Key Points
Expression of IL-15 in a membrane-bound form sustains NK cell survival and expansion in vitro and in vivo without exogenous cytokines.
These NK cells have superior cytotoxicity against leukemia, lymphoma, and solid tumor cells, supporting their clinical testing.
Abstract
Natural killer (NK) cell survival and, hence, cytotoxicity requires cytokine support. We determined whether expression of interleukin-15 (IL-15) in a nonsecretory, membrane-bound form could sustain NK cell growth. We linked the human IL15 gene to that encoding CD8α transmembrane domain (mbIL15). After retroviral transduction, human NK cells expressed mbIL15 on the cell surface; IL-15 secretion was negligible. Survival of mbIL15-NK cells without interleukin-2 (IL-2) after 7-day culture was vastly superior to that of mock-transduced NK cells (P < .001, n = 15) and of NK cells expressing nonmembrane-bound IL-15 (P = .025, n = 9); viable mbIL15-NK cells were detectable for up to 2 months. In immunodeficient mice, mbIL15-NK cells expanded without IL-2 and were detectable in all tissues examined (except brain) in much higher numbers than mock-transduced NK cells (P < .001). Expansion further increased with IL-2. The primary mechanism of mbIL15 stimulation was autocrine; it activated IL-15 signaling and antiapoptotic signaling. NK cells expressing mbIL15 had higher cytotoxicity against leukemia, lymphoma, and solid tumor cells in vitro and against leukemia and sarcoma cells in xenograft models. Thus, mbIL15 confers independent growth to NK cells and enhances their antitumor capacity. Infusion of mbIL15-NK cells would allow NK cell therapy without the potential adverse effects of cytokine administration.
Introduction
Natural killer (NK) cells have shown promise in the treatment of cancer. Besides the strong evidence that donor NK cell alloreactivity can suppress relapse after allogeneic hematopoietic stem cell transplantation,1-5 infusion of allogeneic NK cells has been shown to induce and/or maintain remission in patients with acute myeloid leukemia (AML).6,7 Contrary to T cells, allogeneic NK cells do not exert graft-versus-host disease. This feature, combined with their broad spectrum of anticancer activity,8 renders NK cell therapy an attractive treatment option, which has been recently facilitated by methods that can reliably expand large numbers of NK cells ex vivo.9,10
In vivo survival and proliferation of NK cells requires stimulation by cytokines, such as interleukin-2 (IL-2). For example, activated NK cells injected in immunodeficient mice became undetectable after 1 week but persisted for up to 1 month if human IL-2 was administered.9 Thus, clinical protocols using NK cell infusions typically rely on exogenous IL-2 to prolong NK cell survival in patients.6,7,11,12 However, IL-2 administration can have considerable adverse effects from fever and chills to more serious consequences, such as capillary leak syndrome.13,14 Decreasing the dose of IL-2 might reduce risk but can result in decreased NK cell survival and stimulate regulatory T cells, which can inhibit NK cell function and possibly nullify its anticancer effect.15,16 Hence, it would be important to develop alternative ways to promote NK cell expansion and activity in vivo.
Interleukin-15 (IL-15) can also promote the survival and expansion of NK cells.17-20 Miller et al6 observed that immunosuppressive chemotherapy prior to allogeneic NK cell infusion appeared to induce secretion of endogenous IL-15 and that IL-15 levels correlated with in vivo expansion of infused NK cells. Administration of IL-15 induced NK expansion in nonhuman primates and had apparently limited toxicity21-23 ; its safety profile is being further tested in clinical trials. Physiologically, IL-15 is trans-presented to NK cells on the cell membrane of neighboring cells, such as macrophages and dendritic cells.24-27 In this study, we determined whether human NK cells could self-sustain their survival, proliferation, and functional properties in the absence of exogenous cytokines if they were endowed with the capacity to present IL-15 on their cell membrane.
Materials and methods
Tumor cell lines and NK cells
The human cell lines Nalm-6 (B-lineage acute lymphoblastic leukemia), Daudi (B-cell lymphoma), K562 and U937 (AML), SK-BR-3 (breast carcinoma), and NK-92 (NK cell lymphoma) were from the American Type Culture Collection (Manassas, VA); the Ewing sarcoma cell line ES8, the B-lymphoblastoid cell line 721.221, and its derivative expressing HLA-Cw6 were from the St. Jude Children’s Research Hospital tissue repository. We transduced all cell lines (except 721.221) with a murine stem cell virus-internal ribosome entry site-green fluorescent protein (GFP) retroviral vector (from the St. Jude Vector Development and Production Shared Resource) containing the firefly luciferase gene. GFP-positive cells were selected with a MoFlo (Beckman Coulter, Miami, FL) or a FACSAria (BD Biosciences, San Jose, CA) and cultured in RPMI-1640 (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) and antibiotics. Cell lines were characterized for phenotypic and/or genetic features to ensure their authenticity.
Peripheral blood samples obtained from discarded byproducts of platelet collections from healthy adult donors were used as a source of NK cells. Mononuclear cells were purified by centrifugation with Accu-Prep Lymphocytes (Accurate, Westbury, NY). CD56+ CD3− NK cells were expanded by stimulation with the K562-mb15-41BBL cell line, as previously described.9,28 Residual T cells were removed with Dynabeads CD3 (Invitrogen), resulting in cell populations containing >95% CD56+ CD3− NK cells. This study was conducted in accordance with the Declaration of Helsinki.
Plasmids, gene transduction, and functional analysis of NK cells
IL-15 with long signal peptide was subcloned from a human spleen complementary DNA library (from Dr G. Neale, St. Jude Children’s Research Hospital) by polymerase chain reaction. The complementary DNA encoding the signal peptide of CD8α, the mature peptide of IL-15, and the transmembrane domain of CD8α were assembled using splicing by overlapping extension-polymerase chain reaction to encode a membrane-bound form of IL-15 (mbIL15); a wild-type form of IL-15 (not linked to CD8α transmembrane domain; wtIL15) was also prepared. The expression cassettes were subcloned into EcoRI and XhoI sites of the murine stem cell virus-internal ribosome entry site-GFP vector. Preparation of RD114-pseudotyped retrovirus and retroviral transduction is described in the supplemental Methods, available on the Blood Web site.28,29 Transduced cells were assayed 3 to 29 days after transduction.
Expression of mbIL15 was detected with an anti-human IL-15 antibody (R&D, Minneapolis, MN) and phycoerythrin (PE)-conjugated goat anti-mouse IgG1(Southern Biotech, Birmingham, AL), and visualized with a Fortessa flow cytometer (Becton Dickinson). Levels of IL-15 in culture supernatants were measured with the Quantikine Immunoassay (R&D).
Estimates of NK cell survival, NK cell immunophenotyping, phosphoprotein analysis, staining for CD107a and interferon-γ, and cytotoxicity assays were performed as described in the supplemental Methods.
Expansion and cytotoxicity of NK cells in immunodeficient mice
To test NK cell expansion in vivo, mbIL15- or mock-transduced human NK cells were injected in the tail vein of NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NOD/scid IL2RGnull) mice (Jackson Laboratories, Bar Harbor, ME) (6-9 × 106 cells per mouse). In some mice, we injected 20 000 IU of IL-2 intraperitoneally (i.p.) every 2 days. On day 7 and 11, blood cells were counted with a cell counter (Beckman Coulter); human and mouse CD45+ cells were enumerated by flow cytometry after treating cells with red blood cell lysis solution (Invitrogen) and staining them with allophycocyanin-conjugated mouse-anti-human CD45 and PE-conjugated rat anti-mouse CD45 antibodies (both from BD Biosciences). After euthanasia, human NK cells in bone marrow, liver, spleen, kidney, lung, and brain were enumerated as above. All animal experiments were performed in accordance with a protocol approved by the National University of Singapore Institutional Animal Care and Use Committee.
To test tumor cell killing in mice, we prepared 2 xenograft models. In the first, U937 cells expressing luciferase were i.p. injected in NOD/scid IL2RGnull mice (1 × 104 cells per mouse). Three days later, mbIL15- or mock-transduced NK cells were i.p. injected (1 × 107 cells per mouse); NK cell injection was repeated on day 7. As a control, a group of mice received tissue culture medium instead of NK cells. In the second model, mice were engrafted with ES8 cells (i.p.; 1 × 105 cells per mouse), followed by a single NK cell injection on day 3 as above. Tumor engraftment and progression was evaluated using a Xenogen IVIS-200 system (Caliper Life Sciences, Hopkinton, MA) after i.p. injection of an aqueous solution of d-luciferin potassium salt (3 mg/mouse) (Perkin Elmer, Waltham, MA). Photons emitted from luciferase-expressing cells were quantified using the Living Image 4.3.1 software.
Results
Design of IL-15 constructs and expression in NK cells
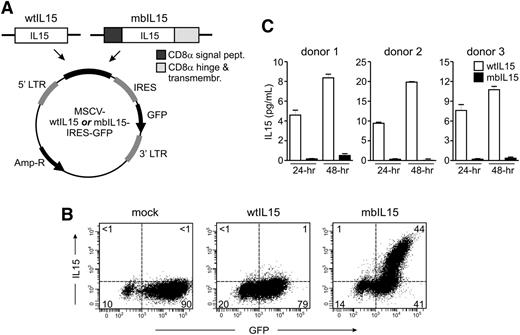
We expressed 2 forms of the IL15 gene in human NK cells: a membrane-bound form, resulting from a construct in which the human IL15 gene was linked to the gene encoding the transmembrane domain of CD8α (mbIL15), and a wild-type unmodified form (wtIL15). Both constructs were inserted in a retroviral vector containing GFP (Figure 1A), which was used to transduce proliferating NK cells obtained after culturing peripheral blood mononucleated cells with the stimulatory cell line K562-mb15-41BBL.28 Median GFP expression in CD56+ CD3− cells was 71% (23% to 97%, n = 64) with the construct containing mbIL15, and 69% (range, 20% to 91%, n = 25) with that containing wtIL15. Median GFP expression in NK cells from the same donors transduced with a vector containing only GFP was 84% (53% to 98%, n = 64) (Figure 1B).
Design and expression of IL-15 constructs. (A) Schematic representation of the wild-type and membrane-bound IL-15 constructs (wtIL15 and mbIL15) used in this study. Amp-R, ampicillin resistance gene; IRES, internal ribosome entry site; LTR, long terminal repeat. (B) Expression of IL-15 on the surface of NK cells transduced with mbIL15. Expanded NK cells were transduced with wtIL15, mbIL15, or a vector containing GFP alone (mock). Flow cytometry dot plots illustrate expression of GFP and IL-15, as detected by an anti-IL15 antibody (R&D Systems) and a PE-conjugated goat-anti-mouse secondary antibody (Southern Biotechnology Associates). Percentage of cells (>98% CD56+ CD3− NK cells) in each quadrant is shown. (C) Secretion of IL-15 by NK cells transduced with wtIL15. NK cells from 3 different donors were tested in triplicate. Bars indicate mean ± SD of enzyme-linked immunosorbent assay measurements performed in supernatants collected after 24 and 48 hours of culture without IL-2. No IL-15 was detected in the supernatants of mock-transduced NK cells.
Design and expression of IL-15 constructs. (A) Schematic representation of the wild-type and membrane-bound IL-15 constructs (wtIL15 and mbIL15) used in this study. Amp-R, ampicillin resistance gene; IRES, internal ribosome entry site; LTR, long terminal repeat. (B) Expression of IL-15 on the surface of NK cells transduced with mbIL15. Expanded NK cells were transduced with wtIL15, mbIL15, or a vector containing GFP alone (mock). Flow cytometry dot plots illustrate expression of GFP and IL-15, as detected by an anti-IL15 antibody (R&D Systems) and a PE-conjugated goat-anti-mouse secondary antibody (Southern Biotechnology Associates). Percentage of cells (>98% CD56+ CD3− NK cells) in each quadrant is shown. (C) Secretion of IL-15 by NK cells transduced with wtIL15. NK cells from 3 different donors were tested in triplicate. Bars indicate mean ± SD of enzyme-linked immunosorbent assay measurements performed in supernatants collected after 24 and 48 hours of culture without IL-2. No IL-15 was detected in the supernatants of mock-transduced NK cells.
After transduction with mbIL15, IL-15 was expressed on the NK cell membrane: 40% to 63% (median, 52; n = 7) of GFP+ NK cells had IL-15 as detected by an anti-IL15 antibody (Figure 1B). By contrast, no IL-15 was detectable in cells transduced with wtIL15 (n = 4) or in mock-transduced NK cells (n = 7). Production of soluble IL-15 by the transduced NK cells was determined by testing supernatants collected after 24 and 48 hours of culture. Cells expressing wtIL15 secreted substantial amounts of IL-15, whereas this was minimal in mbIL15-tranduced NK cells and undetectable in mock-transduced NK cells (Figure 1C).
NK cells expressing IL-15 have autonomous survival and expansion capacity
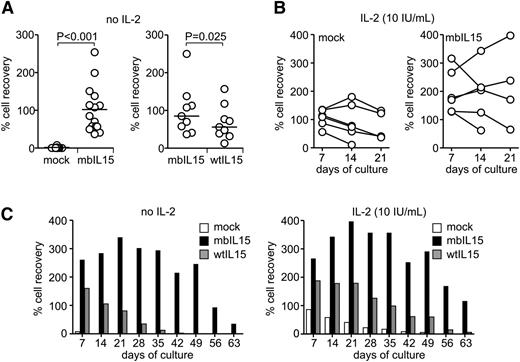
To determine whether expression of IL-15 could replace exogenous IL-2 in maintaining NK cell survival, we transduced NK cells from 15 donors with the mbIL15 construct and cultured them in the absence of IL-2; we then compared cell numbers after culture to those in parallel cultures with mock-transduced NK cells. Expression of mbIL15 dramatically increased NK cell survival: after 7 days of culture, median cell recovery was 85%, whereas essentially no viable mock-transduced NK cell was detectable (<1%; P < .001 by paired t test) (Figure 2A). The effect of mbIL15 significantly decreased if an anti-IL-15 neutralizing antibody was added to the cultures (supplemental Figure 1A). In 9 of the 15 donors, we also compared recovery of mbIL15 NK cells with that of NK cells expressing wtIL15: it was significantly higher with the former (median, 85% vs 56%, P = .025) (Figure 2A).
Survival and expansion of NK cells expressing IL-15 in vitro. (A) Percentage of NK cell recovery compared with input cells after 7-day parallel cultures without IL-2 for mock- and mbIL15-transduced cells from 15 donors (left) and mbIL15- or wtIL15-transduced NK cells from 9 donors (right). Horizontal bars indicate median value. Results of paired t tests are shown. Data of cultures with IL-2 (10 and 100 IU/mL) are shown in supplemental Figure 1. (B) Survival and expansion of mock- and mbIL15-transduced NK cells from 6 donors with low-dose IL-2 (10 IU/mL). (C) Expansion and long-term survival of mbIL15-, wtIL15-, or mock-transduced NK cells from 1 donor cultured with no IL-2 or low-dose IL-2 (results with 100 IU/mL IL2 are shown in supplemental Figure 1). Percentage of NK cell recovery at the indicated days of culture is shown.
Survival and expansion of NK cells expressing IL-15 in vitro. (A) Percentage of NK cell recovery compared with input cells after 7-day parallel cultures without IL-2 for mock- and mbIL15-transduced cells from 15 donors (left) and mbIL15- or wtIL15-transduced NK cells from 9 donors (right). Horizontal bars indicate median value. Results of paired t tests are shown. Data of cultures with IL-2 (10 and 100 IU/mL) are shown in supplemental Figure 1. (B) Survival and expansion of mock- and mbIL15-transduced NK cells from 6 donors with low-dose IL-2 (10 IU/mL). (C) Expansion and long-term survival of mbIL15-, wtIL15-, or mock-transduced NK cells from 1 donor cultured with no IL-2 or low-dose IL-2 (results with 100 IU/mL IL2 are shown in supplemental Figure 1). Percentage of NK cell recovery at the indicated days of culture is shown.
In parallel experiments, we determined the supportive effects of IL-15 expression in the presence of exogenous IL-2. When cultures contained 10 IU/mL of IL-2, 7-day recovery of NK cells expressing either mbIL15 or wtIL15 remained significantly higher than that of mock-transduced cells; under these conditions, no significant differences were noted between the 2 forms of IL-15 (supplemental Figure 1B). Only when exogenous IL-2 was present at a high concentration (100 IU/mL) did 7-day recovery of mock-transduced NK cells match that of NK cells transduced with IL-15 (supplemental Figure 1B).
The capacity of mbIL15 to support NK cell survival beyond 7 days in the presence of low-dose IL-2 (10 IU/mL) was determined in 6 of the 9 donors. On day 14, mbIL15-NK cell numbers were maintained or increased in 4 of the 6 cultures; in 2 of these, cells had further expanded by day 21. Only 2 of the 6 cultures with mock-transduced NK cells from the same donors had maintained cell numbers on day 14 and 21, and no cell growth was observed; median cell recovery on day 21 was 205% for mbIL15-NK cells and 80% for mock-transduced NK cells (Figure 2B). Thus, even in the presence of low-dose IL-2, expression of mbIL15 conferred a considerable survival and growth advantage.
In cultures of NK cells from one donor, we observed a particularly high cell recovery on day 7 when IL-15 was expressed (261% with mbIL15 and 161% with wtIL15 in the absence of IL-2; 266% and 188% with 10 IU/mL IL-2). Even in the absence of IL-2, mbIL15-NK cells continued to proliferate until day 21, and they were still detectable 75 days after initiation of the culture, whereas mock-transduced NK cells had become undetectable on day 14 and wtIL15-transduced NK cells on day 42 (Figure 2C). In the presence of IL-2 at low concentration (10 IU/mL), the number of mbIL15-expressing NK cells 2 months after initiation of the cultures was identical to that originally seeded, whereas viable mock-transduced and wtIL15-transduced NK cells had declined much earlier. With high-dose IL-2 (100 IU/mL), NK cells transduced with either mbIL15 or wtIL15 had a similar persistence profile, both cell types surviving longer than mock-transduced NK cells even under these conditions (supplemental Figure 1C), validating the importance of IL-15 in NK cell survival beyond that of IL-2 alone.
Expansion and homing of mbIL15 NK cells in vivo
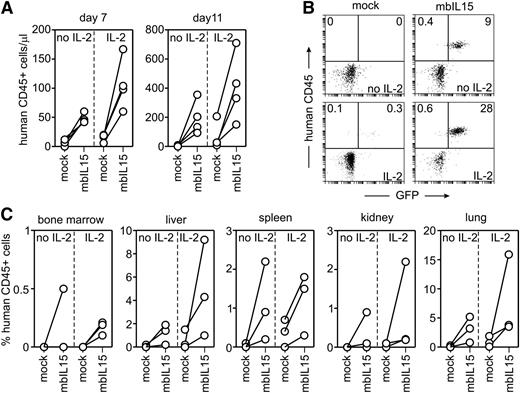
The above experiments indicated that enforced expression of IL-15 in NK cells improved their survival and expansion and that mbIL15 produced overall better stimulation. We determined whether mbIL15 expression would sustain expansion of human NK cells in NOD/scid IL2RGnull mice. Activated NK cells from 4 donors were transduced with mbIL15 (52% to 74% GFP-positive) and injected into 8 mice (2 mice per donor). Eight control mice were injected with mock-transduced NK cells from the same donors; in each group, one-half of the mice i.p. received 20 000 IU human IL-2 every 2 days. In the absence of IL-2, mbIL15-NK cells expanded much more than mock-transduced NK cells: 7 days after injection, the median number of mbIL15 NK cells/μL of blood was 44.5 (range, 42-60) vs 6.5 (0-12) with mock-transduced NK cells (P = .004) (Figure 3A). With IL-2, mbIL15-NK cells expanded even more (median NK cells/μL, 101; range, 60-167), whereas mock-transduced cells remained low (median, 18; range, 6-20; P = .021) (Figure 3A).
Survival and expansion of NK cells expressing mbIL15 in vivo. (A) Absolute number of human CD45+ cells in peripheral blood of mice injected with mock- or mbIL15-transduced NK cells with or without IL-2 (16 mice in total) 7 and 11 days after infusion (P = .004 with no IL-2, P = .021 with IL-2 on day 7; P = .044 and 0.026 on day 11). (B) Flow cytometric dot plots illustrate the presence of human CD45+, GFP+ NK cells in mouse peripheral blood without (top) and with IL-2 treatment (bottom) on day 11. Percentages of human CD45+ cells with or without GFP expression are shown. (C) Percentage of human CD45+ cells in various tissues of mice injected with mock- or mbIL15-transduced NK cells with or without IL-2 collected 11 days after injection. Collectively, percentages of human CD45+ cells were significantly higher with mbIL15 (P < .001 with no IL-2, P = .002 with IL-2).
Survival and expansion of NK cells expressing mbIL15 in vivo. (A) Absolute number of human CD45+ cells in peripheral blood of mice injected with mock- or mbIL15-transduced NK cells with or without IL-2 (16 mice in total) 7 and 11 days after infusion (P = .004 with no IL-2, P = .021 with IL-2 on day 7; P = .044 and 0.026 on day 11). (B) Flow cytometric dot plots illustrate the presence of human CD45+, GFP+ NK cells in mouse peripheral blood without (top) and with IL-2 treatment (bottom) on day 11. Percentages of human CD45+ cells with or without GFP expression are shown. (C) Percentage of human CD45+ cells in various tissues of mice injected with mock- or mbIL15-transduced NK cells with or without IL-2 collected 11 days after injection. Collectively, percentages of human CD45+ cells were significantly higher with mbIL15 (P < .001 with no IL-2, P = .002 with IL-2).
On day 11 after injection, mbIL15-NK cells comprised 168.5 cells/μL (range, 94-355) of peripheral blood mononucleated cells without IL-2 and 382 cells/μL (151-710) with IL-2 (Figure 3A-B). By contrast, mock-transduced NK cells were virtually undetectable on day 11 without IL-2 and present at low levels when IL-2 was also injected (median, 27; range 9-207; P = .026). Of note, in mice injected with mbIL15-NK cells, the proportion of GFP+ among human CD45+ cells had increased from 66.5%± 9.9% before injection to 93.8% ± 4.4% on day 7 and 94.8% ± 3.4% on day 11 (P < .01 for both comparisons).
After euthanasia on day 11, 3 of the 4 mice in each group were examined for the presence of human CD45+ cells in various tissues. When mbIL15 was expressed, considerable numbers of human NK cells were detected in bone marrow, liver, spleen, kidney, and lung; in all tissues, numbers were markedly higher than those seen with mock-transduced cells (Figure 3C): the mean (±SD) percentage of CD45+ cells expressing mbIL15 was 1.2% ± 1.5% with no IL-2 and 3.0% ± 4.3% with IL-2, as compared with 0.04% ± 0.09% and 0.4% ± 0.6% with mock-transduced cells (P < .001 and P = .002, respectively). The only exception was brain, where neither mbIL15- nor mock-transduced NK cells could be detected.
Mechanisms of mbIL15 stimulation
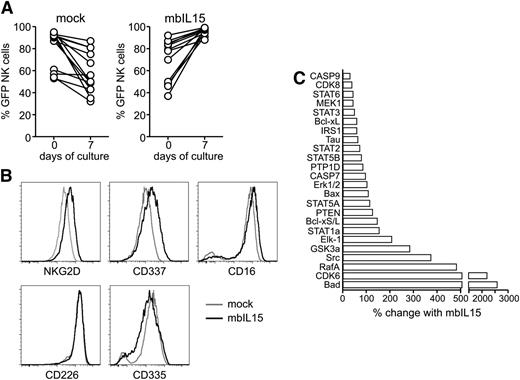
To determine whether mbIL15 predominantly stimulated cells in trans, ie, IL-15 presented on one NK cell stimulating a neighboring cell (a mechanism reported to occur physiologically)24-27,30 or cis, ie, by direct binding of mbIL15 to receptors expressed in the same cell, we evaluated the proportions of GFP+ and GFP− NK cells in our cultures. If the trans mechanism were predominant, the ratio between GFP+ and GFP− NK cells should remain essentially unaltered during culture; if cis were predominant, the proportion of GFP+ cells should increase. After culture without IL-2, the percentage of GFP+ cells among NK cells transduced with mbIL15 consistently increased (95.9%± 3.3% on day 7 as compared with 71.2% ± 19.0% on day 0), whereas it did not in cultures with mock-transduced cells (57.5% ± 18.6% on day 7 vs 80.5% ± 17.1% on day 0; P < .001 (Figure 4A). These results were corroborated by experiments with the NK-92 cell line. When mbIL15-expressing NK-92 cells were mixed at a 1:1 ratio with untransduced cells, the latter decreased in numbers, whereas they expanded in co-culture with wtIL15 NK-92 cells (supplemental Figure 2A). Thus, the predominant mechanism of stimulation by mbIL15 expressed in NK cells is autocrine, explaining the increase in the proportion of GFP+ cells seen in mice injected with these cells. Of note, the cis stimulus provided by mbIL15 on NK-92 cells was superior to the trans stimulus of an identical mbIL15 expressed on K562 cells (supplemental Figure 2B). In the absence of IL-2, NK-92 proliferation driven by mbIL15 expression was similar to that promoted by 5 to 10 ng/mL of exogenous IL-15 (supplemental Figure 2C), an amount corresponding to that measured in animal sera after IL-15 infusion22,31 ; proliferation could not be further enhanced by the addition of IL-2.
Properties of NK cells expressing mbIL15. (A) Relative proportion of GFP+ cells before and after 7 days of culture among NK cell populations transduced with mbIL15 or mock transduced. Results with NK cells from 13 donors are shown (P < .001 for mbIL15, not significant for mock). (B) Immunophenotypic features of mbIL15-transduced NK cells. Cell marker analysis by flow cytometry was performed on NK cells cultured for 48 hours without IL-2. All results are summarized in supplemental Table 1. (C) Mock- and mbIL15-transduced NK cells were cultured for 48 hours without IL-2, and cell lysates were analyzed by Kinex Antibody Microarray (Kinexus, Vancouver, CA). Of 854 antiphosphoprotein antibodies tested, shown are those whose signals had a Z-ratio >0.5 and an error range <100%. Bars indicate percent signal change in NK cells expressing mbIL15 compared with the normalized intensity in mock-transduced NK cells.
Properties of NK cells expressing mbIL15. (A) Relative proportion of GFP+ cells before and after 7 days of culture among NK cell populations transduced with mbIL15 or mock transduced. Results with NK cells from 13 donors are shown (P < .001 for mbIL15, not significant for mock). (B) Immunophenotypic features of mbIL15-transduced NK cells. Cell marker analysis by flow cytometry was performed on NK cells cultured for 48 hours without IL-2. All results are summarized in supplemental Table 1. (C) Mock- and mbIL15-transduced NK cells were cultured for 48 hours without IL-2, and cell lysates were analyzed by Kinex Antibody Microarray (Kinexus, Vancouver, CA). Of 854 antiphosphoprotein antibodies tested, shown are those whose signals had a Z-ratio >0.5 and an error range <100%. Bars indicate percent signal change in NK cells expressing mbIL15 compared with the normalized intensity in mock-transduced NK cells.
Cells expressing mbIL15 essentially retained the immunophenotype of activated NK cells. When examined 2 days after IL-2 withdrawal and compared with mock-transduced NK cells, mbIL15 NK cells expressed moderately higher levels of the activation receptors NKG2D, NKp44 (CD336), and NKp30 (CD337) as well as of CD16, CD56, and CD69, whereas expression of NKp46 (CD335) decreased (Figure 4B; supplemental Table 1). In comparison with mock-transduced NK cells, mbIL-15 NK cells had several highly phosphorylated molecules (Figure 4C). These included molecules known to be phosphorylated in response to IL-15 signaling, such as the transcription factors STAT1, STAT3, and STAT5 and the kinases Src, Erk1/2, and Mek1.17,19 We also observed a marked phosphorylation of Bad as well as phosphorylation of Caspase 7 and 9, collectively indicative of an antiapoptotic effect.32,33 Other highly phosphorylated molecules in mbIL15 NK cells whose role in IL-15 signaling is unclear included CDK6 and RafA.
Expression of mbIL15 did not change the killer immunoglobulin-like receptor profile, and NK cells expressing mbIL15 remained susceptible to killer immunoglobulin-like receptor–mediated inhibitory signals (supplemental Figure 3). Among the CD158a-positive subset, there was a specific reduction in CD107a expression and interferon-γ production in co-culture with the cell line 721.221 expressing the cognate HLA-Cw6.
Effects of mbIL15 on NK cell antitumor cytotoxicity in vitro
The survival, proliferation, and activation features of mbIL15-NK cells implied that they should also have a more powerful antitumor effect than NK cells lacking self-sufficient cytokine support. This notion was first tested in experiments with NK cells from 9 donors targeting the leukemia cell lines Nalm-6 (B-lineage acute lymphoblastic leukemia), U937, and K562 (AML) as well as Daudi (B-cell lymphoma), SK-BR-3 (breast carcinoma), and ES8 (Ewing sarcoma) at different effector:target (E:T) ratios and co-culture durations for a total of 90 experiments. Figure 5A shows results of 24-hour assays: median cytotoxicity was 22% with mock-transduced NK cells at E:T 1:4 and 54% at 1:1; with mbIL15 NK cells, it was 71% and 99%, respectively (P < .001). Results with individual cell lines are shown in supplemental Figure 4. We also observed an increased release of lytic granules by mbIL15-NK cells, as shown by CD107a staining after culture with either K562 or U937 cells (P = .0023) (Figure 5B).
Antitumor capacity of NK cells expressing mbIL15. (A) Results of 24-hour cytotoxicity assays with mbIL15- and mock-transduced NK cells from 9 donors against the Nalm-6, U937, K562, Daudi, SKBR3, and ES8 cell lines at 1:4 and 1:1 E:T ratio (15 experiments at each ratio; P < .001 for both). Results obtained with individual cell lines in 4-hour and 24-hour cytotoxicity assays are shown in supplemental Figure 2. (B) NK cells expressing mbIL15 have an increased release of lytic granules in the presence of target cells. Percentage of CD107a+ NK cells after 4-hour cytotoxicity assays at 1:1 E:T. Results with NK cells from 3 donors against 2 cell lines are shown (P = .002). (C) NK cells expressing mbIL15 exert antitumor activity in vivo. NOD/scid-IL2RGnull mice were i.p. injected with 1 × 104 U937 cells labeled with luciferase. In 3 mice, no treatment was given (No NK), while 4 mice i.p. received mock-transduced NK cells (1 × 107) on days 3 and 7, and 4 other mice received mbIL15-transduced NK cells at the same dose and schedule. Results of in vivo imaging of tumor growth are shown (ventral images). (D) Overall survival comparisons of mice in the different treatment groups. Mice were killed when bioluminescence reached 1 × 1011 photons/second. P values for log-rank test of the 3 curves and for comparisons between each of 2 curves are shown.
Antitumor capacity of NK cells expressing mbIL15. (A) Results of 24-hour cytotoxicity assays with mbIL15- and mock-transduced NK cells from 9 donors against the Nalm-6, U937, K562, Daudi, SKBR3, and ES8 cell lines at 1:4 and 1:1 E:T ratio (15 experiments at each ratio; P < .001 for both). Results obtained with individual cell lines in 4-hour and 24-hour cytotoxicity assays are shown in supplemental Figure 2. (B) NK cells expressing mbIL15 have an increased release of lytic granules in the presence of target cells. Percentage of CD107a+ NK cells after 4-hour cytotoxicity assays at 1:1 E:T. Results with NK cells from 3 donors against 2 cell lines are shown (P = .002). (C) NK cells expressing mbIL15 exert antitumor activity in vivo. NOD/scid-IL2RGnull mice were i.p. injected with 1 × 104 U937 cells labeled with luciferase. In 3 mice, no treatment was given (No NK), while 4 mice i.p. received mock-transduced NK cells (1 × 107) on days 3 and 7, and 4 other mice received mbIL15-transduced NK cells at the same dose and schedule. Results of in vivo imaging of tumor growth are shown (ventral images). (D) Overall survival comparisons of mice in the different treatment groups. Mice were killed when bioluminescence reached 1 × 1011 photons/second. P values for log-rank test of the 3 curves and for comparisons between each of 2 curves are shown.
The higher cytotoxicity of mbIL15-transduced NK cells was also evident when immunotherapeutic antibodies were added to the cultures. Thus, antibody-dependent cell cytotoxicity exerted by mbIL15-NK cells against CD20+ Daudi cells in the presence of the anti-CD20 antibody Rituximab was markedly higher than that of mock-transduced cells (P < .001). Similar results were observed with HER2+ SK-BR-3 cells and the anti-HER2 antibody Trastuzumab (P < .001) (supplemental Figure 5).
Effects of mbIL15 on NK cell antitumor cytotoxicity in vivo
The gains in cytotoxicity associated with expression of mbIL15 were reflected in experiments with NOD/scid IL2RGnull mice engrafted with human tumor cells. In one set of experiments, mice were injected with the human AML cell line U937 and then treated with either mbIL15- or mock-transduced NK cells without exogenous IL-2. Mice receiving mbIL15-transduced NK cells had a slower tumor growth and a significantly longer survival than untreated mice and mice treated with mock-transduced NK cells (P = .01 for both comparisons) (Figure 5C-D). In another xenograft model, we injected NOD/scid IL2RGnull mice with the Ewing sarcoma cell line ES8 (which has a slower growth rate than U937) and treated them with one injection of NK cells and no IL-2. The outcome of mice treated with mbIL15 NK cells (n = 12) was superior to that of mock-transduced NK cells (n = 11) and of untreated mice (n = 7): median survival was 230, 49, and 21 days, respectively (log-rank test for trend, P = .005) (supplemental Figure 6).
Discussion
Among the factors that determine the success of NK cell therapy of cancer, perhaps the most fundamental one is that NK cells persist in sufficient numbers to achieve an E:T ratio likely to produce tumor cytoreduction.34 To lyse tumors cells, infused NK cells must also maintain an activated status.12 In this study, we demonstrated that expression of a membrane-bound form of IL-15 in human NK cells can support their autonomous expansion and extended survival in the absence of IL-2. NK cells expressing mbIL15 could be maintained in vitro for up to 2 months without exogenous IL-2. These cells could also expand in immunodeficient mice and infiltrated multiple tissues, where they could be found in much larger numbers than mock-transduced cells. Expansion of mbIL15 NK cells was further increased by a low concentration of IL-2 both in vitro and in vivo. Expression of mbIL15 did not impair the cytotoxic capacity of NK cells. In fact, in xenograft models, mbIL15 NK cells exerted anticancer activity, which was more powerful than that of mock-transduced cells, suggesting that this approach might improve the antitumor capacity of NK cell infusions while concurrently averting the side effects of higher-dose IL-2 administration.
Previous studies indicated that IL-15 forms complexes with IL-15 receptor α on the surface of monocytes, macrophages, and dendritic cells; IL-15 trans-presentation contributes to the survival of T-cell and NK-cell survival.24,26,27 Kobayashi et al27 found that IL-15 presented in trans augmented the cytotoxicity of murine NK cells, whereas soluble IL-15 at physiologic concentrations did not. Our findings that ectopic expression of IL-15 in human NK cells causes a stronger survival-promoting effect when IL-15 is presented in a membrane-bound form than in a secreted form are in line with these reports. Notably, however, mbIL15 expressed in NK cells preferentially stimulates in cis rather than in the trans mode described when IL-15 is presented by other cells. That is, mbIL15 appears to preferentially engage IL-15 receptors on the same cells, resulting in autocrine stimulation, resembling observations made by Hsu et al35 who transduced T lymphocytes with a soluble form of IL-15. This mechanism explains the IL-15 expression pattern that we consistently observed when we labeled mbIL15-transduced NK cells with an anti-IL-15 antibody, showing a substantial proportion of cells with GFP expression but ostensibly lacking IL-15 (Figure 1B). We speculate that in these cells, IL-15 is expressed but not accessible to antibody because it is bound to its receptor and/or internalized. The capacity of mbIL15 to promote NK cell viability could explain the increased cytotoxicity exerted by these cells, particularly in 24-hour in vitro assays and in vivo. However, the superiority of mbIL15-NK cells was also clear in short-term (4-hour) assays, and these cells also released more lytic granules by CD107a staining. Therefore, expression of mbIL15 is likely to increase NK cell cytotoxicity by other means, possibly by enhancing their activation status.
Clinical administration of NK cells typically relies on IL-2 to support their survival and expansion in vivo.6,7,11,12 The multiple side effects related to IL-2 administration, however, are potentially serious and often render administration of this cytokine poorly tolerated.13,14 Conversely, stopping IL-2 administration or reducing its dose may result in decreased NK cell expansion and inefficient antitumor effect, which may be further inhibited by the stimulation of regulatory T cells.15,16 To this end, replacing IL-2 with IL-15 is potentially attractive, but the clinical formulation of IL-15 is still being tested. Although it was overall well tolerated when administered to rhesus macaques, adverse effects were observed in some animals, including diarrhea, emesis, weight loss, transient neutropenia, increase in transaminases, and hyponatremia.21-23 In this animal model, Lugli et al36 observed T- and NK-cell expansion but also expansion of regulatory T cells. Contrary to NK cells transduced with wtIL15, those transduced with mbIL15 released an exceedingly small amount of IL-15 in the supernatant, and much of the surface protein appeared to be occupying autologous receptors. Thus, any potential side effect that may be caused by the interaction of IL-15 with cells other than NK cells should be minimized by this approach. Of note, Mishra et al37 reported that prolonged exposure of murine large granular lymphocytes to IL-15 leads to their leukemic growth. This poses a potential safety concern for IL-15 administration in patients and also for the use of NK cells expressing IL-15, particularly if such cells were administered to patients at a low risk of relapse. In our experiments, however, NK cells expressing mbIL15 generally survived for much shorter periods than the 1 year or more reported for T-cell clones expressing soluble IL-15.35 Moreover, we did not observe persistent NK expansion in immunodeficient mice, with a follow-up exceeding 12 months.
There is considerable clinical evidence supporting the anticancer potential of NK cells both in hematopoietic stem cell transplantation and as adoptive cellular infusions.1-7,38 NK cells also play a critical role in mediating antibody-dependent cell cytotoxicity in patients treated with monoclonal antibodies.39,40 Thus, infusion of NK cells could be beneficial in multiple settings. Expansion of human NK cells in large numbers ex vivo is feasible28,41 ; robust, large-scale methods for this purpose have been established9,10 and are being used in clinical trials. Genetic modification of NK cells by retroviral transduction28 or electroporation42 is also possible. Therefore, the translation of the approach described here into clinical-grade conditions is realistic, and it is warranted by the predicted superior expansion and cytotoxicity of mbIL15-NK cells, particularly when burrowing into a microenvironment that may be relatively cytokine-depleted, such as the bone marrow or that adjacent to a tumor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities, by the Viva Foundation for Children with Cancer of Singapore, and by a Singapore Translational Research Investigator Award from the National Medical Research Council of Singapore.
Authorship
Contribution: M.I., D.S., T.K., N.S., S.M.H.C., E.C.-S., and C.I. performed experiments and analyzed data; and D.C. initiated the study, analyzed data, and wrote the manuscript with the input of all other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dario Campana, Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Centre for Translational Medicine, 14 Medical Dr, Level 9 South, Singapore 117599; e-mail: paedc@nus.edu.sg.
References
Author notes
M.I. and D.S. contributed equally to this study.