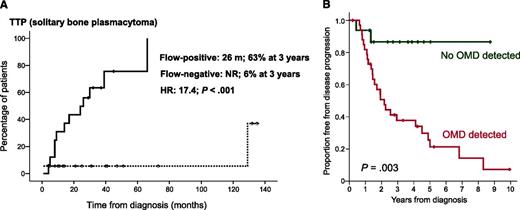
In this issue of Blood, 2 studies, one by Paiva et al (for the Spanish Myeloma Study group) and one by Hill et al (for the Leeds group [UK]), showed independently that flow cytometry of the bone marrow could identify patients with solitary plasmacytomas (SPs) at high risk for progression to active multiple myeloma (MM).1,2 In the first study, 71% of patients with solitary bone plasmacytoma (SBP) who had positive flow cytometry for bone marrow phenotypically aberrant clonal plasma cells progressed to MM at a median time of 26 months, whereas only 6% of patients with negative flow cytometry progressed to myeloma (panel A). The respective values for solitary soft tissue plasmacytomas, outside of the bone, were 20% vs 6%, respectively.1 In the second study, the results were similar: 72% of patients with SBP and occult bone marrow disease detected by flow cytometry vs 12.5% without clonal plasma cells in the bone marrow also progressed to MM at a median time of 26 months (panel B).2 These findings are of great importance because multiparameter flow cytometry may be included in the initial work-up of a patient with a suspected SP.
(A) The median time to progression for patients with SBP and clonal plasma cells detected in the bone marrow by flow cytometry was 26 months in the Spanish study. See Figure 1A in the article by Paiva et al that begins on page 1300. (B) Similarly, in the UK study, the median time to progression for patients with SBP and occult marrow disease was 26 months. See Figure 1A in the article by Hill et al that begins on page 1296.
(A) The median time to progression for patients with SBP and clonal plasma cells detected in the bone marrow by flow cytometry was 26 months in the Spanish study. See Figure 1A in the article by Paiva et al that begins on page 1300. (B) Similarly, in the UK study, the median time to progression for patients with SBP and occult marrow disease was 26 months. See Figure 1A in the article by Hill et al that begins on page 1296.
SPs are rare plasma cell disorders (<5% of the total) that are characterized by the presence of bone or extramedullary soft tissue tumors (EMP), which consist of monoclonal plasma cells, without evidence of systematic MM (absence of clonal plasma cells in the trephine bone marrow biopsy and no CRAB [calcium, renal, anemia, bone] criteria).3 Local radiotherapy, with or without surgical excision, is the recommended treatment of choice for these patients, which achieves high local control rates and high event-free and overall survival rates.3-5 Patients with SBP may progress to MM at a rate of approximately 40% to 50%, with lower rates of progression for EMP.4-7 In one of the largest series in the literature, the Rare Cancer Network described the natural history of 258 patients with SPs. The median time to MM progression was 21 months (range 2-135), with a 5-year probability for progression of 45%. The 5-year overall survival, disease-free survival, and local control rate were 74%, 50%, and 86%, respectively.6 From this and other studies, it is evident that there is great heterogeneity in patients with SBPs, with approximately half of them progressing to MM within 2 to 5 years and another half remaining disease-free for more than 10 to 15 years. Thus it is extremely important to identify those patients who progress to MM to treat them in a different way.
The International Myeloma Working Group has suggested that all patients with SBP need to have a whole-body magnetic resonance imaging (WB-MRI) to better assess the extent of the local disease and to reveal occult lesions elsewhere.8 Positron emission tomography with computed tomography (PET-CT) may also be of importance for the work-up of patients with SBP.9 Despite the progress in the diagnostic ability of imaging techniques, several patients with SBP will finally develop MM because of the growth of previously occult lesions that have not been detected by WB-MRI or PET-CT. Indeed in the Spanish study, in 12 of 28 patients with flow-positive results, the WB-MRI was negative.1 Other prognostic factors for the identification of SBP patients at high risk for progression to MM include the presence and mainly the persistence of monoclonal paraprotein in the serum or urine and the abnormal serum-free light-chain (FLC) ratio at diagnosis.10 In a large study of the Mayo Clinic in 116 patients with SBP, patients with abnormal FLC ratio had a probability of progression at 5 years of 44% vs 26% of those with a normal FLC ratio. In the same study, the persistence of serum M-protein level of 5 g/L or higher was an additional risk factor for progression. A risk stratification model was constructed using the aforementioned 2 variables, and patients who had both of these variables had a risk of progression at 5 years of 62%.10 This was the best prediction so far in the literature. The two studies published in this issue of Blood have reported higher identification rates of patients with high-risk SBPs using flow cytometry of the bone marrow (∼70% at 26 months). More importantly, in the Spanish study, there was no correlation between the level of M-protein and the number of clonal plasma cells in the bone marrow,1 whereas in the UK study, the combination of the presence of clonal plasma cells in the bone marrow by flow cytometry and the presence of urinary FLC identifies a high-risk SBP population with 75% probability of progression.2
Although these findings are very important, there are 2 issues that have to be addressed. First, the different methodology in flow cytometry that is used in the 2 studies: it is important to have a common flow method for the detection of clonal plasma cells in the bone marrow of SBP patients but also for the determination of minimal residual disease in MM. Second, despite the improvement in the identification of high-risk SBP patients with flow cytometry, there are still patients who continue to progress despite negative flow results and negative imaging tests. As far as flow results are concerned, it is well established that a negative test may be either a true-negative result or the consequence of the patchy involvement of the bone marrow by the malignant plasma cells.
Thus, a collaborative effort at an international level should be undertaken to identify which of the previously mentioned tests have an independent value and to develop a more comprehensive predictive model. Over the last 20 years, the diagnosis of SP has become less frequent, because cases with occult myeloma have been excluded. Conversely, the curability rate of SP is increasing because more patients now have a correct diagnosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.