of actin stress fibers and rounding of the cells (Figure 4B). These results show that FXIII-A on the cell surface of preadipocytes promotes cell adhesion, actin stress fiber formation, and focal adhesion assembly. To examine FXIII-A substrates in these cultures, we conducted in situ labeling of preadipocytes with monodansylcadaverine (MDC), which incorporates covalently into TG-reactive Q residues of TG substrate proteins. MDC was found in protein(s) having an MW of 250 kDa (Figure 4C), which corresponds to FN (monomer). FN is one of the major extracellular FXIII-A substrates also linked to cytoskeletal dynamics.54-56 To examine whether the FXIII-A activity crosslinks (and labels) extracellular FN in preadipocyte cultures, MDC-labeled cells were immunoprecipitated using dansyl antibody and detected with FN antibody; this showed that the coated FN is a TG substrate in these cultures (Figure 4C). The effect of NC9 on FN labeling in the cultures was examined by an in situ TG activity assay, where BPA incorporation by the cells onto coated FN was quantified.57 NC9 decreased the amount of BPA incorporated into FN outside the cells by 50% compared with the DM-treated preadipocyte control (Figure 4D). In summary, these results confirmed that FXIII-A activity is predominantly found at the cell surface of preadipocytes and that FN is a major crosslinking substrate in the extracellular matrix.
FXIII-A activity regulates cytoskeletal dynamics; FN is a major extracellular substrate of FXIII-A. (A) Immunofluorescence microscopy of cell morphology and cytoskeletal elements of preadipocytes upon inhibition of FXIII-A activity with NC9. Inhibition attenuates actin stress fiber formation, promotes cortical actin assembly, and reduces focal adhesion formation (actin and vinculin colocalization). F-actin (red), vinculin (green), and focal adhesions (merge, yellow) (n = 3). Scale bar represents 10 µm. (B) Immunofluorescence microscopy of the dansyl group of NC9 (green) shows its incorporation into FXIII-A enzyme at the periphery of preadipocytes, which is accompanied by disappearance of the actin stress fibers seen in control cells. Actin (red) and DAPI (blue) (n = 3). Scale bar represents 10 µm. (C) WB analysis of MDC-labeled preadipocyte extracts, blotted for dansyl, demonstrates that the substrate probe labels a 250-kDa protein(s) in preadipocyte cultures. Immunoprecipitation with dansyl antibody followed by detection with FN antibody shows the labeled substrate is FN (n = 3). Ig, immunoglobulin. (D) In situ extracellular TG activity assay, ie, analysis of BPA incorporation into coated FN by cellular TG activity. Biotin detection with Neutravidin was performed after all cells were removed. NC9 reduces BPA incorporation into coated FN. ***P < .001. Error bars represent SEM (n = 4). DMSO, dimethylsulfoxide.
FXIII-A activity regulates cytoskeletal dynamics; FN is a major extracellular substrate of FXIII-A. (A) Immunofluorescence microscopy of cell morphology and cytoskeletal elements of preadipocytes upon inhibition of FXIII-A activity with NC9. Inhibition attenuates actin stress fiber formation, promotes cortical actin assembly, and reduces focal adhesion formation (actin and vinculin colocalization). F-actin (red), vinculin (green), and focal adhesions (merge, yellow) (n = 3). Scale bar represents 10 µm. (B) Immunofluorescence microscopy of the dansyl group of NC9 (green) shows its incorporation into FXIII-A enzyme at the periphery of preadipocytes, which is accompanied by disappearance of the actin stress fibers seen in control cells. Actin (red) and DAPI (blue) (n = 3). Scale bar represents 10 µm. (C) WB analysis of MDC-labeled preadipocyte extracts, blotted for dansyl, demonstrates that the substrate probe labels a 250-kDa protein(s) in preadipocyte cultures. Immunoprecipitation with dansyl antibody followed by detection with FN antibody shows the labeled substrate is FN (n = 3). Ig, immunoglobulin. (D) In situ extracellular TG activity assay, ie, analysis of BPA incorporation into coated FN by cellular TG activity. Biotin detection with Neutravidin was performed after all cells were removed. NC9 reduces BPA incorporation into coated FN. ***P < .001. Error bars represent SEM (n = 4). DMSO, dimethylsulfoxide.
FXIII-A activity is required for pFN matrix assembly and preadipocyte proliferation
We next investigated the function of FXIII-A activity with regards to the role of FN in preadipocytes. Enzyme-linked immunosorbent assay analysis of FN levels in cell layers showed increased levels of FN associated with initiation of differentiation, reaching a maximum by day 2; by day 4, FN levels decreased to the day 0 level (Figure 5A). This pattern was similar to the pattern of TG activity in the cultures as shown in Figure 2A. FN can be assembled into the cell layers as extracellular matrix from 2 pools of FN: from circulating pFN made by hepatocytes in liver (present in the serum used in cell cultures) and from cFN synthesized by tissue-resident cells.31,37 Both forms can be found as deoxycholate (DOC)-soluble and DOC-insoluble matrix. To investigate whether both pFN and cFN were substrates for FXIII-A activity, cell cultures were labeled with BPA, and the labeled material was affinity purified and detected with FN antibody (detects all FN) and extra domain A (EDA)-FN (cFN) antibody. Detection in this way showed labeling of only total FN but not cFN (EDA-FN), suggesting that only pFN is a substrate for TG activity (Figure 5B). To further confirm that FXIII-A in preadipocytes crosslinks pFN, we used a FXIII-A–specific substrate peptide, bF11, which is a biotinylated peptide containing a reactive glutamine (Q) residue58 ; this peptide is capable of incorporating into substrates only when FXIII-A is active. Cells were labeled with bF11 or control F11QN (where the Q is replaced by asparagine [N]) for 24 hours and pulled down with Neutravidin beads. WB showed clear detection of total FN with bF11 but not with control bF11QN. NC9 blocked FN labeling by bF11 (Figure 5C). To further demonstrate that FXIII-A promotes pFN incorporation into matrix in preadipocyte cultures, cells were given exogenous biotinylated pFN (bpFN) in a pulse/chase experiment. Fluorescence microscopy of the bpFN matrix network showed that FXIII-A inhibition decreased FN assembly in preadipocyte cultures (Figure 5D). Levels of bpFN were analyzed from media and DOC-soluble and DOC-insoluble extracts after 24-hour incubation. These data show (1) a significantly higher level of bpFN retained in the media in NC9-treated cultures (Figure 5E); (2) intracellular FN levels, analyzed from trypsinized cells, which showed no change upon NC9 treatment, demonstrating that FN production was neither increased or decreased (Figure 5F); and (3) significantly lower bpFN levels in both DOC-soluble and DOC-insoluble matrix by NC9 treatment (Figure 5G-H). These data suggest that FXIII-A activity on the preadipocyte surface is specifically directed toward assembling a soluble form of pFN into preadipocyte extracellular matrix. Adding recombinant, soluble FXIII-A (activated) to the 3T3-L1 cultures along with bpFN did not result in organized fibrillogenesis but rather aggregated bpFN, resulting in an increase in lipid accumulation (data not shown). This suggests that soluble FXIII-A may not promote bpFN fibrillogenesis in preadipocyte cultures.
pFN is a substrate for FXIII-A activity. (A) FN detection in total cell protein extracts by enzyme-linked immunosorbent assay during differentiation of preadipocytes to adipocytes during 8 days. FN levels increase in preadipocyte layers during early differentiation and peak at day 2. (B) Affinity-purified preadipocyte culture extracts labeled with BPA shows its incorporation into total FN but not into cFN (EDA-FN), thus demonstrating that cFN/EDA-FN is not a TG substrate and suggesting that pFN is the main crosslinking target in preadipocytes. Total cell extract (Tot.CE) was used as positive control. (C) The FXIII-A–specific substrate peptide bF11 was able to pull down FN, demonstrating that it acts as a specific FXIII-A substrate in preadipocytes. NC9 blocks bF11-mediated FN labeling. The control peptide bF11QN shows no labeling. (D) Immunofluorescence microscopy of bpFN (green) in preadipocytes (actin, red) treated with basic cell culture media (M) (serum-free conditions). Inhibition of TG activity by NC9 decreased bpFN matrix levels (green) in preadipocytes. Nuclei are stained with DAPI (blue). (E) Analysis of exogenous bpFN levels in media using enzyme-linked immunosorbent assay after 24-hour incubation with preadipocytes during differentiation shows a significant increase in media upon NC9 treatment at day 1, indicating that less pFN is incorporating as extracellular matrix. (F) Quantification of intracellular FN levels analyzed from trypsinized cells shows no change in FN levels in cells upon NC9 treatment. (G-H) Assembly of pFN into preadipocyte extracellular matrix is impaired by NC9 treatment. Exogenous bpFN was given to the cells for 24 hours followed by its detection prepared with DOC (DOC-sol) and sodium dodecyl sulfate-containing (DOC-insol) buffers. Quantification was done after WB and detection of biotin. icFN, intracellular FN. All error bars represent SEM (n = 3). *P < .05; **P < .01; ***P < .001.
pFN is a substrate for FXIII-A activity. (A) FN detection in total cell protein extracts by enzyme-linked immunosorbent assay during differentiation of preadipocytes to adipocytes during 8 days. FN levels increase in preadipocyte layers during early differentiation and peak at day 2. (B) Affinity-purified preadipocyte culture extracts labeled with BPA shows its incorporation into total FN but not into cFN (EDA-FN), thus demonstrating that cFN/EDA-FN is not a TG substrate and suggesting that pFN is the main crosslinking target in preadipocytes. Total cell extract (Tot.CE) was used as positive control. (C) The FXIII-A–specific substrate peptide bF11 was able to pull down FN, demonstrating that it acts as a specific FXIII-A substrate in preadipocytes. NC9 blocks bF11-mediated FN labeling. The control peptide bF11QN shows no labeling. (D) Immunofluorescence microscopy of bpFN (green) in preadipocytes (actin, red) treated with basic cell culture media (M) (serum-free conditions). Inhibition of TG activity by NC9 decreased bpFN matrix levels (green) in preadipocytes. Nuclei are stained with DAPI (blue). (E) Analysis of exogenous bpFN levels in media using enzyme-linked immunosorbent assay after 24-hour incubation with preadipocytes during differentiation shows a significant increase in media upon NC9 treatment at day 1, indicating that less pFN is incorporating as extracellular matrix. (F) Quantification of intracellular FN levels analyzed from trypsinized cells shows no change in FN levels in cells upon NC9 treatment. (G-H) Assembly of pFN into preadipocyte extracellular matrix is impaired by NC9 treatment. Exogenous bpFN was given to the cells for 24 hours followed by its detection prepared with DOC (DOC-sol) and sodium dodecyl sulfate-containing (DOC-insol) buffers. Quantification was done after WB and detection of biotin. icFN, intracellular FN. All error bars represent SEM (n = 3). *P < .05; **P < .01; ***P < .001.
After initiation of differentiation, 3T3-L1 cells undergo mitotic clonal expansion for 48 to 72 hours, which coincides with the increased FXIII-A activity (Figure 2A) and increased levels of FN in cell layers. Thus, we hypothesized that FXIII-A activity regulates pFN matrix assembly to promote preadipocyte proliferation. Proliferation was assessed by plating cells under serum-free conditions on pFN-coated plates or by supplementing the media with pFN. Proliferation assays showed that both ways of exposing the cells to pFN increased preadipocyte proliferation in a concentration-dependent manner (Figure 6A). A blocking antibody against EDA-FN further increased preadipocyte proliferation, suggesting that pFN and EDA-FN may have opposing functions in preadipocytes (Figure 6B). Cell proliferation can be induced by FN and soluble mitogens such as INS.59 Because INS was a component of the DM for preadipocytes, we examined the combined effects of INS and pFN on preadipocyte proliferation. The analyses showed a significant fourfold increase in proliferation by INS-pFN treatment compared with pFN treatment alone, demonstrating a synergistic effect. The proproliferative effect was dependent on FXIII-A activity, because NC9 significantly attenuated these effects for both the pFN treatment alone and the INS-pFN treatment combination (Figure 6C). It is known that INS mediates cell proliferation via activating the mitogen-activated protein kinase/extracellular signal-regulated kinase (Erk) pathway.60 Analysis of Erk phosphorylation levels in pFN- and INS-treated preadipocytes showed that NC9 decreased the sustained Erk phosphorylation in these cells in both serum and serum-free conditions (supplemental Figure 7). To examine whether INS effects are mediated through the INS-like growth factor receptor or through the INS receptor, we inhibited both receptors and assessed cell proliferation. As seen in Figure 6D, only the INS receptor inhibitor HNMPA-(AM)3 was able to decrease the combined proproliferative effects of pFN and INS. Inhibition of INS-like growth factor receptor with its specific inhibitor PPP had an opposite effect and promoted cell proliferation. These results indicate that FXIII-A–mediated assembly of pFN is required for proliferation of preadipocytes and can potentiate the proproliferative effects of INS.
FXIII-A activity regulates proliferation of preadipocytes by promoting pFN assembly into preadipocyte extracellular matrix. (A) Exogenous pFN immobilized onto culture plates (white circles) or added to media (black circles) under serum-free conditions promotes preadipocyte proliferation in a concentration-dependent manner (n = 4). (B) Blocking antibody toward EDA-FN (white triangle) further increased pFN-mediated preadipocyte proliferation, suggesting that the 2 forms of FN have opposing functions. Control treatment; isotype antibody (black circles) (n = 3). **P < .01. (C) Media supplemented with pFN potentiates the proproliferative effect of INS on preadipocytes under serum-free conditions. The combined effect of pFN and INS on preadipocyte proliferation is inhibited by NC9 (n = 3). *** or ###P < .001 (D) The proproliferative effect of INS on preadipocytes under serum-free conditions is mediated by the INS receptor (IR) and not by the INS-like growth factor receptor (IGFR), as demonstrated by the ability of respective receptor inhibitors to block the effect. Only the IR inhibitor HNMPA-(AM)3 (10 µM) reduced proliferation caused by combined pFN and INS treatment; addition of the IGFR inhibitor (PPP, 10 µM) had the opposite effect. Proproliferative effects of pFN alone were not mediated by either IR or IGFR. n = 3, *P < .05; **P < .01; N.S, not significant. Error bars represent SEM.
FXIII-A activity regulates proliferation of preadipocytes by promoting pFN assembly into preadipocyte extracellular matrix. (A) Exogenous pFN immobilized onto culture plates (white circles) or added to media (black circles) under serum-free conditions promotes preadipocyte proliferation in a concentration-dependent manner (n = 4). (B) Blocking antibody toward EDA-FN (white triangle) further increased pFN-mediated preadipocyte proliferation, suggesting that the 2 forms of FN have opposing functions. Control treatment; isotype antibody (black circles) (n = 3). **P < .01. (C) Media supplemented with pFN potentiates the proproliferative effect of INS on preadipocytes under serum-free conditions. The combined effect of pFN and INS on preadipocyte proliferation is inhibited by NC9 (n = 3). *** or ###P < .001 (D) The proproliferative effect of INS on preadipocytes under serum-free conditions is mediated by the INS receptor (IR) and not by the INS-like growth factor receptor (IGFR), as demonstrated by the ability of respective receptor inhibitors to block the effect. Only the IR inhibitor HNMPA-(AM)3 (10 µM) reduced proliferation caused by combined pFN and INS treatment; addition of the IGFR inhibitor (PPP, 10 µM) had the opposite effect. Proproliferative effects of pFN alone were not mediated by either IR or IGFR. n = 3, *P < .05; **P < .01; N.S, not significant. Error bars represent SEM.
F13a1−/− MEFs show reduced cell adhesion and proliferation and increased adipogenesis
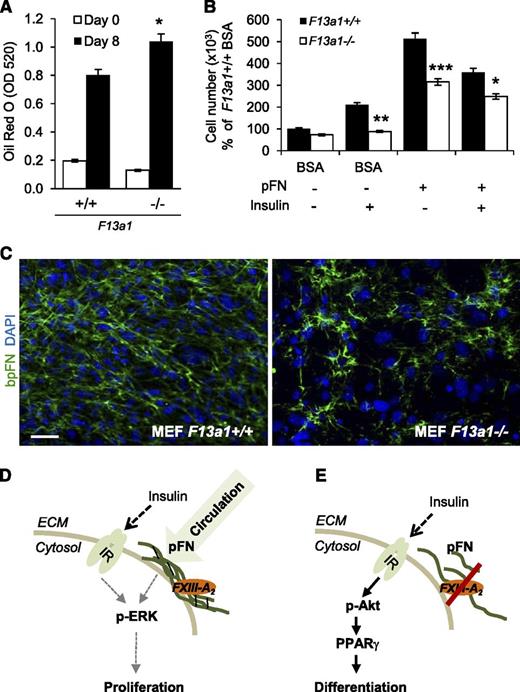
To confirm the role of FXIII-A in adipocyte function, we examined the ability of F13a1−/− MEFs to proliferate and differentiate into adipocytes. Compared with F13a1+/+ cells, F13a1−/− MEFs exhibited a 30% increase in lipid accumulation (Figure 7A; supplemental Figure 8A) and decreased cell adhesion to pFN (supplemental Figure 8B). mRNA expression of F13a1 and Tgm2 were not altered during differentiation of F13a1+/+ MEFs (supplemental Figure 8C). F13a1−/− MEFs also showed a significant decrease in their ability to proliferate and a decreased response to the proliferative effects of exogenous pFN supplemented into the serum-free media. The F13a1−/− MEFs also showed an overall reduced proliferative response to INS with or without exogenous pFN (Figure 7B). Fluorescence microscopy of bpFN in MEFs showed that F13a1−/− cells assembled bpFN poorly into the fibrillar matrix compared with F13a1+/+ cells (Figure 7C). These data show that pFN constitutes the majority of the total FN extracellular matrix in preadipocytes/MEFs and requires FXIII-A for its assembly.
F13a1−/− MEFs show increased adipogenesis, decreased proliferation, and a defect in pFN matrix assembly. (A) F13a1−/− MEFs accumulate significantly more lipids in 8 days when subjected to adipogenic differentiation. Lipid accumulation was visualized and quantified by Oil Red O staining on day 8 of differentiation (n = 3). *P < .05. (B) F13a1−/− MEFs show a significantly poorer response to the proproliferative effect of exogenous pFN given alone to cells or in combination with INS (n = 3). *P < .05; **P < .01; ***P < .001. Error bars represent SEM. (C) pFN assembly is impaired in F13a1−/− MEF cultures compared with F13a1+/+ cultures as assessed by incorporation of exogenous bpFN (green) into extracellular matrix on day 1 of differentiation. Nuclei are stained in blue (DAPI) (n = 2). Scale bar represents 100 µm. (D) Proposed mechanism for the role of FXIII-A in preadipocytes. FXIII-A acts on the cell surface of preadipocytes where it promotes liver-derived, circulating pFN assembly into preadipocyte extracellular matrix. pFN matrix promotes cell proliferation and potentiates the proproliferative effects of INS via the IR and activation of the Erk pathway. (E) In the absence of FXIII-A TG activity, pFN assembly is reduced, which switches INS signaling to activation of the Akt pathway, resulting in increased PPARγ expression and adipocyte differentiation. Thus, the extent of FXIII-A–mediated pFN assembly in preadipocytes and adipocytes can modulate the mitogenic and metabolic effects of INS.
F13a1−/− MEFs show increased adipogenesis, decreased proliferation, and a defect in pFN matrix assembly. (A) F13a1−/− MEFs accumulate significantly more lipids in 8 days when subjected to adipogenic differentiation. Lipid accumulation was visualized and quantified by Oil Red O staining on day 8 of differentiation (n = 3). *P < .05. (B) F13a1−/− MEFs show a significantly poorer response to the proproliferative effect of exogenous pFN given alone to cells or in combination with INS (n = 3). *P < .05; **P < .01; ***P < .001. Error bars represent SEM. (C) pFN assembly is impaired in F13a1−/− MEF cultures compared with F13a1+/+ cultures as assessed by incorporation of exogenous bpFN (green) into extracellular matrix on day 1 of differentiation. Nuclei are stained in blue (DAPI) (n = 2). Scale bar represents 100 µm. (D) Proposed mechanism for the role of FXIII-A in preadipocytes. FXIII-A acts on the cell surface of preadipocytes where it promotes liver-derived, circulating pFN assembly into preadipocyte extracellular matrix. pFN matrix promotes cell proliferation and potentiates the proproliferative effects of INS via the IR and activation of the Erk pathway. (E) In the absence of FXIII-A TG activity, pFN assembly is reduced, which switches INS signaling to activation of the Akt pathway, resulting in increased PPARγ expression and adipocyte differentiation. Thus, the extent of FXIII-A–mediated pFN assembly in preadipocytes and adipocytes can modulate the mitogenic and metabolic effects of INS.
Discussion
Recent genome-wide association studies of human WAT identified F13A1 as a potentially causative gene for obesity,10 suggesting that FXIII-A may be linked to adipose tissue function. In our study, we provide the first set of evidence showing that WAT has abundant FXIII-A activity and how FXIII-A can be linked to adipogenesis. FXIII-A enzyme was localized to the preadipocyte surface, where it assisted in assembling pFN into the matrix to promote cell proliferation and to potentiate the proproliferative effects of INS. This antagonized the prodifferentiating effects of INS on preadipocytes (Figure 7 D-E). FXIII-A, jointly with pFN, maintained preadipocytes in an undifferentiated state by modulating cytoskeletal dynamics. Thus, we conclude that FXIII-A acts as a negative regulator of adipogenesis. Our study also demonstrated that preadipocytes express TG2; however, TG activity probe and inhibitor NC9 did not covalently incorporate into TG2 based on immunofluorescence data, indicating that it is not active as a TG enzyme. However, because its function has been strongly linked to cytoskeletal dynamics,46 it may also contribute to maintenance of the cytoskeleton of preadipocytes/adipocytes via a mechanism that does not involve its TG activity.
Preadipocyte differentiation into lipid-accumulating mature adipocytes is part of normal adipose tissue function and is critical for storage and elimination of lipids from the circulation. Preadipocyte proliferation is required for adipose tissue expansion to accommodate the increased requirement for energy storage in obesity. Failure to accumulate lipids or to expand adipose tissue results in increased circulating fatty acids and their ectopic storage in nonmetabolic tissues, which is a major contributor to the development of INS resistance.61-65 Our results show that FXIII-A, jointly with pFN, increases preadipocyte proliferation, but inhibits lipid accumulation. The role of FN as a negative regulator of adipogenesis in vitro has been demonstrated in mouse and human preadipocytes, where it inhibits lipid accumulation by blocking the morphological and cytoskeletal changes necessary for lipid accumulation.36,48,66-68 Our work is the first to demonstrate that pFN, synthesized by hepatocytes in liver, contributes to adipogenesis. This adds to the list of tissues (liver, brain, testis, heart, lungs, and bone37,38 ) and cell types that have been shown to accumulate pFN for their function.32,33 Furthermore, although FN matrix is clearly an important component of WAT and preadipocyte cultures, its actual function has remained elusive. Our work shows that preadipocytes use pFN matrix for proliferation and that this matrix sensitizes the cells for the proproliferative effects of insulin. Vascularization of WAT is critical for adipose tissue expansion during increased need for energy storage; it is likely that pFN is one of the circulating factors that can regulate this tissue expansion.
The transition of preadipocytes from a proliferation phase to a differentiation phase is reflected by changes in cell morphology accompanied by major remodeling of extracellular matrix components. Whereas preadipocytes themselves regulate synthesis and degradation of collagen and laminin matrices,69 the pFN matrix accumulation appears to be regulated by the presence of FXIII-A in the cells. pFN levels in preadipocyte cultures follow the pattern of F13a1 mRNA and enzyme activity, and a decrease in FN matrix was associated with decreased F13a1 mRNA levels by the cells. Thus, FXIII-A regulation in preadipocytes may be part of the transition between the proliferative and differentiation states (Figure 7D-E). The preadipocyte FXIII-A is found on the cell surface mostly as a complex form. Similar HMW FXIII-A was found to form upon activation of human FXIII in vitro, suggesting that preadipocyte cell surface FXIII-A may be a covalent, active dimer. Whether this preadipocyte FXIII-A requires further proteolytic activation remains unknown; however, it is possible that the observed dimerization/complexation, together with increased Ca2+ levels, and binding to substrate in the extracellular space suffices to induce activity. Of interest is also the observation that preadipocytes produce mostly FXIII-A monomer of lower MW. This form is also found in platelets, which produce 2 forms as per to our antibody detection data. Thus, it is possible that this smaller monomer FXIII-A, in both platelets and adipocytes, is a proteolytically cleaved form of the full-length FXIII-A and that the cleavage process could be linked to mechanisms on how FXIII-A is anchored to the cell surface.
FXIII-A deficiency in humans results in a rare blood-clotting defect.70 There are no reports of energy metabolism dysregulation or BMI-linked abnormalities in FXIII-A–deficient patients; however, circulating FXIII-A levels are increased in type 2 patients with diabetes.71 Thus, it is possible that obesity-linked F13A1 SNPs in WAT discovered in the ENGAGE study10 may have effects on FXIII-A function only locally in adipose tissue while having no effects on coagulation or other cellular processes. Indeed, a specific regulation, modification, and function of FXIII-A in WAT is supported by the observation that the Finnish twins discordant in BMI and having altered FXIII-A expression in WAT have normal FXIII-A levels in blood.6 Similarly, the FXIII-A Val34Leu polymorphism, which results in increased enzyme activation, has a protective effect against coronary artery disease12,72 but is not linked to obesity.10 In conclusion, our study shows the presence and relevance of FXIII-A in adipose tissue and preadipocytes, suggesting a mechanism by which FXIII-A might be linked to obesity and weight gain. Elucidating the full metabolic phenotype of F13a1−/− mice, and understanding how FXIII-A is modulated, processed, secreted, and anchored to the cell surface in adipocytes in the normal vs obese state, can provide valuable information about how to regulate adipose tissue health.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Aisha Mousa for assistance.
This study was supported by grants to M.T.K. from the Canadian Institutes of Health Research (CIHR) and the CIHR Institute of Genetics. K.H. was supported by a Grant-in-Aid for Scientific Research (B) (no. 23380200) from JSPS, Japan (KH). V.D.M. received stipends from Faculty of Dentistry and the CIHR Systems Biology Training Program.
Authorship
Contribution: V.D.M. designed and conducted the experiments, analyzed the data, and drafted the manuscript; M.T.K. supervised the study, analyzed the data, and edited the manuscript and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and K.H. contributed peptide reagents for the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mari T. Kaartinen, Faculty of Dentistry, McGill University, 3640 University St, M/72, Montreal, QC, Canada, H3A 0C7; e-mail: mari.kaartinen@mcgill.ca.