Key Points
ICOS-based CARs program bipolar TH17/TH1 cells with augmented effector function and in vivo persistence.
The expression of selected CAR endodomains can program T cells for their subsequent differentiation fates and effector functions.
Abstract
With the notable exception of B-cell malignancies, the efficacy of chimeric antigen receptor (CAR) T cells has been limited, and CAR T cells have not been shown to expand and persist in patients with nonlymphoid tumors. Here we demonstrate that redirection of primary human T cells with a CAR containing the inducible costimulator (ICOS) intracellular domain generates tumor-specific IL-17-producing effector cells that show enhanced persistence. Compared with CARs containing the CD3ζ chain alone, or in tandem with the CD28 or the 4-1BB intracellular domains, ICOS signaling increased IL-17A, IL-17F, and IL-22 following antigen recognition. In addition, T cells redirected with an ICOS-based CAR maintained a core molecular signature characteristic of TH17 cells and expressed higher levels of RORC, CD161, IL1R-1, and NCS1. Of note, ICOS signaling also induced the expression of IFN-γ and T-bet, consistent with a TH17/TH1 bipolarization. When transferred into mice with established tumors, TH17 cells that were redirected with ICOS-based CARs mediated efficient antitumor responses and showed enhanced persistence compared with CD28- or 4-1BB-based CAR T cells. Thus, redirection of TH17 cells with a CAR encoding the ICOS intracellular domain is a promising approach to augment the function and persistence of CAR T cells in hematologic malignancies.
Introduction
Significant progress has been achieved during the past few years demonstrating the potential for adoptive T-cell transfer to treat cancer. One of the most promising approaches is the introduction of chimeric antigen receptors (CARs) to redirect T-cell specificity with high affinity antibody-based recognition units.1 CARs are synthetic molecules containing 3 distinct modules: an extracellular target binding module, a transmembrane module that anchors the molecule into the cell membrane, and an intracellular signaling module that transmits activation signals.2 Transmembrane modules are most commonly derived from molecules involved in T-cell function such as CD8 and CD28. The intracellular module almost always contains the CD3ζ chain and other costimulatory domains linked in cis. After initial reports demonstrating safety, with disappointing clinical results,3-5 the most recent clinical results with CAR-redirected T cells show remarkable antitumor effects in patients with neuroblastoma, chronic lymphocytic leukemia, non-Hodgkin lymphoma, and acute lymphoid leukemia.6-10
Since the mid-2000s, a new effector CD4+ T helper cell subset that secretes IL-17 was discovered,11,12 and it has become clear that TH17 cells represent an independent subset of T helper cells. TH17 cells regulate host defense and exacerbate autoimmune diseases. Naturally arising endogenous TH17 cells have been found in various human tumors, however their function in cancer immunity is unclear. When adoptively transferred into tumor-bearing mice, TH17 cells have been found to be more potent at eradicating melanoma than TH1 or nonpolarized (TH0) T cells.13-15 Importantly, TH17 cells have considerable plasticity and can acquire certain type 1 characteristics (such as IFN-γ production) depending on the inflammatory conditions. The ability of TH17 cells to acquire TH1 cell-like features appears to be a prerequisite for potent antitumor activity.13 One obstacle to the use of TH17 cells for adoptive cell transfer is the identification of robust culture conditions that limit the inherent plasticity of this subset.16-18
Two properties of CAR T cells that correlate with potency are the specific lymphocyte subsets that are infused and the signaling domains of the CAR. Preclinical studies indicate that cells with extensive proliferative capacity are more potent.19-21 Adoptive transfer experiments in mice indicate that TH17 cells have higher in vivo survival and self-renewal capacity than TH1 polarized cells.14 In studies using CAR T cells, incorporation of signaling domains from CD28 or from tumor necrosis factor (TNF) family members CD137 (4-1BB) or CD134 (OX40) has been shown to prevent anergy and to enhance antitumor effects.2,22
Inducible costimulator (ICOS, also called CD278) is a member of the CD28 family. We have previously shown that ICOS, but not CD28, is necessary for optimal expansion and function of human TH17 cells.15 ICOS is constitutively expressed on TH17 cells and anti-CD3/ICOS stimulation induced RORγt and T-bet expression in these cells, leading to increased secretion of IL-17A, IL-21, and IFN-γ compared with CD3/CD28 stimulation. We herein report that TH17 cells expressing CARs bearing ICOS signaling domains exhibit enhanced stability as TH17/TH1 cells and increased persistence after transfer into tumor-bearing mice.
Materials and methods
Generation of SS1-CARs and lentivirus production
Mesothelin-specific SS1-based CARs containing the TCR-ζ signal-transduction domain alone (ζ) or in combination with the CD28 (28ζ) or the 4-1BB (BBζ) intracellular domains were generated as previously described.23 The third generation self-inactivating lentival expression vector containing the SS1-ICOSz CAR was generated as described in the supporting information text. High-titer replication-defective lentiviral vectors were produced and concentrated as previously described.24
Isolation, polarization, transduction, and expansion of TH17 and TC17 cells
Blood samples were obtained from the Human Immunology Core of the University of Pennsylvania. The study was conducted in accordance with the Declaration of Helsinki. Peripheral blood CD4+ and CD8+ T cells were negatively isolated using RosetteSep Kits (Stemcell Technologies) and cultured under TH17 conditions as previously described15 (Figure 1B). For stimulation, CD4+ and CD8+ T cells were cultured with activating beads coated with antibodies to CD3 (eBioscience, San Diego, CA) and ICOS (Japan Tobacco Inc., Japan, and eBioscience) at a 1:3 cell-to-bead ratio.
Redirection of TH17 cells with SS1-CARs. (A) Schematic representation of a panel of chimeric receptors that contain the SS1 single chain fragment and differ in the transmembrane and the intracellular domains. (B) Schematic of the experimental protocol. Peripheral blood CD4+ T cells were stimulated with antibodies to CD3/ICOS beads and cultured under TH17 polarizing conditions. Human IL-2 was added 3 days after activation. T cells were transduced with lentiviral vectors 24 hours following stimulation. After T cells rested down, they were cryopreserved. For functional assays, T cells were thawed and stimulated with surrogate antigen in media without cytokine supplementation. (C) Surface expression of the SS1 scFv fusion proteins on human CD4+ T cells at the time of functional evaluation.
Redirection of TH17 cells with SS1-CARs. (A) Schematic representation of a panel of chimeric receptors that contain the SS1 single chain fragment and differ in the transmembrane and the intracellular domains. (B) Schematic of the experimental protocol. Peripheral blood CD4+ T cells were stimulated with antibodies to CD3/ICOS beads and cultured under TH17 polarizing conditions. Human IL-2 was added 3 days after activation. T cells were transduced with lentiviral vectors 24 hours following stimulation. After T cells rested down, they were cryopreserved. For functional assays, T cells were thawed and stimulated with surrogate antigen in media without cytokine supplementation. (C) Surface expression of the SS1 scFv fusion proteins on human CD4+ T cells at the time of functional evaluation.
Cytokine production of restimulated T cells
T cells (4 × 105) were cocultured with 2 × 105 K562, K562.meso, or mesothelin-expressing tumor cells, and supernatants were harvested 24 hours later. Concentrations of IL-17A, IL17-F, IL-22, IL-2, IFN-γ, TNF-α, IL-10, IL-4, and CCL20 were determined using the DuoSet enzyme-linked immunosorbent assay (ELISA) Development Systems (R&D Systems, Minneapolis, MN). Concentrations of IL-21 were determined using Human IL-21 ELISA Ready-SET-Go! (eBioscience).
Flow cytometry
The following conjugated antibodies were purchased from eBioscience: anti-CD161 phycoerythrin (PE), Ig (PE), anti-CD8 allophycocyanin (APC), anti-CD4 PerCp-Cy5.5, anti-CD4-PE, anti-CD45 PerCp-Cy5.5, and anti-CD45 APC. Expression of the various SS1 scFv fusion proteins on T cells was detected using biotinylated goat anti-mouse IgG (specific for scFvs of murine origin) from Jackson ImmunoResearch (West Grove, PA). Streptavidin (PE) and streptavidin (APC) were purchased from BD Biosciences. Cells were stained for viability with aqua amine-reactive viability dye (Invitrogen, Frederick, MD) for 30 minutes at room temperature. Surface staining was performed at 4°C for 30 minutes in phosphate-buffered saline (PBS) supplemented with 3% fetal bovine serum. Samples were analyzed in the LSRII flow cytometer using the DiVa software (BD Biosciences, San Jose, CA), and results were evaluated using the FlowJo software (TreeStar, Eugene, OR).
Microarray studies
Redirected T cells from 3 different normal human donors were stimulated with immobilized yeast-derived recombinant mesothelin.25 Cell pellets were collected and frozen at indicated time points on antigen recognition. Gene expression levels were determined with GeneChip Human Gene 1.0 ST arrays according to manufacturer's protocols (Affymetrix, Santa Clara, CA). Description of methods and statistical analysis is included in supplemental data. (Gene Expression Omnibus accession number: GSE58867.)
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) for gene lists that were ranked by log2-fold changes were constructed, representing the expression patterns of TH17 cells expressing 3 different CARs. Analysis was performed using a modified R version of the GSEA code provided by the Broad Institute. In all cases we used a list of 74 TH17-relevant genes that was previously curated from the literature.26
Multidimensional scaling analysis
We performed multidimensional scaling (MDS) analysis on 886 differentially expressed genes among 3 CARs (P < .05, Student t test) using Euclidean distances, and the R function cmdscale() with parameters set as k = 2 dimensions, and eigen values = TRUE.
Quantitative gene expression analysis by RT-PCR
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was used to validate select microarray results, using the same RNA samples. cDNA was synthesized with the High Capacity RNA-to-cDNA Kit (Applied Biosystems). qRT-PCR was performed in duplicate using TaqMan Universal PCR Master Mix, in a ViiA 7 Real Time PCR system following manufacturer protocols. All probes used are commercially available (Applied Biosystems).
Flow cytometry-based assay to quantify cell-mediated cytolysis
Target cells (M108) were stained with CFSE and seeded at 150 000 cells/well in 48-well plates. After 16 hours, effector T cells and CFSE-labeled target cells were cocultured at multiple Effector:Target ratios for 18 hours. Total cells were trypsinized and stained for viability with aqua amine-reactive viability dye for 30 minutes. After washing, cells were stained with annexin V, and T cells were stained with an anti-CD45 antibody. Finally, cells were resuspended in 1% HuSA PBS and a known number of counting beads. Data were collected on an LSRII flow cytometer. 4000 beads were collected for each sample.
Mice
The University of Pennsylvania Institutional Animal Care and Use Committee approved all animal experiments. NOD scid gamma (NSG) mice were purchased from The Jackson Laboratory and were bred in the vivarium at the University of Pennsylvania. The mice were housed under specific pathogen-free conditions in microisolator cages and were provided ad libitum access to autoclaved food and acidified water.
In vivo assessment of anti-mesothelin CAR T cells
Xenograft tumors were established by subcutaneous injection of 5 × 106 M108 mesothelioma cells or 5 × 106 L55 nonsmall cell lung cancer cells in the presence of a 50% Matrigel solution (BD Biosciences) in PBS. Tumor dimensions were measured with calipers, and tumor volumes were calculated using the formula V = 1∕2 × L × W × W, where L is length (longest dimension) and W is width (shortest dimension). Peripheral blood was obtained from retro-orbital bleeding or intracardiac puncture and was stained for the presence of human CD45+, CD4+, and CD8+ T cells. After gating on the human CD45+ population, the CD4+ and CD8+ subsets were quantified using TruCount tubes (BD Biosciences). All experiments were performed in a blinded, randomized fashion.
Statistical analysis
All results were expressed as means ±standard deviation (SD) or standard error of the mean (SEM), as indicated. Data were analyzed with a 2-tailed Student t test using Prism (GraphPad Software).
Results
Construction and expression of ICOS-based CARs
The CAR constructs used in this study contain the SS1 scFv that recognizes human mesothelin (Figure 1A). The scFv was fused to the TCR-ζ signal transduction domain with the CD28, 4-1BB, or ICOS signaling domains in tandem. As negative control for signal transduction, we used a chimeric receptor containing a truncated form of the TCR-ζ intracellular domain (delζ). CD4+ T cells were activated with beads coated with anti-CD3 and anti-ICOS antibodies in the presence of TH17 polarizing cytokines, transduced with lentiviral vectors encoding SS1-based chimeric receptors, and expanded (Figure 1B). For all functional assays, T-cell cultures were normalized for equivalent CAR expression by the addition of mock transduced T cells as required. The surface expression of all CAR constructs was similar (Figure 1C).
In vitro function of ICOS-based CAR cells
One of the functional hallmarks of TH17 cells is the production of large amounts of IL-17A, IL-17F, and IL-22. To test the cytokine response of the mesothelin redirected T cells, we cocultured them with tumor cells that expressed mesothelin and quantified the concentrations of cytokines in the supernatants by ELISA (Figure 2A). We observed a significant enhancement of IL-17A, IL17-F, and IL-22 secretion by the ICOSζ cells, consistent with a predominantly TH17 phenotype. The secretion of IL-17 required surrogate antigen signaling because cells expressing a CAR with a truncated ζ chain did not secrete IL-17 (data not shown). Importantly, the addition of the CD28 or the 4-1BB costimulatory domains to CD3ζ domain impaired expression of TH17 cytokines, whereas the addition of the ICOS domain significantly increased IL-17A production. In contrast, no differences in IL-17A secretion were observed when T cells were stimulated through their native TCR receptor (Figure 2B), confirming that differences observed in IL-17A secretion following antigen encounter require signaling through CAR costimulatory domains. The enhancement of IL-17A secretion following ligation of the ICOSζ CAR was consistently observed on T-cell exposure to cancer cells derived from various tissues including lung, mesothelioma, pancreas, ovary, and breast (Figure 2C), and when using ICOSζ−based CARs with distinct antigen specificities (supplemental Figure 1; available on the Blood Web site). In addition, ICOSζ CART cells cultured under TH17 polarizing conditions, but stimulated with anti-CD3/CD28 beads rather than anti-CD3/ICOS beads, showed enhanced IL-17A secretion when compared with ζ alone, 28ζ-, and BBζ-redirected T cells, although the amount of IL-17A secreted was 20-fold lower (Figure 2D). These TH17 cells expressed similar levels of IFN-γ compared with cells preexpanded with anti-CD3/ICOS beads.
TH17 cells redirected with an ICOS-based CAR release high amounts of IL17-A, IL-17F, and IL-22 but low amounts of IL-2. (A) TH17 cells transduced with various CARs were cocultured with surrogate antigen, APC cells transduced with mesothelin (K562meso). Supernatants from several different healthy donors (n = 4-9) were obtained 24 hours after coculture, and cytokine production was analyzed by ELISA. Box plots show median (line) and 25th to 75th percentile (box). The end of the whiskers represents the minimum and the maximum of all of the data. (B) Redirected TH17 cells were stimulated with plate bound anti-CD3 (OKT3). IL-17A was analyzed by ELISA 24 hours after stimulation. Data represent the means ±SD for 2 different normal donors. (C) TH17 cells were cultured with the indicated tumor cells expressing mesothelin. IL-17A and IL-2 production were analyzed by ELISA 24 hours on antigen recognition. Error bars indicate SD in duplicate samples. Results are representative of at least 2 different experiments. (D) CD4+ T cells from different normal donors (n = 3) were activated with anti-CD3/CD28 beads, cultured under TH17 polarizing conditions, and redirected with the different CARs. After their primary expansion, CD4+ T cells were cultured with K562meso cells. Secretion of IL17-A and INF-γ production was analyzed by ELISA 24 hours on antigen recognition. Error bars indicate SD. *P < .05, **P < .01, and ***P < .001.
TH17 cells redirected with an ICOS-based CAR release high amounts of IL17-A, IL-17F, and IL-22 but low amounts of IL-2. (A) TH17 cells transduced with various CARs were cocultured with surrogate antigen, APC cells transduced with mesothelin (K562meso). Supernatants from several different healthy donors (n = 4-9) were obtained 24 hours after coculture, and cytokine production was analyzed by ELISA. Box plots show median (line) and 25th to 75th percentile (box). The end of the whiskers represents the minimum and the maximum of all of the data. (B) Redirected TH17 cells were stimulated with plate bound anti-CD3 (OKT3). IL-17A was analyzed by ELISA 24 hours after stimulation. Data represent the means ±SD for 2 different normal donors. (C) TH17 cells were cultured with the indicated tumor cells expressing mesothelin. IL-17A and IL-2 production were analyzed by ELISA 24 hours on antigen recognition. Error bars indicate SD in duplicate samples. Results are representative of at least 2 different experiments. (D) CD4+ T cells from different normal donors (n = 3) were activated with anti-CD3/CD28 beads, cultured under TH17 polarizing conditions, and redirected with the different CARs. After their primary expansion, CD4+ T cells were cultured with K562meso cells. Secretion of IL17-A and INF-γ production was analyzed by ELISA 24 hours on antigen recognition. Error bars indicate SD. *P < .05, **P < .01, and ***P < .001.
In contrast to the enhanced secretion of TH17 cytokines by the ICOSζ cells, we found that only 28ζ CAR T cells secreted robust amounts of IL-2 after coculture with mesothelin-expressing targets (Figure 2A,C). 28ζ cells secreted significantly more TNF-α and consistently more IL-21 (although significance was not reached because of donor variability) than cells transduced with the other CARs. This is consistent with studies showing that stimulation by natural ligand or agonistic antibodies to CD28 induces IL-2 and IL-21 secretion, whereas ligation of the endogenous ICOS molecule does not.27 Cells expressing either ICOSζ or 28ζ CARs secreted similar amounts of CCL20, IFN-γ, and IL−10, whereas cells expressing the BBζ CAR produced low-level secretion of all cytokines. IL-4 secretion could not be detected in any of the conditions tested.
Surface phenotype of ICOS-based CAR T cells
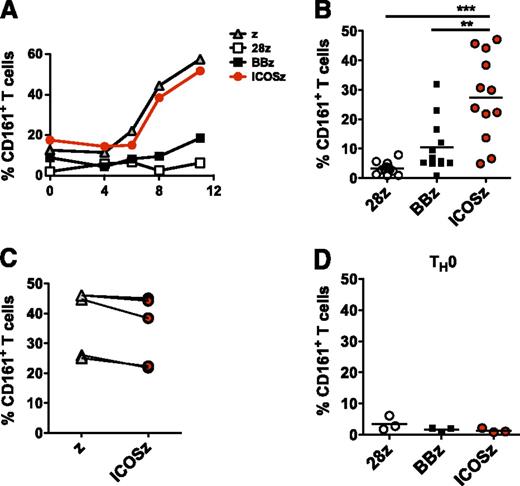
Human TH17 cells can be identified by their surface expression of the lectin-like receptor CD161/NKRP1A, a marker of all human IL-17-producing T-cell subsets.34 Therefore, we measured the frequency of CD161+ T cells after surrogate antigen recognition. We observed a progressive enrichment of CD161+ cells in the ζ and ICOSζ CAR cultures. In contrast, cells expressing CD161 were not enriched in the cultures containing BBζ or 28ζ CAR T cells (Figure 3A). This enrichment was reproducible, as it was observed in ICOSζ CAR expressing T cells from 12 healthy donors (Figure 3B). In contrast to TH17 cytokine production, no differences in CD161 expression were observed between cells redirected with ζ or ICOSζ CARs (Figure 3C), indicating that signaling through 4-1BB and CD28 are detrimental for the expansion of CD161+ cells, whereas ICOS is not necessary but not detrimental. Enrichment required initial exposure to TH17 polarizing conditions, as T cells transduced with the panel of CARs under nonpolarizing conditions did not exhibit subsequent enrichment for CD161 expressing cells when cultured with mesothelin-expressing tumor cells (Figure 3D).
TH17 cells redirected with ICOS endodomain show increased expression of CD161. Redirected TH17 cells were cocultured with irradiated APC expressing mesothelin. (A) CD161 expression by CAR+CD4+ T cells in response to mesothelin-specific stimulation was analyzed by flow cytometry at indicated time points. The percentage of CAR+ TH17 (B-C) or TH0 (D) cells expressing CD161 at day 8 after stimulation in several different normal donors is plotted. **P < .01 and ***P < .001.
TH17 cells redirected with ICOS endodomain show increased expression of CD161. Redirected TH17 cells were cocultured with irradiated APC expressing mesothelin. (A) CD161 expression by CAR+CD4+ T cells in response to mesothelin-specific stimulation was analyzed by flow cytometry at indicated time points. The percentage of CAR+ TH17 (B-C) or TH0 (D) cells expressing CD161 at day 8 after stimulation in several different normal donors is plotted. **P < .01 and ***P < .001.
Transcriptional signature of T cells expressing ICOS-based CARs
The enhanced expression of CD161 together with the selective secretion of IL-17 suggests that the ICOSζ CAR can be used to maintain a TH17 cell phenotype. To characterize fully the spectrum of Th17-related markers in our cells, we next used gene expression analysis to perform an unbiased characterization of the effects of the CAR signaling domains on T-cell activation after antigen recognition. Redirected CD4+ T cells from 3 healthy donors were restimulated with surrogate antigen using immobilized recombinant mesothelin-Fc and gene expression levels determined before activation and at multiple time points after stimulation. Signaling through CARs induced the differential expression of 886 genes compared with resting T cells at 4 hours on antigen recognition (false discovery rate [FDR] <0.05, >twofold change [FC] in expression). MDS analysis of these 886 genes showed that T cells redirected with ICOSζ and 28ζ signaling domains presented a closely related gene expression profile, with only 15 differentially expressed genes (Figure 4A). By contrast, BBζ T cells exhibited a distinct gene expression profile, with 163 and 140 differentially expressed genes when compared with ICOSζ and 28ζ T cells, respectively. Supplemental Tables I-VIII display all genes differentially expressed in ICOSζ CAR CD4+ T cells vs 28ζ and BBζ CAR T cells at different time points following stimulation. Supplemental Figures 2-4 show the expression of selected genes.
Transcriptional signature of T cells expressing ICOS-based CARs. Redirected TH17 cells from 3 different human normal donors were stimulated with immobilized recombinant mesothelin. Gene expression levels were determined previous to stimulation (day 0) and 4, 8, 24, and 96 hours on antigen recognition. (A) MDS analysis of 886 differentially expressed genes between the 3 sets of CAR T cells at 4 hours on activation show 3 distinct clusters. The number of differentially expressed genes (FDR < 0.05, FC > 2) between a pair of CARs is indicated with pair-connecting arrows. (B) Relative log2 expression of Il17a at indicated time points on antigen recognition. #FDR < 0.05, FC > 2 compared with both 28ζ and BBζ. The cytokine levels of IL-17A were validated by ELISA. Error bars represent SEM (3 different normal donors). *P < .05, **P < .01. (C) Expression of genes that were found significantly upregulated in ICOSζ T cells compared with both 28ζ and BBζ T cells was validated in at least 1 of the 3 healthy donors by quantitative reverse transcription polymerase chain reaction (qRT-PCR) at indicated time points and shown as ratio vs β-actin. (D) The significant regulatory inputs for Ncs1 identified in the TH17 differentiation network model are displayed. Blue and orange nodes depict the extent of TH17 upregulation and downregulation, respectively (compared with a TH0 control). Edges connecting 2 genes are green for activation and red for repression. Edges emitting from IRF4, STAT3, BATF, MAF, and Fosl2 are supported by both TF ChIP-seq and TF knockout RNA-seq and thus should be considered as validated. Edges emitted from other TFs were inferred from transcriptomic analysis of a large compendium of mouse immune cells and thus should be considered as likely but not validated.
Transcriptional signature of T cells expressing ICOS-based CARs. Redirected TH17 cells from 3 different human normal donors were stimulated with immobilized recombinant mesothelin. Gene expression levels were determined previous to stimulation (day 0) and 4, 8, 24, and 96 hours on antigen recognition. (A) MDS analysis of 886 differentially expressed genes between the 3 sets of CAR T cells at 4 hours on activation show 3 distinct clusters. The number of differentially expressed genes (FDR < 0.05, FC > 2) between a pair of CARs is indicated with pair-connecting arrows. (B) Relative log2 expression of Il17a at indicated time points on antigen recognition. #FDR < 0.05, FC > 2 compared with both 28ζ and BBζ. The cytokine levels of IL-17A were validated by ELISA. Error bars represent SEM (3 different normal donors). *P < .05, **P < .01. (C) Expression of genes that were found significantly upregulated in ICOSζ T cells compared with both 28ζ and BBζ T cells was validated in at least 1 of the 3 healthy donors by quantitative reverse transcription polymerase chain reaction (qRT-PCR) at indicated time points and shown as ratio vs β-actin. (D) The significant regulatory inputs for Ncs1 identified in the TH17 differentiation network model are displayed. Blue and orange nodes depict the extent of TH17 upregulation and downregulation, respectively (compared with a TH0 control). Edges connecting 2 genes are green for activation and red for repression. Edges emitting from IRF4, STAT3, BATF, MAF, and Fosl2 are supported by both TF ChIP-seq and TF knockout RNA-seq and thus should be considered as validated. Edges emitted from other TFs were inferred from transcriptomic analysis of a large compendium of mouse immune cells and thus should be considered as likely but not validated.
Our results in stimulating T cells through the CAR signaling domains are similar to previous studies after ICOS and CD28 costimulation through the endogenous receptors.27 We identified only 2 genes that were markedly upregulated by CD28ζ-expressing T cells, compared with both ICOSζ and BBζ CAR T cells: IL-2 and IL-8 (supplemental Figure 2). In contrast, we found 11 genes that were differentially upregulated in ICOSζ cells compared with both 28ζ and BBζ T cells. Of note, 5 of the 11 genes upregulated in the ICOSζ cells were TH17-associated transcripts, such as Il17a, Il17f, Ccl20, Il1r1, and Klrb1 (which encodes CD161) (Figure 4B-C and supplemental Figure 3). As predicted by the cytokine secretion data, Il17a was the most differentially expressed transcript in ICOSζ-redirected cells, and was induced 16-fold (4 hours vs 0 hours) (Figure 4B). Other genes that were preferentially activated by ICOS include Thbs1, Xcl2, Fam184a, Cldn1, and klrk1, although their specific roles for TH17 biology are currently unknown. Differences in gene expression observed by microarray analysis were validated independently using ELISA or RT-PCR analysis (Figure 4B-C).
The TH17 developmental regulatory network in the mouse has been characterized,26 and it was shown that a gene's involvement in TH17 function can be predicted using a network-based “KCRI” score.26 This score quantifies the extent to which each gene is regulated by 5 TH17 core transcription factors (TFs): Stat3, Batf, Irf4, Maf, and Rorc. Using this network resource, we tested whether the 6 uncharacterized genes we identified were regulated by the 5 core TH17 TFs. We found that Ncs1 was significantly upregulated in TH17 cells compared with TH0 cells, and was ranked 46 out of more than 22 000 tested genes for TH17 function based on the “KCRI” scoring scheme, similar to well-established TH17 genes including: Il17a (ranks in parentheses; 6), Il23r (7), Il17f (8), Rorc (11), Il1r1 (19), Ahr (107), and Ccl20 (143). Figure 4D shows the significant regulatory inputs for Ncs1 identified in the TH17 differentiation network model.
CD4+ T cells redirected with ICOSζ showed a TH17 core molecular signature with a TH1 bias
We next performed GSEA of the microarray data obtained using cells at 4 hours following antigen recognition (Figure 5A). We observed a nonrandom distribution of genes associated with TH17 cells at the top of the ranked gene list for all groups. Cells expressing the ICOSζ CAR had the highest maximal enrichment score (ES) of 0.784, followed by 28ζ and BBζ, confirming that redirection of TH17 with an ICOSζ-based CAR can be used to sustain a TH17 core molecular signature.
TH17 cells redirected with ICOS endodomain showed increased expression of TH17/TH1-related genes. (A) GSEA for gene lists ranked by log2-fold change capturing expression patterns from TH17 cells with 3 different CAR signaling domains. Shaded area around each line reflects the mean ES ±1 SD for each corresponding CAR from 3 donor replicates, respectively. The verticle red lines indicate where in the ranked list human orthologous of 74 literature-derived TH17-related mouse genes were recovered. Blue line shows the performance of 10 000 randomly permuted gene sets in the ranked gene list from TH17 cells with ICOS. (B) Heat map of log2-fold change in expression of T helper signature genes at 4 hours relative to 0 hours. (C-D) Expression of selected genes was measured by qRT-PCR and was expressed relative to β-actin. The cytokine levels of IFN-γ were validated by ELISA. *P < .05, **P < .01.
TH17 cells redirected with ICOS endodomain showed increased expression of TH17/TH1-related genes. (A) GSEA for gene lists ranked by log2-fold change capturing expression patterns from TH17 cells with 3 different CAR signaling domains. Shaded area around each line reflects the mean ES ±1 SD for each corresponding CAR from 3 donor replicates, respectively. The verticle red lines indicate where in the ranked list human orthologous of 74 literature-derived TH17-related mouse genes were recovered. Blue line shows the performance of 10 000 randomly permuted gene sets in the ranked gene list from TH17 cells with ICOS. (B) Heat map of log2-fold change in expression of T helper signature genes at 4 hours relative to 0 hours. (C-D) Expression of selected genes was measured by qRT-PCR and was expressed relative to β-actin. The cytokine levels of IFN-γ were validated by ELISA. *P < .05, **P < .01.
Because it has been suggested that TH17 cells must acquire TH1 cell-related features to eradicate tumors in mice,13 we next analyzed whether signaling through ICOS could induce the expression of TH1-related genes, as well as TH2- and Treg-related genes. Heat map representation of log2-fold changes of T helper signature gene expression at 4 hours after stimulation relative to nonstimulated cells demonstrated that TH17 cells redirected with ICOSζ highly overexpressed TH17-related genes, including Rorc, Il22, and Il26 (Figure 5B-C). Notably, they also overexpressed some TH1-related genes, including Ifnγ, Tnfα, and Tbx21 (T-bet) (Figure 5B-D). Importantly, ICOS signaling in TH17 cells did not induce the expression of Treg- or TH2-related genes, with the exception of Il13 (Figure 5B). Also, contrary to cytokine and transcription-factor expression, signaling through CAR did not induce significant changes in expression of most cell surface markers, including Ccr6, Ccr4, and Ccr10 (supplemental Figure 4A). Collectively, these results suggest that ICOS-based CARs can sustain TH17 function with a TH1 bias.
Comparison of antitumor efficacy of TH17/TH1 polarized T cells expressing CD28, 4-1BB, and ICOS-based CARs
We next analyzed the antitumor activity of retargeted TH17 cells. For this experiment, in addition to redirected TH17 cells, we also redirected TC17 cells (CD8+ T cells cultured under TH17 polarizing conditions).28 Both TH17 and TC17 redirected cells efficiently lysed mesothelioma (M108) tumor cells, and no differences in cytotoxicity were observed between CAR signaling domains (Figure 6A). TC17 cells secreted robust amounts of IFN-γ following antigen recognition, although IL-17 secretion was not detected (supplemental Figure 5). Next, the antitumor activity of mixed redirected TH17 and TC17 cells was analyzed in NSG mice bearing M108 tumors (Figure 6B-C). A potent antitumor effect was observed in all groups treated with mesothelin-specific CAR T cells. T cells redirected with BBζ showed a relatively slow tumor decline, with all mice showing significant tumor shrinkage after treatment. 28ζ and ICOSζ treatments showed a more rapid tumor decline, leading to tumor eradication in most mice (Figure 6B and supplemental Figure 6). Treated mice were sacrificed on day 51 after T-cell injection as they developed xenogeneic graft-versus-host disease (GVHD), and mice treated with the ICOSζ CAR T cells had the most severe GVHD (supplemental Figure 7). We have previously shown that this tumor does not regress following injection of nongene-modified allogeneic T cells.23
TH17 /TC17 cells redirected with mesothelin-specific CARs eradicate advanced preestablished tumors. (A) TC17 and TH17 cells were cocultured with M108 target cells stained with CFSE for 18 hours at the indicated effector-target (E:T) ratios. Specific cytolysis was determined using a flow cytometry-based assay. Percent specific lysis was calculated as [experimental release (cpm) − spontaneous release (cpm)]/[maximal release (cpm) − spontaneous release (cpm)] × 100. Error bars indicate SD in duplicate samples. Representative of 2 different experiments. (B) As shown schematically, human primary M108 tumors were established in the flanks of NSG mice. After 8 weeks, when the tumors reached a large volume of 500 mm3, mice were treated with 2 intratumoral injections of 10 × 106 TH17 and TC17 cells (80% and 60% CAR+) or PBS on days 0 and 7. (C) Tumor volume was analyzed at indicated time points. Results are expressed as a mean tumor volume (± SD) with n = 9 for all groups. Representative of 2 different experiments.
TH17 /TC17 cells redirected with mesothelin-specific CARs eradicate advanced preestablished tumors. (A) TC17 and TH17 cells were cocultured with M108 target cells stained with CFSE for 18 hours at the indicated effector-target (E:T) ratios. Specific cytolysis was determined using a flow cytometry-based assay. Percent specific lysis was calculated as [experimental release (cpm) − spontaneous release (cpm)]/[maximal release (cpm) − spontaneous release (cpm)] × 100. Error bars indicate SD in duplicate samples. Representative of 2 different experiments. (B) As shown schematically, human primary M108 tumors were established in the flanks of NSG mice. After 8 weeks, when the tumors reached a large volume of 500 mm3, mice were treated with 2 intratumoral injections of 10 × 106 TH17 and TC17 cells (80% and 60% CAR+) or PBS on days 0 and 7. (C) Tumor volume was analyzed at indicated time points. Results are expressed as a mean tumor volume (± SD) with n = 9 for all groups. Representative of 2 different experiments.
CAR TH17 cells reprogramed with ICOS endodomains have long-lived persistence in vivo
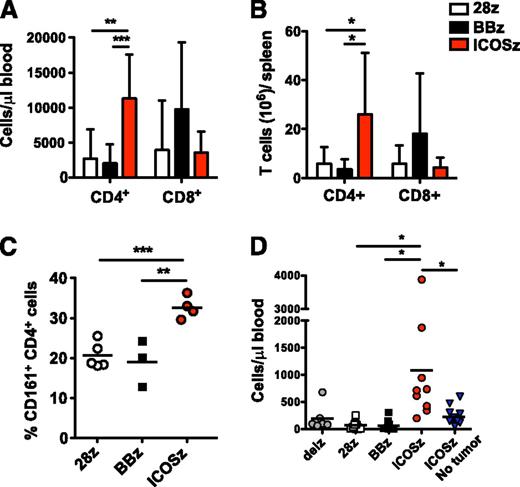
Long-term persistence of adoptively transferred T cells correlates with antitumor effects in clinical trials.29,30 Because it has been shown that natural TH17 cells are long lived in vivo,14 we next sought to assess the in vivo persistence of CAR-modified TH17 and TC17 cells in the peripheral blood of treated mice (Figure 7A-B). The average number of CD4+ T cells per µL of blood and per spleen at the time of sacrifice (day 51) was higher in mice treated with ICOSζ-based CARs than when compared with 28ζ and BBζ groups. Of note, TH17 cells redirected with ICOS also showed a higher frequency of CD161+ cells, correlating with in vitro results (Figure 7C). CD8+ T cells redirected with BBζ persisted better, although differences were not significant (Figure 7A).
TH17 cells programmed with an ICOS-based CAR show enhanced persistence that is dependent on antigen encounter. (A) NSG mice from the experiment shown in Figure 6, which were treated with intratumoral injections of redirected TH17 /TC17 cells, were killed on day 51 after T-cell infusion. Peripheral blood was quantified for the presence of human CD4+ and CD8+ T cells by a FACS Trucount assay. Results are expressed as a mean absolute T-cell count per μL of peripheral blood ± SD (n = 9 for all groups). Representative of 2 different experiments. (B-C) Redirected T cells were obtained from mouse spleens on day 51 after treatment. The number of CD4+ and CD8+ T cells (B) and the percentage of CD161+ CAR+ CD4+ T cells (C) were analyzed by flow cytometry. Error bars represent SD. (D) Human L55 non–small cell lung cancers were established in the flanks of NSG mice. After 3 weeks, when the tumors reached a volume of 150 mm3, mice without tumors (empty mice) or with preestablished tumors were treated with 2 IV injections of 10 × 106 TH17 /TC17 cells. The concentration of CD4+ T cells was determined in the blood of treated animals 3 weeks postinfusion. *P < .05, **P < .01, and ***P < .001. Representative of 1 experiment.
TH17 cells programmed with an ICOS-based CAR show enhanced persistence that is dependent on antigen encounter. (A) NSG mice from the experiment shown in Figure 6, which were treated with intratumoral injections of redirected TH17 /TC17 cells, were killed on day 51 after T-cell infusion. Peripheral blood was quantified for the presence of human CD4+ and CD8+ T cells by a FACS Trucount assay. Results are expressed as a mean absolute T-cell count per μL of peripheral blood ± SD (n = 9 for all groups). Representative of 2 different experiments. (B-C) Redirected T cells were obtained from mouse spleens on day 51 after treatment. The number of CD4+ and CD8+ T cells (B) and the percentage of CD161+ CAR+ CD4+ T cells (C) were analyzed by flow cytometry. Error bars represent SD. (D) Human L55 non–small cell lung cancers were established in the flanks of NSG mice. After 3 weeks, when the tumors reached a volume of 150 mm3, mice without tumors (empty mice) or with preestablished tumors were treated with 2 IV injections of 10 × 106 TH17 /TC17 cells. The concentration of CD4+ T cells was determined in the blood of treated animals 3 weeks postinfusion. *P < .05, **P < .01, and ***P < .001. Representative of 1 experiment.
To extend these results, we performed a similar experiment with NSG mice bearing non–small cell lung tumors. To confirm that T-cell persistence was a result of tumor recognition, and not a result of recognition of xenoantigens by the endogenous TCR of these cells, this experiment was performed in duplicate using mice with or without tumors (empty mice). TH17 cells redirected with ICOSζ showed an increased in vivo expansion and persistence that was significantly greater than the 28ζ and BBζ groups. Importantly, ICOSζ T-cell expansion was dependent on tumor antigen encounter, as empty mice showed significantly fewer ICOSζ cells compared with mice with tumors (Figure 7D). In conclusion, these results suggest that reprogramming CD4+ T cells with an ICOS-based CAR signaling domain promotes TH17 cell function and phenotype and enhances T-cell persistence in tumor-bearing mice.
Discussion
A central issue in the field of adoptive cell therapy is optimizing the proliferative capacity of the infused T cells while stabilizing the phenotype and limiting the plasticity of the infused cells so that they are resistant to the induction of regulatory or exhausted phenotypes that commonly emerge during cancer therapy.31,32 Here we find that CAR T cells expressing an ICOS signaling domain in TH17 cells are preferable to CAR T cells expressing CD28 or 4-1BB domains resulting from a stabilized TH17 core molecular signature and an enhanced persistence of the CAR T cells in tumor-bearing mice. CAR T cells with ICOS domains retain a “bipolar” bias toward a TH1 and TH17 signature, which is a plasticity that appears to be a prerequisite for potent antitumor activity in several preclinical models.17 Shen et al constructed an ICOS-based CAR that recognizes EGFRvIII,33 and demonstrated that TH1 cells redirected with this CAR could release IFN-γ and destroy glioma cells in an antigen-dependent manner. Our results confirm and extend this work by performing a comprehensive analysis of CARs expressing ICOS signaling domains. We show for the first time that ICOS based CARs have better persistence than the signaling domains currently being tested in the field, principally CD28 and the TNF family member 4-1BB.
We cultured CAR T cells under conditions that are used for clinical trials using antimesothelin redirected CAR T cells. When the cells were cultured in TH17 polarizing conditions, only ICOS-based CARs secreted high amounts of IL-17A, IL17-F, IL-22, and expressed CD161 after encounter with the redirected antigen. In humans, interleukin 17-producing cells have been shown to originate from a CD161+CD4+ T-cell precursor.34 Consistent with TH17 polarization, CARs with ICOS domains did not secrete IL-2 in contrast to CAR T cells with CD28 domains. IL-2 is generally considered to be essential in adoptive therapy protocols that involved transfer of CD8+ T cells; however, IL-2 can also promote the outgrowth of Tregs, so that competing agonistic and antagonistic effects might be observed in vivo. In this regard, Kofler et al showed that deletion of the lck-binding moiety in the CD28 endodomain of a CD28-ζ CAR abrogated IL-2 secretion on CAR engagement, and improved antitumor activity in tumors infiltrated by Treg cells.35 Our finding that ICOS-based CAR T cells don’t secrete IL-2 may provide a strategy for the treatment of hematologic and solid tumors heavily infiltrated by Tregs.
Related to our findings are reports that treatment of cancer patients with antagonistic CTLA-4 antibodies results in an enrichment of CD4+ T cells that express ICOS and produce augmented levels of IFN-γ.36 Of note, a report showed that concomitant CTLA-4 blockade and ICOS engagement by tumor cell vaccines engineered to express ICOS ligand significantly improved rejection of established melanoma and prostate cancer in mice.37 Our findings are consistent with reports suggesting that ICOS expression on CD4+ T cells is a predictive biomarker for CD4+ effector T-cell responses generated after CTLA-4 blockade.38
Cioani et al created a validated regulatory network that describes the master transcription factors and genes expressed in TH17 cells.26 We have used those results as a framework to characterize the human orthologs expressed in CAR TH17 cells expressing various signaling domains. We found that the gene expression analysis of human ICOS CAR bearing T cells was closely related to the orthologous set of genes expressed in mouse TH17 cells.26 CAR cells bearing ICOS domains specifically retained a genotype of TH17 cells with the expression of Rorc and Il1r1 in the absence of Foxp3 after surrogate antigen stimulation. IL-1 signaling is reported to promote proliferation and survival of antigen-stimulated TH17 cells. This process involves activation of the serine-threonine protein kinase Akt-mammalian target of rapamycin (Akt-mTOR) pathway.39 In addition, the ICOS CAR cells also retained selective expression of Tbx21 and IFN-γ, consistent with the maintenance of a TH17/TH1 bipolar phenotype. We also found that stimulation of ICOS CAR cells with surrogate antigen promoted the expression of Ncs1. NCS1 is a protein that belongs to a large Ca2+-binding protein family, implicated in the regulation of Ca2+-dependent exocytosis, with a previously unknown function in T-cell biology. However, based in our results, NCS1 is likely to carry an important function in the development and function of TH17 cells, as it was also implicated in the mouse TH17 cell network.26
Enhanced in vivo persistence is a desirable effect for long-term antitumor effects. Our studies suggest that the ex vivo manipulation of CAR T cells with an ICOS signaling domain can stabilize TH17 function and promote CAR T-cell persistence in mice bearing human tumor xenografts. This enhanced persistence required stimulation through the CAR receptor. In our microarray data we observed similar expressions of antiapoptotic genes in all of the CAR T-cell signaling domains tested. However, it is likely that ICOS-CAR TH17 cells had a proliferation advantage because they were consistently recovered in larger numbers from the spleens of humanized mice. This may have important translational implications, especially in patients with nonlymphoid tumors, where the functional persistence of CAR T cells has not yet been observed. A limitation of the present studies is that we do not yet know whether this will be predictive of the results after CAR T-cell transfer to patients, and therefore a future clinical trial will be required to test these principles.
To our knowledge, our findings are the first to demonstrate that the expression of selected CAR endodomains can program T cells for their subsequent differentiation fates and effector functions. The balance of Tregs to effector T cells is an important biomarker for the outcome of many immunotherapies,40 and the ability of ICOS to limit IL-2 secretion may be an important feature in improving this ratio. The observation that CAR T cells bearing ICOS signaling domains have enhanced survival after adoptive transfer provides the rationale to test this concept in human trials in the setting of solid tumors and nonlymphoid hematologic malignancies, where improved persistence of CAR T cells remains as an unmet medical need.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chrystal Paulos for helpful discussions, Dr Katsunari Tezuka (Japan Tobacco, Inc.) for the ICOS mAb, Daniel Powell for the generous gift of Mov19 vectors, Brian Keith for helpful comments on the manuscript, and Ronghua Liu, Anthony Secreto, Omkar Kawalekar, Kathleen Haines, and Shree Joshi for expert technical assistance.
This work was supported by the National Institutes of Health Common Fund Nanomedicine program (PN2 EY016586, RO1CA120409) and the PA DOH award 410005172. This work was supported by a Marie Curie International Outgoing Fellowship within the 7th Framework Programme of the European Union (IOF-GA-2010-275139) (S.G.).
Authorship
Contribution: S.G. and C.H.J. designed the study and wrote the manuscript; S.G., C.C., S.E.M., M.J.F., J.L., A.D.P.Jr, and J.S. performed experiments; X.C., A.M., and R.B. performed bioinformatics analysis; and N.S. provided vital new reagents.
Conflict-of-interest disclosure: S.G., J.S., and C.H.J. are inventors of intellectual property licensed by the University of Pennsylvania to Novartis. The remaining authors declare no competing financial interests.
Correspondence: Sonia Guedan, 3400 Civic Center Blvd, 8th Floor, Room 08-123, Philadelphia, PA 19104-5156; e-mail: sgued@mail.med.upenn.edu; and Carl H. June, 3400 Civic Center Blvd, 8th Floor, Room 08-123, Philadelphia, PA 19104-5156; e-mail: cjune@exchange.upenn.edu.





![Figure 6. TH17 /TC17 cells redirected with mesothelin-specific CARs eradicate advanced preestablished tumors. (A) TC17 and TH17 cells were cocultured with M108 target cells stained with CFSE for 18 hours at the indicated effector-target (E:T) ratios. Specific cytolysis was determined using a flow cytometry-based assay. Percent specific lysis was calculated as [experimental release (cpm) − spontaneous release (cpm)]/[maximal release (cpm) − spontaneous release (cpm)] × 100. Error bars indicate SD in duplicate samples. Representative of 2 different experiments. (B) As shown schematically, human primary M108 tumors were established in the flanks of NSG mice. After 8 weeks, when the tumors reached a large volume of 500 mm3, mice were treated with 2 intratumoral injections of 10 × 106 TH17 and TC17 cells (80% and 60% CAR+) or PBS on days 0 and 7. (C) Tumor volume was analyzed at indicated time points. Results are expressed as a mean tumor volume (± SD) with n = 9 for all groups. Representative of 2 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/7/10.1182_blood-2013-10-535245/5/m_1070f6.jpeg?Expires=1765904272&Signature=Ov38eh1rEVoHG-mrdQ1qDI-rmzC9UZj6opaVVXC8uD1WQ3I7H-LJcUHAOMr0N2Z~v39wXC6CGxtv5zTxHsYsziQc4~qhO9~QzndLBGGQx-7E8WAiWGuSZAl80tY5tjuoeWs91eqTtdEJzo6pxFl-fdKJqq6B5fQ9ssbXyq1stxwssHQME5ZDUIWDyjZpwW3j6OhCY1ipPyEONqzJjCYKtZfsDWFC35gGtORHgw9cy1vIv5MAl6DmtE6S5qrvlwnAn6buXG1kcq2ymAKIGWVW7lYgo7TMK9K3G4CHeg0300aibkeanxBhKD27yVmCSIgBbjMph1vZqLZW1UhTTchBHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal