Key Points
5hmC levels vary considerably in patients with AML.
High levels of 5hmC independently correlate with inferior overall survival in AML.
Abstract
Patients with acute myeloid leukemia (AML) frequently harbor mutations in genes involved in the DNA (hydroxy)methylation pathway (DNMT3A, TET2, IDH1, and IDH2). In this study, we measured 5-hydroxymethylcytosine (5hmC) levels in 206 clinically and molecularly well-characterized younger adult AML patients (≤60 years) included in the European Organization for Research and Treatment of Cancer/Gruppo Italiano Malattie Ematologiche dell'Adulto (EORTC/GIMEMA) AML-12 06991 clinical trial and correlated the 5hmC levels with mutational status and overall survival (OS). In healthy control cells, 5hmC levels were confined to a narrow range (1.5-fold difference), whereas in AML cells, a much wider range was detected (15-fold difference). We identified 3 5hmC subpopulations in our patient cohort (low, intermediate, and high). The low 5hmC group consisted almost entirely of patients with TET2 or IDH mutations. As expected, TET2 and IDH mutated patients had significantly lower levels of 5hmC compared with patients without mutated TET2 and IDH1/2 (both P < .001). Interestingly, high 5hmC levels correlated with inferior OS (high vs intermediate 5hmC: P = .047, hazard ratio [HR] = 1.81). Multivariate analysis revealed that high 5hmC is an independent poor prognostic indicator for OS (high vs intermediate 5hmC: P = .01, HR = 2.10). This trial was registered at www.clinicaltrials.gov as NCT00004128.
Introduction
Alterations in DNA methylation occur in many types of cancer, including hematological malignancies. Global hypomethylation as well as promoter hypermethylation leading to silencing of various tumor suppressor genes have been described.1,2 Because DNA methylation is reversible, it represents a promising target for therapy. In several clinical trials, the effect of hypomethylating agents (azacitidine and decitabine) on the treatment of patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia (AML) has been assessed,3-6 which may result in the reactivation of silenced genes. These trials showed promising results for intermediate-1 to high-risk MDS and elderly AML patients by improving complete remission rates, event-free survival, and overall survival (OS).
In the last few years, our knowledge of DNA (de)methylation has grown extensively. It has been known for a few decades that DNA methyltransferases (DNMTs) are responsible for the conversion of cytosine into methylcytosine. DNMT3A and DNMT3B are de novo DNMTs, whereas DNMT1 is mainly implicated in maintaining methylcytosine marks during DNA replication.7 DNA demethylation can take place as a passive process when the 5-methylcytosine (5mC) mark is not sustained during DNA replication. Only recently, evidence for an active DNA demethylation process has been presented. In 2009 it was discovered that the Ten-Eleven-Translocation (TET) protein family, consisting of TET1, TET2, and TET3, mediates the conversion of 5mC into 5-hydroxymethylcytosine (5hmC).8 5hmC can be further oxidized by TET proteins to 5-formylcytosine and 5-carboxylcytosine, which are both recognized by thymine DNA glycosylase. Subsequently, thymine DNA glycosylase activates the base excision repair pathway, which replaces the modified cytosine with an unmodified cytosine, thereby completing the active demethylation process.9-11 Neuronal cells and stem cells display remarkably high levels of 5hmC, suggesting that 5hmC may be a rather stable mark with a more versatile function besides being an intermediate in the active DNA demethylation process.8,12 TET proteins belong to the group of α-ketoglutarate (α-KG) and iron [Fe(II)]-dependent dioxygenases. The co-substrate α-KG is produced by the isocitrate dehydrogenase (IDH) proteins, which catalyze the conversion of isocitrate into α-KG.13
Intriguingly, 4 genes (TET2, IDH1, IDH2, and DNMT3A) important for regulating DNA (hydroxy)methylation are frequently mutated in myeloid malignancies (supplemental Figure 1, available on the Blood Web site). Loss-of-function mutations in TET2 have been described in various hematological malignancies,14-17 including 7% to 10% of AML patients.18-20 Heterozygous mutations in IDH1 and IDH2 occur in 10% to 20% of AML patients and are mutually exclusive with TET2 mutations.19 Mutant IDH proteins are unable to catalyze the formation of α-KG but gain the function to convert α-KG into the oncometabolite 2-hydroxyglutarate (2HG).21 In vitro studies have shown that 2HG is a competitive inhibitor of the TET proteins.22,23 This implies that both TET2 and IDH1/2 mutations can impair 5hmC generation, which may explain why mutations in these genes are mutually exclusive. Indeed, AML patients with TET2 or IDH mutations showed decreased 5hmC levels.24-27 Recently, mutations affecting the methyltransferase DNMT3A were described in 20% to 25% of AML patients.19,28,29 Interestingly, DNMT3A mutations frequently co-occurred with TET2 or IDH1/2 mutations,18 suggesting that an additive effect may result from the combination of these mutations. In most AML studies, DNMT3A mutations have been associated with a poor prognosis18,19,28-30 except for a recent study by Gaidzik et al.31 Also, TET2 mutations were associated with a poor prognosis in most studies,18,19,32-34 whereas the effect of IDH mutations is less clear. In several studies, IDH1 mutations correlated with a poor survival in cytogenetically normal AML patients, whereas the impact of IDH2 mutations on survival remains inconclusive.18,35-37
Several studies have assessed the effect of these mutations on DNA methylation. AML patients harboring IDH1/2 mutations showed a promoter hypermethylation phenotype and clustered together based on their common DNA methylation profile.38 The effect of TET2 mutations on overall DNA methylation remains controversial. In some studies, TET2 mutated patients displayed a hypermethylation signature overlapping with IDH1/2 mutated patients,23 whereas others described no major effect on global methylation or even hypomethylation.24,39 In vitro, most DNMT3A mutations led to a decreased capability of the protein to produce 5mC,29,40 although global 5mC levels in patients with DNMT3A mutations were not decreased.28 Methylated DNA immunoprecipitation experiments showed several differentially methylated regions between patients with and without DNMT3A mutations.28,29 No information is available concerning the consequences of DNMT3A mutations on 5hmC levels.
The impact of 5hmC levels on survival of AML patients has not been addressed so far. In this study, we measured 5-(hydroxy)methylcytosine (5[h]mC) levels in a prospectively collected cohort of 206 AML patients included in the European Organization for Research and Treatment of Cancer/Gruppo Italiano Malattie Ematologiche dell'Adulto (EORTC/GIMEMA) AML-12 clinical trial and assessed whether 5hmC levels in AML correlated with clinical and molecular parameters.
Materials and methods
Patient samples
Bone marrow (BM) and peripheral blood (PB) from AML patients (n = 206) included in the EORTC/GIMEMA AML-12 06991 clinical trial (NCT0004128, 1999-2009)41 were obtained after informed consent. To avoid bias, only patients from participating centers where DNA was collected from all consecutive cases were included. In total we obtained DNA from 206 AML patients. After DNA collection, we performed the 5hmC analysis. A consort diagram of the trial and an overview of the patients included and excluded in our study is presented in supplemental Tables 1 and 2 and supplemental Figures 2 and 3. The observed OS rate was slightly lower in the included cohort of patients compared with the nonincluded group (38.6% vs 41.9% 5-year OS rate; P = .22).The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Radboud University Nijmegen Medical Centre. The cohort of AML patients (age 16 to 60 years) contained samples of all French-American-British classification subtypes, except for AML M3. The median follow-up at the time of analysis was 6.4 years. PB and BM mononuclear cells, as well as G-CSF–mobilized CD34-positive cells, were obtained from healthy donors (age 18-60 years for PB and BM donors and 44-67 years for donors of CD34-positive cells).
DNA and RNA isolation
For genomic sequencing and 5(h)mC measurements, DNA was isolated from whole PB or BM of AML patients (at diagnosis or in remission) using salt extraction. We compared the 5hmC values in PB and BM and observed that the levels were comparable (supplemental Figure 4). RNA for expression analysis was isolated using RNA-Bee (Tel-Test, Inc., Friendswood, TX). DNA from G-CSF–mobilized CD34-positive cells was isolated using salt extraction and DNA from PB and BM mononuclear cells of healthy donors was isolated using the NucleoSpin Blood QuickPure kit (Macherey Nagel, Düren, Germany).
Sequence analysis
Sanger sequencing was performed on polymerase chain reaction (PCR)-amplified genomic DNA fragments spanning the entire coding region of TET2 (NM_001127208.2) as previously described.14 For IDH1 (ENST00000345146) and IDH2 (ENST00000330062) the mutational hotspot regions were sequenced. DNMT3A exons 7 to 23 (ENST00000264709) were sequenced using Sanger sequencing or Roche 454-based next generation sequencing (NGS) (454 Life Sciences, Branford, CT). Primers used for IDH1, IDH2, and DNMT3A Sanger sequencing can be found in supplemental Tables 3 and 4. DNMT3A NGS was performed as previously described.42 In short, this encompasses PCR-based amplicon-library preparation followed by emulsion PCR and pyrosequencing. NGS data were analyzed using R453Plus1Toolbox,43 GS Variant Analyzer Software 2.6 (454 Life Sciences), and Sequence Pilot, version 3.5.2 (JSI Medical Systems, Kippenheim, Germany). For TET2 and DNMT3A, all intron-exon boundaries were included to identify possible splice site mutations. The variations were compared with the single nucleotide polymorphism database (dbSNP) to determine whether they represented known single nucleotide polymorphisms.
TET expression analysis
Messenger RNA (mRNA) levels of TET2 isoform 1 and isoform 2, TET1, and TET3 were determined using quantitative PCR. Specific PCR primers and probes were designed to discriminate between the different TET2 isoforms (supplemental Table 5). Taqman assays Hs00286756_m1 and Hs00379125_m1 (Life Technologies, Carlsbad, CA) were used to determine TET1 and TET3 expression. Quantitative PCR was conducted using an Applied Biosystems Taqman 7900HT Fast Real-Time PCR system (Life Technologies). Expression levels were normalized to GAPDH and calculated using the 2ΔCT method.
5mC and 5hmC HPLC-MS/MS measurement
For each patient, 2 µg of DNA (isolated from ∼1 × 106 cells) was degraded into individual nucleosides using DNA degradase plus (Zymo Research, Irvine, CA). The individual nucleosides were measured using a high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) system consisting of an Acquity UPLC (Waters, Milford, MA) containing a Waters Atlantis Hilic column (2.1 mm × 100 mm 3 µm) connected to a Micromass Quattro Premier XE (Waters). Quantification was performed using area-based linear regression curves derived from calibration standards containing internal standard solutions. The 5mC and 5hmC levels were calculated as a concentration percentage ratio of % 5-methyl-2′-deoxycytidine/2′-deoxyguanosine (%mdC/dG) and % 5-hydroxymethyl-2′-deoxycytidine/2′-deoxyguanosine (%hmdC/dG), respectively. The inter-assay coefficient of variation over a 1-year period was 2% for 5mC and 5% for 5hmC (n = 49). A detailed description is presented in the supplemental Methods.
2HG HPLC-MS/MS measurement
A detailed description is presented in the supplemental Methods.
Bisulfite sequencing
Bisulfite conversion of 500 ng of DNA was performed using the Methyldetector kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. A semi-nested PCR on the converted DNA was carried out to amplify a CpG-rich promoter region in 2 genes frequently hypermethylated in AML: P15 and MyoD. Primer sequences are listed in supplemental Table 6. PCR products were cloned into the pDrive vector (Qiagen, Valencia, CA), transformed in competent DH12α cells, and plated on a Luria-Bertani agar plate containing 50 µg/mL ampicillin. Per patient, DNA was isolated from 10 colonies and sequenced using Sanger sequencing.
Statistical analysis
5(h)mC differences between 2 patient groups were statistically tested by performing unpaired t tests or Mann-Whitney U tests. More than 2 groups were compared using a 1-way analysis of variance or Kruskal Wallis test. Pearson and Spearman correlation coefficients were used to calculate correlations between 2 continuous variables. Two-sided P values are reported. For negative results that may be scientifically relevant, we determined minimal detectable differences with a power of 80% (http://biomath.info/power/ttest.htm). To divide the AML patients into subgroups based on their 5hmC level, we fitted a normal mixture model to the data using the mixtools R package.44 The optimal number of components was determined using the Akaike information criterion.45 The results of the OS analysis have been presented in a Kaplan-Meier curve. OS was computed from the date of registration in the EORTC 06991 study until death (by any cause) or until last follow-up (censored observation). The Cox Proportional Hazards Model was used to determine the prognostic importance of each factor analyzed and to obtain hazard ratio (HR) estimates as well as corresponding 95% confidence intervals (95% CIs). The following variables were considered: 5hmC levels as continuous or categorical variable, cytogenetic/FLT3-ITD risk group (good = inv16, t(8;21); poor = −5/5q-, −7/7q-, complex, FLT3-ITD; intermediate = NN, -Y; others = other cytogenetic abnormalities), white blood cell count (<25, 25 to <100, >100), percent of BM blasts (20-<40%, 40 to <60%, 60 to <80%, >80%), and age (<30, 30 to <40, 40 to <50, 50-60 years). Statistical analyses were performed using SAS (Cary, NC), R (R Development Core Team, www.r-project.org), and SPSS software (IBM Corporation, Armonk, NY).
Results
Wide distribution of 5hmC levels in AML patients
5hmC levels were measured in 206 AML patients (see Table 1 for patient characteristics), in healthy purified CD34-positive progenitor cells, and healthy BM and PB cells using HPLC-MS/MS. In CD34-positive cells (Figure 1A, range 0.037% to 0.045%; mean 0.041%), and in healthy control cells from BM and PB (Figure 1B, range 0.024% to 0.033%; mean 0.029%) 5hmC levels were tightly clustered, whereas in AML cells a wide range of 5hmC levels was detected (Figure 1A, range 0.006% to 0.091%; mean 0.039%). To determine whether these aberrant 5hmC levels were normalized in remission, we measured 5hmC levels in paired samples at diagnosis and remission from 25 AML patients. For 19/25 patients, the type of material (BM or PB) at remission was matched to the material used at diagnosis. In all cases, the remission samples showed 5hmC levels comparable with the levels in healthy BM and PB (Figure 1B). In addition, 5hmC was measured at diagnosis from 5 patients during remission and at relapse. For all 5 patients, 5hmC levels in remission were comparable with healthy controls (Figure 1C). At relapse, 5hmC returned to the levels obtained at diagnosis in all but 1 patient (UPN128). We found that this patient harbored an IDH1 mutation at diagnosis that was absent at relapse, which may explain the difference in 5hmC levels between diagnosis and relapse (see next paragraph). For patients with similar 5hmC levels at diagnosis and relapse, the same mutations were present at both time points (supplemental Table 7).
Patient characteristics
| . | Overall population (n = 206) . | |
|---|---|---|
| . | No. . | % . |
| Age (years) at diagnosis | ||
| Median (range) | 48.0 (16.0-60.0) | |
| <30 | 21 | 10.2 |
| 30 to <40 | 36 | 17.5 |
| 40 to <50 | 59 | 28.6 |
| 50-60 | 90 | 43.7 |
| Sex | ||
| Male | 101 | 49.0 |
| Female | 105 | 51.0 |
| WHO-ECOG performance status | ||
| 0 | 122 | 59.2 |
| 1 | 74 | 35.9 |
| 2 | 7 | 3.4 |
| 3-4 | 3 | 1.5 |
| WBC (×109L) at diagnosis | ||
| Median (range) | 24.7 (0.5-312.5) | |
| <25 | 104 | 50.5 |
| 25-99.9 | 69 | 33.5 |
| ≥100 | 33 | 16.0 |
| FAB subtype | ||
| M0 | 11 | 5.3 |
| M1 | 45 | 21.8 |
| M2 | 65 | 31.6 |
| M4 | 25 | 12.1 |
| M5 | 48 | 23.3 |
| M6 | 6 | 2.9 |
| M7 | 1 | 0.5 |
| Unknown | 5 | 2.4 |
| Cytogenetic/FLT3-ITD risk group | ||
| Good | 24 | 11.7 |
| Intermediate (NN,-Y) | 62 | 30.1 |
| Poor (-5/5q-,-7/7q-,complex,FLT3-ITD) | 67 | 32.5 |
| Others | 46 | 22.3 |
| UNK/ND/failure | 7 | 3.4 |
| . | Overall population (n = 206) . | |
|---|---|---|
| . | No. . | % . |
| Age (years) at diagnosis | ||
| Median (range) | 48.0 (16.0-60.0) | |
| <30 | 21 | 10.2 |
| 30 to <40 | 36 | 17.5 |
| 40 to <50 | 59 | 28.6 |
| 50-60 | 90 | 43.7 |
| Sex | ||
| Male | 101 | 49.0 |
| Female | 105 | 51.0 |
| WHO-ECOG performance status | ||
| 0 | 122 | 59.2 |
| 1 | 74 | 35.9 |
| 2 | 7 | 3.4 |
| 3-4 | 3 | 1.5 |
| WBC (×109L) at diagnosis | ||
| Median (range) | 24.7 (0.5-312.5) | |
| <25 | 104 | 50.5 |
| 25-99.9 | 69 | 33.5 |
| ≥100 | 33 | 16.0 |
| FAB subtype | ||
| M0 | 11 | 5.3 |
| M1 | 45 | 21.8 |
| M2 | 65 | 31.6 |
| M4 | 25 | 12.1 |
| M5 | 48 | 23.3 |
| M6 | 6 | 2.9 |
| M7 | 1 | 0.5 |
| Unknown | 5 | 2.4 |
| Cytogenetic/FLT3-ITD risk group | ||
| Good | 24 | 11.7 |
| Intermediate (NN,-Y) | 62 | 30.1 |
| Poor (-5/5q-,-7/7q-,complex,FLT3-ITD) | 67 | 32.5 |
| Others | 46 | 22.3 |
| UNK/ND/failure | 7 | 3.4 |
FAB, French–American–British classification; ND, not determined; No., number; WBC, white blood cell count; WHO-ECOG, World Health Organization-Eastern Cooperative Oncology Group; UNK, unknown.
Aberrant 5hmC levels in AML. (A) In AML cells, a broad range of 5hmC levels was detected at diagnosis when compared with healthy CD34-positive cells. (B) Aberrant 5hmC levels at diagnosis were restored to normal levels in remission. (C) For 5 AML patients, 5hmC levels were measured at diagnosis, remission, and relapse. The 5hmC levels were in 4 of 5 cases comparable at diagnosis and relapse. For unique patient number (UPN) 128, the 5hmC level at relapse was remarkably higher than at diagnosis due to an IDH1 mutation at diagnosis that was absent at relapse. Dashed lines indicate the range of 5hmC values measured in healthy BM and healthy PB.
Aberrant 5hmC levels in AML. (A) In AML cells, a broad range of 5hmC levels was detected at diagnosis when compared with healthy CD34-positive cells. (B) Aberrant 5hmC levels at diagnosis were restored to normal levels in remission. (C) For 5 AML patients, 5hmC levels were measured at diagnosis, remission, and relapse. The 5hmC levels were in 4 of 5 cases comparable at diagnosis and relapse. For unique patient number (UPN) 128, the 5hmC level at relapse was remarkably higher than at diagnosis due to an IDH1 mutation at diagnosis that was absent at relapse. Dashed lines indicate the range of 5hmC values measured in healthy BM and healthy PB.
Decreased global 5hmC levels in patients with TET2 or IDH1/2 mutations
The entire TET2 gene and the mutational hotspot regions in IDH1 and IDH2 were sequenced in 206 AML patients using Sanger sequencing.18 TET2 mutations were present in 7% of the patients (n = 15), IDH1 R132 in 10% (n = 21), IDH2 R140 in 11% (n = 22), and IDH2 R172 in 2% (n = 4) of the patients. In most cases TET2, IDH1, and IDH2 mutations were mutually exclusive, except for 1 patient with an IDH1 and subclonal IDH2 mutation (supplemental Table 8).
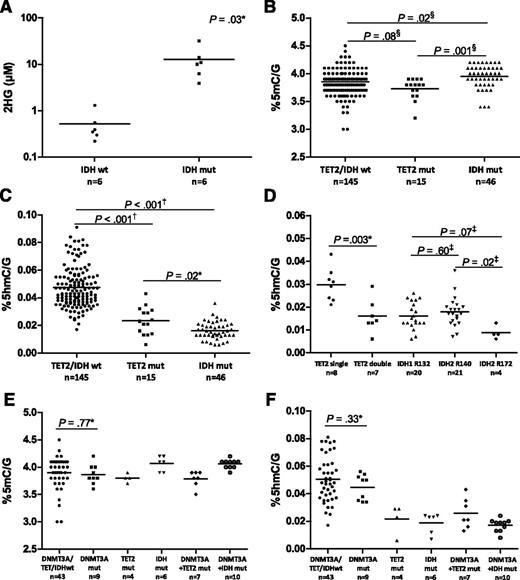
Using HPLC-MS/MS, we confirmed that patients with IDH mutations produce 2HG, which is known to inhibit TET proteins (Figure 2A; supplemental Table 9).22,23 Patients with IDH mutations had up to 150 times higher plasma 2HG levels compared with IDH wild-type patients (P = .03). Because a reduced function of TET2, either by mutations or by 2HG-mediated inhibition, may affect both 5mC and 5hmC levels, we compared these levels in patients with and without TET2 or IDH mutations. We observed small but statistically significant differences in 5mC levels between the different patient groups (Figure 2B). Patients with IDH mutations displayed slightly increased 5mC values compared with TET2/IDH wild-type and TET2 mutated patients (P = .02 and P = .001, respectively). In contrast, substantial differences in 5hmC levels were found. Patients with TET2 mutations had significantly lower 5hmC levels compared with patients without TET2 or IDH mutations (P < .001) (Figure 2C). Patients with 2 TET2 aberrations showed a significantly lower 5hmC than patients with a single affected allele (P = .003) (Figure 2D). Furthermore, 5hmC values were decreased in patients with IDH1/2 mutations compared with TET2/IDH wild-type patients (P < .001) (Figure 2C). Comparison of the 3 different IDH mutational hotspots revealed a slightly lower 5hmC in the subgroup of patients with IDH2 R172 mutations (n = 4) (Figure 2D).
5hmC levels are decreased in TET2 and IDH mutated patients. (A) Plasma 2HG levels measured by LC-MS/MS were up to 150 times higher in patients with IDH mutations compared with IDH wild-type patients. (B) Small differences in 5mC values were present between AML patients. Patients with IDH mutations showed higher 5mC levels than TET2 mutated and TET2/IDH wild-type patients. (C) Patients with TET2 or IDH1/2 mutations showed significantly lower 5hmC levels than TET2/IDH wild-type patients. (D) Patients with 2 affected TET2 alleles showed lower 5hmC values than patients with only 1 affected allele. All types of IDH mutations led to decreased 5hmC levels. The 4 patients with an IDH2 R172 mutation had slightly lower 5hmC values than most patients with an IDH1 R132 or IDH2 R140 mutation. (E) DNMT3A mutations frequently co-occurred with TET2 or IDH mutations. DNMT3A mutations did not significantly affect global 5mC levels. (F) Mutant DNMT3A also did not strongly affect 5hmC values, but the levels were influenced by the presence or absence of accompanying TET2 or IDH mutations. Mut, mutated; wt, wild type. Symbols indicate unpaired t test (*), Mann-Whitney U test (†), 1-way analysis of variance (‡), and Kruskal Wallis test (§).
5hmC levels are decreased in TET2 and IDH mutated patients. (A) Plasma 2HG levels measured by LC-MS/MS were up to 150 times higher in patients with IDH mutations compared with IDH wild-type patients. (B) Small differences in 5mC values were present between AML patients. Patients with IDH mutations showed higher 5mC levels than TET2 mutated and TET2/IDH wild-type patients. (C) Patients with TET2 or IDH1/2 mutations showed significantly lower 5hmC levels than TET2/IDH wild-type patients. (D) Patients with 2 affected TET2 alleles showed lower 5hmC values than patients with only 1 affected allele. All types of IDH mutations led to decreased 5hmC levels. The 4 patients with an IDH2 R172 mutation had slightly lower 5hmC values than most patients with an IDH1 R132 or IDH2 R140 mutation. (E) DNMT3A mutations frequently co-occurred with TET2 or IDH mutations. DNMT3A mutations did not significantly affect global 5mC levels. (F) Mutant DNMT3A also did not strongly affect 5hmC values, but the levels were influenced by the presence or absence of accompanying TET2 or IDH mutations. Mut, mutated; wt, wild type. Symbols indicate unpaired t test (*), Mann-Whitney U test (†), 1-way analysis of variance (‡), and Kruskal Wallis test (§).
Effect of DNMT3A mutations on global 5mC and 5hmC levels
Because the DNMT3A protein forms 5mC, we investigated whether mutations in DNMT3A would affect global 5mC and 5hmC levels. DNMT3A exons 7 to 23 were sequenced in 79 patients (supplemental Table 8). Interestingly, DNMT3A mutations frequently co-occurred with TET2 or IDH mutations; 64% of TET2 mutated and 63% of IDH mutated patients harbored a DNMT3A mutation. The frequent co-occurrence suggests that an additive oncogenic effect may result from the combination of these mutations. When patients were divided into different subgroups based on co-occurring mutations, mutant DNMT3A alone had no strong effect on global 5mC levels (Figure 2E). The detected difference between means (DNMT3A/TET2/IDH wild-type vs DNMT3A mutant group) was 0.03 and was not significant (the minimal detectable difference in our cohort was 0.29%5mC/G with a power of 80%). In addition, DNMT3A mutations also did not significantly affect global 5hmC values (Figure 2F). The observed difference between means was 0.0056 (the minimal detectable difference with a power of 80% was 0.016% 5hmC/G).
High 5hmC levels confer poor prognosis in AML
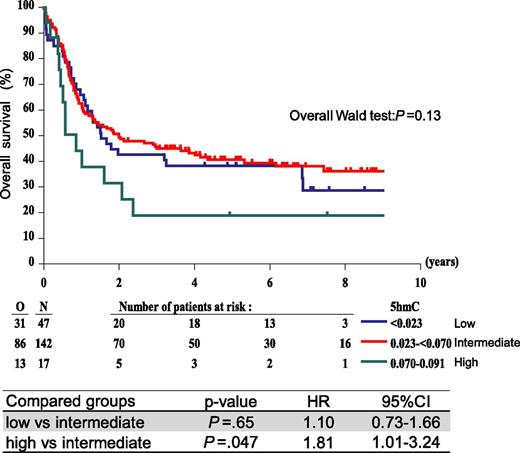
In AML, a wide range of 5hmC levels was observed when compared with healthy control cells (Figure 1A). As the 5hmC values were not normally distributed, we used a mixture model analysis to divide the patient cohort in subgroups. Three subpopulations were identified: low 5hmC (0.006 to <0.023), intermediate 5hmC (0.023 to <0.070), and high 5hmC (0.070-0.091) (see supplemental Table 10 for patient characteristics). We questioned whether 5hmC levels correlated with OS by examining the survival of these 3 groups. Univariate analysis showed that the low and intermediate 5hmC groups had comparable 5-year OS rates (38.3% vs 40.7%) (Figure 3). Interestingly, the high 5hmC group showed a considerably lower 5-year OS rate compared with the intermediate group (18.9% vs 40.7%) (Figure 3) resulting in a significant difference in OS (P = .047, HR = 1.81, 95% CI = 1.01-3.24). A similar trend was observed when event-free survival was analyzed (supplemental Table 11). Multivariate Cox regression analysis showed that, when adjusted for cytogenetic/FLT3-ITD features, high 5hmC levels independently predicted poor OS (high vs intermediate 5hmC: P = .01, HR = 2.10, 95% CI = 1.16-3.80) (Table 2).
OS according to 5hmC level. Patients were divided into 3 groups based on a mixture model analysis. Univariate analysis indicates that patients with high 5hmC levels (0.070-0.091) showed a poor OS compared with patients with low (<0.023) and intermediate (0.023 to <0.070) 5hmC.
OS according to 5hmC level. Patients were divided into 3 groups based on a mixture model analysis. Univariate analysis indicates that patients with high 5hmC levels (0.070-0.091) showed a poor OS compared with patients with low (<0.023) and intermediate (0.023 to <0.070) 5hmC.
Multivariate Cox regression analysis
| Parameter . | P value . | HR . | 95% HR . | |
|---|---|---|---|---|
| Confidence limits . | ||||
| 5hmC: <0.023 vs 0.023 to <0.070 | .44 | 1.18 | 0.77 | 1.81 |
| 5hmC: ≥0.070 vs 0.023 to <0.070 | .01 | 2.10 | 1.16 | 3.80 |
| Cytogenetics/FLT3-ITD: good vs intermediate | .21 | 0.60 | 0.25 | 1.36 |
| Cytogenetics/FLT3-ITD: others vs intermediate | .17 | 1.44 | 0.86 | 2.39 |
| Cytogenetics/FLT3-ITD: poor vs intermediate | <.0001 | 3.00 | 1.90 | 4.68 |
| Cytogenetics/FLT3-ITD: unknown vs intermediate | .02 | 2.96 | 1.21 | 7.21 |
| Parameter . | P value . | HR . | 95% HR . | |
|---|---|---|---|---|
| Confidence limits . | ||||
| 5hmC: <0.023 vs 0.023 to <0.070 | .44 | 1.18 | 0.77 | 1.81 |
| 5hmC: ≥0.070 vs 0.023 to <0.070 | .01 | 2.10 | 1.16 | 3.80 |
| Cytogenetics/FLT3-ITD: good vs intermediate | .21 | 0.60 | 0.25 | 1.36 |
| Cytogenetics/FLT3-ITD: others vs intermediate | .17 | 1.44 | 0.86 | 2.39 |
| Cytogenetics/FLT3-ITD: poor vs intermediate | <.0001 | 3.00 | 1.90 | 4.68 |
| Cytogenetics/FLT3-ITD: unknown vs intermediate | .02 | 2.96 | 1.21 | 7.21 |
Akaike information criterion for this model = 1238.
Addition of age, treatment arm (data not shown), and/or percentage BM blasts (supplemental Table 12) in the model did not improve its prognostic discrimination value. Considering 5hmC as a continuous variable in the model (linear and quadratic effect), and adjusting for cytogenetic/FLT3-ITD risk groups, provided similar results as the model indicated above (supplemental Table 12).
Correlation of 5hmC levels with clinical and molecular parameters
As shown in Figure 2C, low 5hmC levels could in many patients be explained by TET or IDH mutations. In patients without TET2 or IDH mutations, 5hmC levels still varied considerably. Therefore, we examined whether the range of 5hmC values in patients without TET2 or IDH mutations correlated with specific clinical or molecular parameters. There was no significant correlation between 5hmC levels and sex, age, initial performance status, white blood cell count, or cytogenetic categories (supplemental Figure 5). Furthermore, we assessed whether 5hmC levels correlated with the percentage of blasts at diagnosis, because it has been shown that stem cells may contain more 5hmC than differentiated cells.46 A positive correlation was present between 5hmC levels and the percentage of blasts in BM (P = .003) and PB (P = .02) at diagnosis (supplemental Figure 6). The percentage of BM blasts did, however, not appear to be of prognostic importance for OS, neither in uni- nor in multivariate analysis. When blast percentage was included in the multivariate model (supplemental Table 12, model 2), high 5hmC remained an independent poor prognostic factor, thereby showing that 5hmC is not a surrogate marker for percentage of BM blasts. To investigate whether the level of TET expression affected the level of 5hmC in AML, we measured mRNA expression of TET1 (n = 31), TET2 (isoform 1 and 2, n = 109), and TET3 (n = 31) in patients without TET2 or IDH mutations. For all TET family members, no strong correlation was found with the level of 5hmC (supplemental Figure 7). Finally, we considered whether 5hmC levels correlated with other specific molecular aberrations. Interestingly, MLL aberrations and AML1-ETO translocations were enriched in patients with high levels of 5hmC (Figure 4). Of note, there was one patient who carried an IDH2 mutation together with an MLL translocation. Of all patients with MLL aberrations, this patient showed the lowest value of 5hmC, possibly indicating that the effect of IDH mutations (correlating with low 5hmC) overrides the effect of MLL translocations (correlating with high 5hmC).
Molecular markers in different groups of 5hmC levels. Patients are ranked from low to high 5hmC levels, and the different 5hmC groups used for the OS analysis are indicated. Per patient, all mutations are indicated with small colored boxes. Light gray boxes represent no mutation and dark gray boxes not determined/unknown. TET2 and IDH mutations are present in patients with low 5hmC, whereas AML1-ETO and MLL translocations are more frequently found in patients with high 5hmC levels. ITD, internal tandem duplication; transl, translocation.
Molecular markers in different groups of 5hmC levels. Patients are ranked from low to high 5hmC levels, and the different 5hmC groups used for the OS analysis are indicated. Per patient, all mutations are indicated with small colored boxes. Light gray boxes represent no mutation and dark gray boxes not determined/unknown. TET2 and IDH mutations are present in patients with low 5hmC, whereas AML1-ETO and MLL translocations are more frequently found in patients with high 5hmC levels. ITD, internal tandem duplication; transl, translocation.
Increased P15 promoter methylation in patients with low 5hmC levels
We examined the methylation status of 2 genes that are frequently hypermethylated in AML (P15 and MyoD)47 in 10 patients with low and 10 with high 5hmC levels. The majority of patients with low 5hmC (8/10) showed considerable methylation on the P15 promoter compared with only 2/10 patients with high 5hmC levels (at least 1 CpG >50% methylated) (Figure 5A), which suggests that demethylation of the P15 promoter is altered in most patients with low 5hmC. In contrast, for MyoD no difference in promoter methylation was observed between the low and high 5hmC groups (Figure 5B), indicating that this correlation is promoter specific. Therefore, genome-wide analysis should be performed to determine which genes are differentially methylated between the low and high 5hmC groups.
P15 and MyoD promoter methylation.P15 (A) and MyoD (B) promoter methylation were determined in 10 patients with low and 10 with high 5hmC levels. For each CpG, the methylation status is indicated with shaded boxes. Patients with low 5hmC show more P15 promoter methylation than patients with high 5hmC levels, whereas there is no difference in MyoD promoter methylation. UPN, unique patient number.
P15 and MyoD promoter methylation.P15 (A) and MyoD (B) promoter methylation were determined in 10 patients with low and 10 with high 5hmC levels. For each CpG, the methylation status is indicated with shaded boxes. Patients with low 5hmC show more P15 promoter methylation than patients with high 5hmC levels, whereas there is no difference in MyoD promoter methylation. UPN, unique patient number.
Discussion
In this study, we correlated global 5(h)mC levels with various clinical and molecular parameters in a large, prospectively collected cohort of younger adult AML patients (age ≤60 years) included in the EORTC/GIMEMA AML-12 clinical trial. No major differences in global 5mC levels were observed between patients, but 5hmC levels were distributed over a wide range. In remission, 5hmC values were normalized to levels comparable with healthy BM and PB, indicating that the aberrant 5hmC levels at diagnosis were an intrinsic property of the leukemic cells. In line with previous reports, mutations in TET2 and IDH1/2 were mutually exclusive,19,23 and patients with these mutations displayed significantly decreased 5hmC levels.24-27 Among the patients that did not harbor TET2 or IDH mutations, a wide variation in 5hmC levels was still observed.
Remarkably, IDH or TET2 mutations co-occurred frequently with mutations in DNMT3A, indicating that they may cooperate during malignant transformation. The majority of DNMT3A mutations are heterozygous missense mutations affecting one specific amino acid (R882), but nonsense and frameshift mutations have also been described.48 We did not observe significant effects of DNMT3A mutations on global 5mC levels in patients with either mono- and bi-allelic mutations. This suggests that there is still enough remaining DNMT protein (DNMT3B, DNMT1, and in most cases a remaining DNMT3A allele) to form most of the 5mC marks. In addition, we did not find strong correlations between global 5mC levels and survival (supplemental Figure 8). This lack of correlation does not necessarily mean that 5mC levels are not relevant but may rather point to the fact that global DNA methylation patterns obscure local effects at specific genes. To identify the important loci for (deregulated) 5mC levels, more targeted strategies, such as MeDIP, should be used.
TET2 and IDH mutations both lead to defective conversion of 5mC into 5hmC, which may result in impaired demethylation of DNA, contributing to the malignant transformation of cells. Using bisulfite sequencing, we showed a high level of P15 promoter methylation in patients with low 5hmC. However, no difference was observed for the MyoD gene, indicating that the correlation is locus specific. Genome-wide methylation and hydroxymethylation patterns should be determined to see which genes are differentially (hydroxy)methylated between patients with low and high 5hmC levels. Apart from affecting the methylation status of the DNA, other processes might be influenced by 5hmC as well. Several studies showed that the 5hmC mark may attract specific proteins like MECP2 and UHRF2.49,50 The role of these interactions is not clear yet, but protein complexes that recognize the 5hmC mark might serve as “readers” of the local epigenetic DNA code and induce further chromatin alterations.
After excluding patients with mutations in TET2 or IDH, 5hmC levels still varied considerably in the remaining patients. The cause of this broad range of 5hmC levels could not be attributed to differences in TET expression, as no strong correlation was found between TET1, 2, or 3 mRNA expression and 5hmC levels. A positive correlation was, however, observed between blast count at diagnosis and 5hmC levels. As it has been shown that stem cells contain more 5hmC than differentiated cells, this might explain the correlation.46
We assessed whether 5hmC levels correlated with OS by comparing the survival of the 3 5hmC subpopulations present in our cohort (Figure 1A). We showed that high 5hmC levels conferred a poor prognosis in both uni- and multivariate analysis. On the other hand, low 5hmC levels did not affect survival. The low 5hmC group mainly consists of patients with TET2 or IDH1/2 mutations (45 of 47 patients). TET2 mutations in AML are often associated with a poor prognosis,32-34 as was also observed in our study.18 The impact of IDH mutations on OS was less pronounced, with IDH1 mutations showing a trend toward a poor prognosis, whereas IDH2 mutations had no effect.18 The differences in OS between these 3 types of mutations indicates that low 5hmC is not the only variable that affects survival in these patients. Interestingly, AML1-ETO and MLL translocations were more frequently present in patient cells with high 5hmC levels. These findings remain to be confirmed in future studies.
Finally, it would be particularly interesting to investigate how patients with aberrant 5hmC respond to hypomethylating agents, such as decitabine and azacitidine, as these compounds have shown promising results in MDS and elderly AML.3-6 If a correlation between 5hmC levels and response to these therapeutics could be shown, this would allow a more tailored therapeutic approach.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Liv Meert and Hilde Breyssens from the EORTC Headquarters for data collection.
This work was supported by a grant from the EORTC Charitable Trust and by a grant from the Netherlands Institute for Regenerative Medicine.
Authorship
Contribution: L.I.K., M.G.A., G.H., T.d.W., R.A.W., B.A.v.d.R., and J.H.J. designed the study; C.J.M.H. and J.-P.M. provided patient material and T.C. the stabile 5hmC isotope; L.I.K., M.G.A., T.N.K.-S., and M.M. performed the expression and sequence analysis; L.I.K., T.N.K.-S., A.v.R., and R.A.W. contributed to the 2HG, 5mC, and 5hmC LC-MS/MS measurements; J.B.B. and S.S. performed statistical analysis; and J.H.J. and L.I.K. wrote the paper; and all authors discussed the results and commented on the manuscript at all stages.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. H. Jansen, Laboratory of Hematology, Department of Laboratory Medicine, Radboud University Medical Center, Radboud Institute for Molecular Life Sciences, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: joop.jansen@radboudumc.nl.
References
Author notes
L.I.K. and M.G.A. contributed equally to this study.