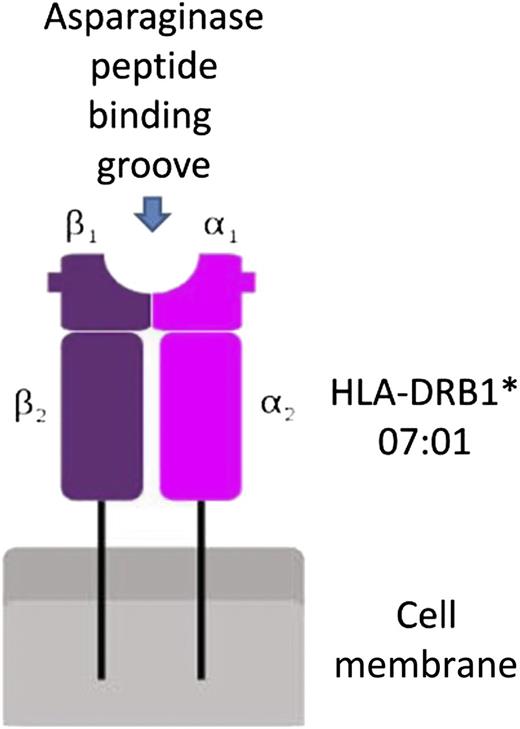
In this issue of Blood, Fernandez et al demonstrate that human leukocyte antigen (HLA) DRB1 alleles confer high-affinity binding to asparaginase epitopes, leading to higher frequency of allergic reactions.1 The authors initially examined HLA data from European ancestry patients enrolled onto St. Jude Children’s Research Hospital (n = 541) and the Children’s Oncology Group (n = 1329) clinical trials and identified a higher incidence of allergic reactions and anti-asparaginase antibodies in patients with HLA-DRB1*07:01 alleles. They then analyzed the structure of the HLA protein to show high-risk amino acids located within the binding pocket (see figure), possibly affecting the interaction between asparaginase epitopes and the HLA-DRB1 protein.
Schematic representation of the interaction between HLA-DRB1 and asparaginase epitopes.
Schematic representation of the interaction between HLA-DRB1 and asparaginase epitopes.
A relationship between genetic aberrations and adverse drug reactions is not novel, as was demonstrated for 6-mercaptopurine and its metabolizing enzyme, thiopurine S-methyltransferase.2 Specifically, defects in the thiopurine S-methyltransferase gene lead to decreased inactivation of 6-mercaptopurine and enhanced toxicity, which can cause severe life-threatening myelosuppression. Further, HLA-B alleles were associated with drug allergies,3 including HLA-DRB*5701 and abacavir, HLA-DRB*1502 and carbamazepine, and HLA-DRB*5801 and allopurinol. The novelty of the work by Fernandez et al is the finding that HLA-DRB1*07:01 is associated with asparaginase allergic reactions and elucidating the mechanism of asparaginase-induced allergies. This work could not have been accomplished without the outstanding databases of St. Jude Children’s Research Hospital and the Children’s Oncology Group meticulously collecting allergic reaction information and, more importantly, samples on all patients accrued to their clinical trials.
A role for HLA-B1 proteins in autoimmune diseases was previously demonstrated, for example, in multiple sclerosis,4 substantiating that HLA-DRB1 amino acids predispose immune responses. This finding led to the hypothesis that altering the binding affinity for HLA-DRB1 may modify the immune response. Copaxone, an immunomodulatory polypeptide that binds to HLA-DRB1 proteins on the surface of antigen-presenting cells and competes with myelin antigens for activation of effector T cells, was shown to be beneficial in patients with relapsing-remitting multiple sclerosis,5 especially those with HLA-DRB1*1501.6 This serves as a proof of concept that modulating HLA-DRB1 proteins’ binding can affect immune response.
The story with asparaginase is somewhat different. The native asparaginase, used in the pediatric studies,1 was recently removed from the market. The alternative, pegylated asparaginase was developed for patients with allergic reactions to the native enzyme and is associated with allergic reactions to the pegylated moiety,7 which exists in canned food and cosmetic products. Further, Erwinia asparaginase was recently approved for patients with allergic reactions to the native enzyme,8 but patients may react to both formulations. In addition, HLA-DRB1 does not encompass all the explanations for asparaginase allergic reactions, as was demonstrated by the same group.9 Specifically, Chen et al studied 485 children and have shown that 5 single nucleotide polymorphisms in the glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunit 1 (GRIA1) gene were associated with asparaginase allergic reactions. Finally, some clinicians may have used either antihistamines or glucocorticoids to mitigate initial allergic reactions in the pediatric studies,1 affecting the true denominator of allergic reactions. In spite of all of this, and based on the current data from Fernandez et al,1 it will be interesting to see if a prospective trial will identify patients predisposed to developing immune responses to asparaginase based on their HLA-DRB1 alleles and whether those patients will benefit from offering Erwinia asparaginase or another intervention.
Conflict-of-interest disclosure: The author served as consultant and received honoraria from Sigma-Tau and Jazz Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal