In this issue of Blood, Zhang et al from Cincinnati Children’s Hospital report the occurrence of presumably synergistic single heterozygous mutations in two genes involved in cytotoxic function of lymphocytes and natural killer (NK) cells in patients with hemophagocytic lymphohistiocytosis (HLH), representing a previously unrecognized form of inheritance for this condition.1
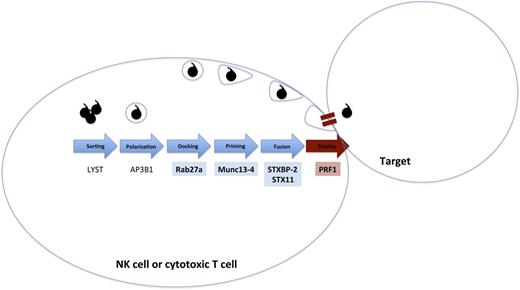
HLH-associated genes and digenic inheritance. A simplified pathway of formation, polarization, and transport of cytotoxic granules that is essential for lymphocyte cytotoxicity is shown. Biallelic mutations have been reported for all of these genes in patients with fHLH. Zhang et al1 describe digenic heterozygous mutations in the highlighted genes. Digenic mutations in degranulation pathway genes (blue/blue) in this series had earlier onset of disease than digenic mutations in a degranulation pathway gene and PRF1 (blue/red).
HLH-associated genes and digenic inheritance. A simplified pathway of formation, polarization, and transport of cytotoxic granules that is essential for lymphocyte cytotoxicity is shown. Biallelic mutations have been reported for all of these genes in patients with fHLH. Zhang et al1 describe digenic heterozygous mutations in the highlighted genes. Digenic mutations in degranulation pathway genes (blue/blue) in this series had earlier onset of disease than digenic mutations in a degranulation pathway gene and PRF1 (blue/red).
Regarding a previous controversy arising out of Cincinnati, US Supreme Court Justice Stewart coined the phrase “I know it when I see it” (Jacobellis v. Ohio, 1964). Some may argue that HLH falls into this category of critically important phenomenon for which precise definitions remain intangible. Working diagnostic criteria for HLH are derived from the Histiocyte Society HLH-2004 protocol. That protocol helps identify a patient with extreme inflammation with subsequent immunopathology: fever, splenomegaly, cytopenias, hypertriglyceridemia, hypofibrinogenemia, decreased NK function, elevated ferritin, elevated soluble interleukin-2 receptor α, and eponymous hemophagocytosis (reviewed in Henter et al2 and Jordan et al3 ).
The paradigm for HLH pathogenesis has been that in the case of inherited familial HLH (fHLH), extreme inflammation arises in babies or toddlers from inherited predisposing immune deficiencies caused by defects in genes expressing proteins that control the formation and release of cytotoxic granules, including AP3, LYST, MUNC13-4, PRF1, Rab27a, STX11, and STXBP2 (reviewed in Chandrakasan and Filipovich4 and Meeths et al5 ) (see figure). By contrast, acquired or secondary HLH describes older patients without known HLH-associated gene defects in whom significant immune activation may be caused by autoimmune disease, persistent infection, malignant antigen-presenting cells, or acquired loss of inhibitory mechanisms (reviewed in Jordan et al3 ). However, the distinction between presumed fHLH and secondary HLH is becoming increasingly blurred. Hypomorphic biallelic mutations in HLH-associated genes have been identified in adults,6 and polymorphisms have been described in patients with autoimmune disease and macrophage activation syndrome.7
The study by Zhang et al1 further blurs the fHLH vs secondary HLH dichotomy by identifying an additional potential mechanism of inheriting risk for developing HLH. They retrospectively analyzed their extensive genetic database (n = 2701 patients) and associated immune function results to identify 28 patients with single heterozygous mutations in 2 distinct cytotoxic pathway genes (see figure). The authors separated single-allele mutations into genes involved in degranulation (MUNC13-4, STXBP2, STX11, and Rab27a) and single-allele mutations in PRF1. Heterogyzous deg/deg patients had an age of onset typical of that of most patients with fHLH compared with PRF1/deg patients who had later onset. Functional implications of deg/deg mutations were supported by defective degranulation assays in a limited series, whereas PRF1/deg patients had normal degranulation. Not surprisingly, perforin expression was decreased in the PRF1/deg patients. Some patients with PRF/deg presented as late as 20 to 30 years of age. Defective NK function was observed across all heterozygous patients.
These data support the potential for a digenic mode of inheritance in fHLH. Prospective, uniform sample collection and testing in HLH patients and families as well as recapitulation of these observations in murine models would be helpful in proving the functional significance of the observed associations in this study. However, this study is an important first step toward expanding diagnostic strategies for patients suspected of having HLH beyond Justice Stewart’s metrics of personal sensibilities. As clinical applications of deep sequencing expand, genetic evaluations will include complex inheritance coupled with functional assays to define HLH in affected patients and determine risk in family members.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal