Key Points
Infection is the major cause of treatment-related mortality in pediatric acute lymphoblastic leukemia and is greatest during the induction phase.
Children with Down syndrome are at high risk for infection-related mortality throughout all treatment phases, including the low-intensity maintenance phase.
Abstract
Although infection is the major cause of treatment-related mortality (TRM) in childhood acute lymphoblastic leukemia, factors associated with infection-related mortality (IRM) are poorly understood. To address this, we report an analysis of all 75 cases of IRM in the United Kingdom Childhood Acute Lymphoblastic Leukaemia Randomised Trial 2003 (UKALL 2003). The 5-year cumulative incidence of IRM was 2.4% (95% confidence interval [CI], 1.9%-3.0%), accounting for 75 (30%) of 249 trial deaths and 75 (64%) of 117 TRM deaths. Risk for IRM as a proportion of TRM was greater in induction than other phases (77% vs 56%; P = .02). Sixty-eight percent of cases were associated with bacterial infection (64% Gram-negative) and 20% with fungal infection. Down syndrome was the most significant risk factor for IRM (odds ratio [OR], 12.08; 95% CI, 6.54-22.32; P < .0001). In addition, there was a trend toward increased IRM in girls (OR, 1.63; 95% CI, 1.02-2.61; P = .04), as well as increasing treatment intensity (regimen B vs A: OR, 2.11 [95% CI, 1.24-3.60]; regimen C vs A: OR, 1.41 [95% CI, 0.76-2.62]; P = .02). Importantly, patients with Down syndrome were at significantly higher risk for IRM during maintenance (P = .048). Our results confirm Down syndrome as a major risk factor for IRM. Enhanced supportive care and prophylactic antibiotics should be considered in high-risk patient groups and during periods of increased risk. This study was registered at http://www.controlled-trials.com/ as #ISRCTN07355119.
Introduction
Outcome in acute lymphoblastic leukemia (ALL) in children has shown a steady improvement, with recent trials demonstrating excellent survival in standard-risk patients.1-3 This has been achieved through a combination of enhanced risk stratification and improved supportive care. However, despite the advances in disease outcome, treatment-related toxicity remains unacceptably high. Therefore, recent trials have focused on treatment reduction in patients with favorable prognosis in an attempt to reduce treatment-related toxicity.4,5
The incidence of treatment related mortality (TRM) on contemporary ALL trials is reported to be between 2% and 4%.2,3,6,7 The most common cause of TRM is infection. The 5-year event-free survival in low-risk patients, who have favorable cytogenetics and a good response to treatment, is now higher than 93%, with death from TRM being as common as relapse.8-10 As such, reducing infection-related mortality (IRM) is paramount to improving future outcomes in childhood ALL.
Despite the increasing importance of IRM in ALL, little is known about the clinical characteristics associated with IRM. In pediatric acute myeloid leukemia, several large studies have investigated risk factors associated with IRM in an attempt to inform on interventions to reduce incidence.11,12 However, similar studies in pediatric ALL have been limited by small sample size.6,13 Furthermore, in a recent systematic analysis, Ethier et al found that only 6.3% of pediatric ALL clinical trials provided definitions and rates of TRM.14 This illustrates the current paucity of data available on TRM in pediatric ALL trials and highlights the difficulty in making comparisons between different trials.
To address the current lack of evidence, we performed a retrospective analysis of the clinical characteristics of all cases of IRM in the recently reported large, multicenter, randomized controlled trial, Medical Research Council United Kingdom Childhood Acute Lymphoblastic Leukaemia Randomised Trial 2003 (MRC UKALL 2003). This trial represents the largest cohort of deaths caused by sepsis reported in pediatric ALL studies.
Methods
Patients
The population investigated in this study included all patients enrolled in the MRC UKALL 2003 trial, which has recently been reported.10 The trial focused on the use of treatment stratification using minimal residual disease (MRD) monitoring, including reduction of treatment intensity in MRD low-risk patients. The trial recruited consecutive children and young people with ALL diagnosed at 45 centers in the United Kingdom and Ireland between October 2003 and June 2011. Patients aged younger than 1 year or with mature B-cell ALL were not eligible. Initially, the upper age limit of entry was 18 years, but this was increased to the 20th birthday in April 2006 and the 24th birthday in September 2007 in view of evidence for improved outcome of young adults treated on pediatric protocols. Patients with Philadelphia-chromosome-positive ALL were transferred to other protocols such as the European Study for Philadelphia-chromosome-positive ALL (EsPhALL) or UKALL XII once their Philadelphia chromosome status was known.
The protocol was approved by the Multi-Centre Research Ethics Committee for Scotland. Patients were enrolled at individual treatment centers by principal investigators after written informed consent from carers or patients was obtained. The trial was monitored by an independent data monitoring committee, which reviewed safety and efficacy data annually. The study was conducted in accordance with the Declaration of Helsinki.
Patients were stratified according to initial clinical risk for relapse, based on the National Cancer Institute (NCI) risk criteria (NCI standard risk: patients younger than 10 years with a white blood cell count of less than 50 × 109/L; NCI high risk: patients aged 10 years or older and those with a white blood cell count of at least 50 × 109/L) and leukemia cytogenetics (all patients with a cytogenetic abnormality involving rearrangement of the MLL gene, hypodiploidy [<45 chromosomes], or intrachromosomal amplification of chromosome 21 were classified as clinical high risk). In the absence of high-risk cytogenetics, NCI standard-risk patients were allocated to the clinical standard-risk group, regimen A. NCI high-risk patients were allocated to the intermediate-risk group, regimen B, and those with high-risk cytogenetics were allocated to the clinical high-risk group, regimen C. An overview of the 3 treatment regimens is shown in supplemental Figure 1, available on the Blood website. In addition, standard- or intermediate-risk patients with a slow early response to induction therapy were reclassified to the clinical high-risk group. Further details of the chemotherapy regimens can be found in supplemental Tables 2–4 and in the recent publication by Vora et al.10
Supportive care
Supportive care guidance was detailed in the protocol. This included the use of Pneumocystis jiroveci pneumonitis prophylaxis and the hematological parameters required at the start of each phase of chemotherapy. Antifungal prophylaxis was not used. Treatment of infective episodes was based on local policies and protocols for management of febrile neutropenia. It is standard practice in the United Kingdom for all immunocompromised children to receive the annual influenza vaccine. The varicella zoster vaccine does not form part of the routine vaccination program in the United Kingdom.
The overall treatment intensity for patients with Down syndrome (DS) was reduced after July 2009 because of excess treatment-related mortality; from that time, patients with DS were registered on the trial but did not undergo randomization and were treated as clinical standard-risk patients, with adjustment of postinduction treatment according to response to induction therapy. Increased supportive care measures were also recommended, including prophylactic antibiotics and regular review during periods of intensive chemotherapy.
Definitions
Within the trial, treatment-related mortality was defined as any death occurring at any point after the start of treatment that did not occur as a result of relapse or secondary malignancy. IRM was defined as any death that occurred in the presence of clinical or microbiological documented infection. Induction death was defined as any death occurring between commencement of treatment and the start of the consolidation period.
Analysis included all cases of IRM that occurred as a first event during first remission. Infection-related deaths were identified from the trial database, using information provided by the severe adverse event reporting form. Further information was then obtained from the relevant treatment centers, using a standardized proforma. Additional information obtained included clinical findings during the septic episode, microbiological and radiological results, and the treatment given. Completed proformas were returned on all identified cases of IRM.
As defined by the Centers for Disease Control and Prevention, infections were classified as catheter-associated blood stream infections (CABSIs) if there were positive blood culture results, a central venous catheter (CVC) was in situ, and there was no evidence of infection at another site.15 All infections classified as fungal were diagnosed by microbiological isolation, and therefore met the European Organisation for Research and Treatment of Cancer criteria for “proven” infections.16 Neutropenia was defined as neutrophil count lower than 0.5 × 109/L, in accordance with recent National Institute for Health and Clinical Excellence guidance.17
Statistical analysis
The cumulative incidence of death resulting from various causes was determined after adjusting for competing risks, as previously described.18 Death was categorized as resulting from sepsis, resulting from other treatment-related causes, or other. Categorical variables were compared with the χ-squared test and continuous variables with an appropriate nonparametric test. The relationship between the binary outcome, sepsis death, and other variables was examined using multivariate logistic regression.
Follow-up was to October 31, 2012, with a median follow-up (for surviving patients) of 4 years, 10 months (range, 1.5 months - 9 years, 1 month).
Results
Patient demographics
The UKALL 2003 trial included 3126 eligible patents. Patient demographics are shown in Table 1. In total, there have been 249 deaths, of which 132 (53.0%) were disease-related and 117 (47.0%) were caused by TRM. Sepsis was the most common cause of TRM, with 75 deaths. This constitutes 2.4% of all trial patients, 30.1% of the 249 deaths overall, and 64.1% of the 117 treatment-related deaths. There was slight fluctuation in the rate of IRM per year of the trial, but no significant trend was observed (P = .2).
Patient demographics and univariate analysis to identify risk for IRM
| Demographics . | All patients (n = 3126) . | IRM (n = 75 . | P-value (IRM vs all other patients) . |
|---|---|---|---|
| Sex | |||
| Female | 1350 (43.2%) | 39 (52.0%) | .1 |
| Male | 1776 (56.8%) | 36 (48.0%) | |
| Age group (age at treatment start), years | |||
| <2 | 210 (6.7%) | 5 (6.7%) | .5 |
| 2-9 | 2077 (66.4%) | 44 (58.7%) | .1* |
| 10-15 | 610 (19.5%) | 19 (25.3%) | |
| 16+ | 229 (7.3%) | 7 (9.3%) | |
| White blood cell group (×109/L) | |||
| <10 | 1407 (45.0%) | 31 (41.3%) | .1† |
| 10-19 | 502 (16.1%) | 7 (9.3%) | |
| 20-49 | 526 (16.8%) | 16 (21.3%) | |
| 50-99 | 315 (10.1%) | 9 (12.0%) | |
| 100+ | 376 (12.0%) | 12 (16.0%) | |
| NCI risk group | |||
| Standard | 1816 (58.1%) | 34 (45.3%) | .02 |
| High | 1310 (41.9%) | 41 (54.7%) | |
| DS | |||
| No | 3041 (97.2%) | 59 (78.7%) | <.0001 |
| Yes | 86 (2.8%) | 16 (21.3%) | |
| Immunophenotype | |||
| T | 387 (12.4%) | 8 (10.7%) | .6 |
| non-T | 2739 (87.6%) | 67 (89.3%) | |
| Treatment regimen given | |||
| A | 1538 (49.2%) | 27 (36.0%) | .01 |
| B | 845 (27.0%) | 31 (41.3%) | |
| C | 743 (23.8%) | 17 (22.7%) |
| Demographics . | All patients (n = 3126) . | IRM (n = 75 . | P-value (IRM vs all other patients) . |
|---|---|---|---|
| Sex | |||
| Female | 1350 (43.2%) | 39 (52.0%) | .1 |
| Male | 1776 (56.8%) | 36 (48.0%) | |
| Age group (age at treatment start), years | |||
| <2 | 210 (6.7%) | 5 (6.7%) | .5 |
| 2-9 | 2077 (66.4%) | 44 (58.7%) | .1* |
| 10-15 | 610 (19.5%) | 19 (25.3%) | |
| 16+ | 229 (7.3%) | 7 (9.3%) | |
| White blood cell group (×109/L) | |||
| <10 | 1407 (45.0%) | 31 (41.3%) | .1† |
| 10-19 | 502 (16.1%) | 7 (9.3%) | |
| 20-49 | 526 (16.8%) | 16 (21.3%) | |
| 50-99 | 315 (10.1%) | 9 (12.0%) | |
| 100+ | 376 (12.0%) | 12 (16.0%) | |
| NCI risk group | |||
| Standard | 1816 (58.1%) | 34 (45.3%) | .02 |
| High | 1310 (41.9%) | 41 (54.7%) | |
| DS | |||
| No | 3041 (97.2%) | 59 (78.7%) | <.0001 |
| Yes | 86 (2.8%) | 16 (21.3%) | |
| Immunophenotype | |||
| T | 387 (12.4%) | 8 (10.7%) | .6 |
| non-T | 2739 (87.6%) | 67 (89.3%) | |
| Treatment regimen given | |||
| A | 1538 (49.2%) | 27 (36.0%) | .01 |
| B | 845 (27.0%) | 31 (41.3%) | |
| C | 743 (23.8%) | 17 (22.7%) |
Age <10 vs 10+ years.
P-value for trend.
As IRM is usually an early event, the 3- and 5-year cumulative incidence rates were also calculated. However, as the majority of patients have completed treatment and there was minimal loss to follow-up, the figures were similar to the absolute numbers (Table 2). At 5 years, the cumulative incidence of death from all causes was 8.5%; the incidence of death from TRM was 3.8%, accounting for 44.7% of all deaths, and death from IRM was 2.4%.
Cumulative incidence of mortality at 3 and 5 years
| Mortality . | Cumulative incidence, % . | 95% confidence interval . |
|---|---|---|
| 3 years | ||
| Infection-related | 2.4% | 1.9%-3.0% |
| Other treatment- related | 1.3% | 0.9%-1.7% |
| Non-treatment-related | 2.9% | 2.3%-3.6% |
| Overall | 6.6% | |
| 5 years | ||
| Infection-related | 2.4% | 1.9%-3.0% |
| Other treatment-related | 1.4% | 1.0%-1.9% |
| Non-treatment-related | 4.7% | 3.9%-5.6% |
| Overall | 8.5% |
| Mortality . | Cumulative incidence, % . | 95% confidence interval . |
|---|---|---|
| 3 years | ||
| Infection-related | 2.4% | 1.9%-3.0% |
| Other treatment- related | 1.3% | 0.9%-1.7% |
| Non-treatment-related | 2.9% | 2.3%-3.6% |
| Overall | 6.6% | |
| 5 years | ||
| Infection-related | 2.4% | 1.9%-3.0% |
| Other treatment-related | 1.4% | 1.0%-1.9% |
| Non-treatment-related | 4.7% | 3.9%-5.6% |
| Overall | 8.5% |
Factors associated with IRM
A univariate analysis was performed to identify significant relationships between patient factors and IRM. As shown in Table 1, NCI risk group, treatment regimen, and DS all showed significant association with IRM. There was also a trend toward an increased risk for IRM in patients older than 10 years; however, this did not reach significance (P = .1).
Given the highly significant result for DS and differences in some patient characteristics for patients with DS, the association between patient characteristics and sepsis death was reanalyzed, controlling for DS. This additionally highlighted patient sex to be of borderline significance (P = .05), with an increased risk in girls. The factors predicting IRM in DS were otherwise the same as in the non-DS population.
Multivariate analysis
To verify the factors independently associated with IRM, a multivariate analysis was performed using the factors identified as significant in the univariate analysis: DS, NCI risk group, treatment regimen, and sex. The multivariate analysis confirmed the significance of all factors. However, because of a very high correlation between NCI risk group and treatment regimen, only treatment regimen is included in the final model in Table 3. DS conferred the greatest risk for IRM, with an odds ratio (OR) of 12.08 (95% confidence interval [CI], 6.54-22.32; P < .0001). Increasing intensity of treatment regimen given (regimen B vs A: OR, 2.11 [95% CI, 1.24-3.60]; regimen C vs A: OR, 1.39 [95% CI, 0.75-2.60]; P = .02) and female sex (OR, 1.63; 95% CI, 1.02-2.61; P = .04) were also associated with an increased risk for IRM but had a much weaker magnitude of effect.
Multivariate analysis identifying factors associated with IRM
| Subgroup . | Odds ratio . | 95% confidence interval . | P-value . |
|---|---|---|---|
| DS | |||
| No | 1.00 | <.0001 | |
| Yes | 12.08 | 6.54-22.32 | |
| Treatment given | |||
| A | 1.00 | .02 | |
| B | 2.11 | 1.24-3.60 | |
| C | 1.41 | 0.75-2.60 | |
| Sex | |||
| Male | 1.00 | .04 | |
| Female | 1.62 | 1.02-2.61 |
| Subgroup . | Odds ratio . | 95% confidence interval . | P-value . |
|---|---|---|---|
| DS | |||
| No | 1.00 | <.0001 | |
| Yes | 12.08 | 6.54-22.32 | |
| Treatment given | |||
| A | 1.00 | .02 | |
| B | 2.11 | 1.24-3.60 | |
| C | 1.41 | 0.75-2.60 | |
| Sex | |||
| Male | 1.00 | .04 | |
| Female | 1.62 | 1.02-2.61 |
Timing of IRM
Sepsis was the most common cause of TRM in both the induction and postinduction phases. In total, 48.0% (36/75) of all infectious-related deaths occurred during induction, more than in any other treatment phase (induction, 48.0%; consolidation, 9.3%; delayed intensification, 22.6%; maintenance, 20%). The proportion of TRM caused by sepsis was significantly higher in the induction phase than in the overall postremission period (76.6% vs 55.7%, respectively; P = .02).
The majority of patients (74.2%) were neutropenic (neutrophil count, <0.5×109/L) at the time of presentation of the septic episode. Notably, the majority of deaths (55%; 41/75) occurred within 48 hours of initial presentation. Further analysis failed to identify any significant associations between time to death and other factors.
Site and type of infection
The clinical site of infection was evident in 64 cases, including 8 cases that were diagnosed on post mortem. Thirty-five percent of cases were respiratory infections (26/75), 11% were gastrointestinal infections (8/75), 11% were central nervous system infections (8/75) and 29% were CABSIs (22/75).
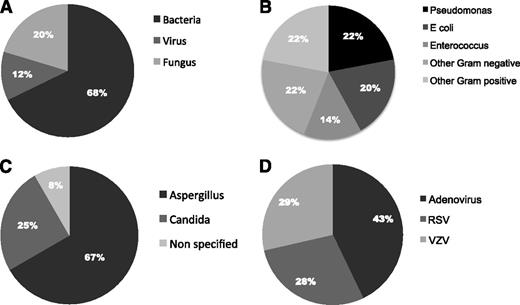
An infective pathogen was identified in 75% of IRM cases (56/75), with dual pathogens identified in 3 cases. Of these, the majority were bacterial infections (68%), 20% were fungal, and 12% were viral (Figure 1A). Gram-negative organisms represented the most frequent type of bacteria, accounting for 64% of bacterial infections. The most common specific bacteria detected were Pseudomonas (22%), Escherichia coli (20%), and Enterococcus (14%; Figure 1B). A number of other bacteria, including Clostridium. Klebsiella, Haemophilus, and Streptococcus, were detected in a minority of cases. Fungal infections represented the second most common cause of IRM (20%; 12 cases). Aspergillus was the most frequent fungal pathogen detected (8 cases), whereas the remaining cases were mainly caused by candida (3 cases; Figure 1C). Viral pathogens were isolated in 7 cases of TRM (12%). Of these, adenovirus was found in 3 cases, and respiratory syncytial virus and varicella zoster virus were found in 2 cases each (Figure 1D). Further analysis failed to find any significant associations between the type of infective pathogen and other factors.
Pathogens isolated in 75 cases of IRM. (A) Type of pathogen isolated in cases of IRM with positive microbiological findings. (B) Types of bacteria isolated in cases of IRM (n = 45). (C) Types of fungus isolated in cases of IRM (n = 12). (D) Types of virus isolated in cases of IRM (n = 8).
Pathogens isolated in 75 cases of IRM. (A) Type of pathogen isolated in cases of IRM with positive microbiological findings. (B) Types of bacteria isolated in cases of IRM (n = 45). (C) Types of fungus isolated in cases of IRM (n = 12). (D) Types of virus isolated in cases of IRM (n = 8).
DS
Of the factors identified by multivariate analysis, DS conferred the greatest risk for sepsis death (OR, 12.08; 95% CI, 6.54-22.32; P < .0001). Of 86 patients with DS in the trial, 25 have died, 17 as a result of TRM, giving a 5-year cumulative incidence of TRM in patients with DS of 21.6% (95% CI, 12.8%-30.4%). Of the cases of TRM, 16 were a result of IRM. Therefore, 18.6% of patients with DS died of IRM compared with only 1.9% of non-DS patients (P < .0001). In patients with DS, there were significantly more deaths from sepsis (16/86; 18.6%) than from other treatment-related deaths 1/86 (1.2%; P = .005) and from other deaths overall (9/86 [10.5%]; P < .0001), making sepsis the leading cause of death in patients with DS treated on this trial.
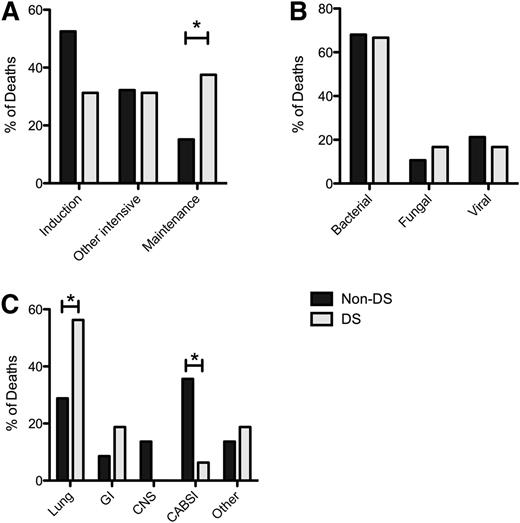
Patients with DS were at increased risk for IRM compared with non-DS patients in all trial phases. However, although most IRM occurred in the induction phase in non-DS patients, IRM in patients with DS was spread relatively evenly throughout all trial phases (Figure 2A). Importantly, a significantly greater proportion of infectious-related deaths occurred in the relatively low-intensity maintenance phase in patients with DS than in non-DS patients (37.5% vs 15.2%; P = .048).
IRM in DS and non-DS patients. (A) Timing of IRM in DS and non-DS patients. (B) Type of pathogen in DS and non-DS patients. (C) Site of infection in DS and non-DS patients. * P < .05
IRM in DS and non-DS patients. (A) Timing of IRM in DS and non-DS patients. (B) Type of pathogen in DS and non-DS patients. (C) Site of infection in DS and non-DS patients. * P < .05
Discussion
This article represents the largest cohort of sepsis-related deaths to be reported in pediatric ALL. Although the data were collected retrospectively, we were able to collect detailed information on all cases of IRM that occurred in this large, multicenter trial. The results demonstrate that DS is a highly significant factor for IRM on contemporary childhood ALL protocols. Other factors such as intensity of treatment regimen given and sex were associated with an increased risk for IRM, but a much weaker magnitude of effect.
The comparison of rates of IRM and TRM across different trials is difficult; there is significant heterogeneity in the definitions and data capture methods used, along with differences in patient cohorts, in terms of the age range included and the proportion of high-risk patients. Furthermore, as the majority of groups do not report IRM rates for each phase, it is difficult to make detailed comparisons. Nevertheless, the 5-year cumulative incidence of IRM on UKALL 2003 overall was 2.4%, which is comparable to other trials in which IRM has been reported (1.7% - 2.3%).3,6,13
Our analysis demonstrated that a significant proportion of sepsis deaths occurred during induction and periods of neutropenia. Thus, increased awareness of the potential for septic complications is required when caring for patients during these high-risk periods. Furthermore, the majority of deaths occurred within the first 48 hours of presentation, emphasizing the need for rapid recognition and treatment of infective episodes. The United Kingdom’s National Institute for Health and Clinical Excellence classifies neutropenic sepsis as a medical emergency and recommends administration of antibiotics within 1 hour.17
Unexpectedly, although IRM was highest during the induction phase, it was also seen in low-intensity periods including maintenance. UKALL2003 included regular pulses of dexamethasone and vincristine throughout the maintenance period. However, it is unclear whether this contributed to IRM. To address this issue, the current UK study UKALL 2011 is randomly allocating to pulses or not, which will therefore determine its significance.
Our analysis confirms the increased risk for infectious-related complications in patients with DS treated on ALL protocols, as seen in other studies.6,19-22 However, both TRM and IRM appear slightly higher in our cohort than in other trials. A recent retrospective analysis by the Ponte di Legno group of 653 DS-ALL patients treated on various protocols found a 2-year TRM of 7%, which is lower than that seen on UKALL 2003 (5-year cumulative incidence of TRM 21.6%).7 However, the relapse rate on UKALL 2003 was lower, resulting in similar overall survival and suggesting that the higher rate of TRM is offset by a lower relapse rate.
There are a number of possible reasons for the high IRM in the DS population, including an increased sensitivity to chemotherapy-induced mucositis and prolonged myelosuppression.19,20 This may in part be a result of heightened methotrexate sensitivity and reduced renal clearance of methotrexate.23,24 In addition, a recent report found an association between hyperglycemia and episodes of sepsis during the induction period on the UKALL 2003 trial, particularly in patients with DS.25 Several factors are likely to contribute to hyperglycemia, including the use of corticosteroids and asparaginase, and it is therefore important that further research is conducted to assess this link. We also found that patients with DS were at particular risk for IRM secondary to respiratory infection. This is likely to be because of other comorbidities associated with DS, including cardiac abnormalities, immunodeficiency, gastroesophageal reflux, and tracheomalacia.26
Importantly, the timing of IRM differs in patients with DS compared with in the non-DS population. Although the risk for IRM in induction is still higher in patients with DS than in non-DS patients, IRM in patients with DS is also significantly higher in the relatively low-intensity maintenance phase. A similar result was found in the Ponte di Legno analysis, with around half of all TRM occurring during maintenance.7 This indicates that patients with DS require enhanced supportive care during the maintenance period and that further studies are required to assess the value of prophylactic antibiotics or immunoglobulin during this period. In the United Kingdom, the maintenance period has already been reduced from 3 years to 2 years in boys with DS to reduce potential risk for IRM in this group.
In this trial, we have previously reported that MRD risk status and relapse risk were no greater in patients with DS, and so reduction in IRM is therefore essential to improve outcomes.27 The COG group have introduced a series of measures to reduce the IRM in the DS population, including the use of leucovorin rescue after intrathecal methotrexate doses, a single delayed intensification phase, and expanded supportive care guidelines.22 This resulted in a reduction in IRM from 11.5% to 1.7% in standard-risk disease. However, IRM in high-risk disease only decreased from 15% to 10%. Within the United Kingdom, similar alterations in chemotherapy were implemented, including omission of daunorubicin from induction in high-risk patients and restriction to a single delayed intensification phase. Furthermore, enhanced supportive care guidance was given, including the use of prophylactic antibiotics during periods of intensive chemotherapy, clinical review 3 times each week, and intensive treatment of all episodes of febrile neutropenia. Preliminary data indicate that these modifications have reduced induction deaths (4/46 DS deaths premodifications vs 1/40 DS deaths postmodifications).27 Further follow-up is required to ascertain the long-term effects of the changes. Interestingly, the Ponte di Legno found no difference in TRM rates between patients with DS treated using a 3-drug or 4-drug induction, indicating that the inclusion of an anthracycline in induction had no effect on TRM. This suggests that further modifications are required to reduce TRM in patients with DS and that work is now underway to develop a specific DS ALL treatment protocol.7
The multivariate model also found a small increase in IRM in girls and in those treated on high-risk protocols. Similarly, the Nordic group described an increased rate of TRM in girls, largely because of sepsis.6 The reason for this association is unclear, although the authors of the Nordic study speculate that this may be a result of sex differences in immunological response to infections or differences in toxicity after cytotoxic chemotherapy.
Although we found a trend toward increased IRM with increasing intensity of treatment, when this was analyzed on the basis of individual regimens, significance was limited to the regimen B vs regimen A comparison, but not regimen C vs regimen A. The reason for this discrepancy is unclear. Previous studies have seen more infections with the use of dexamethasone, as used in the UKALL 2003 trial,28,29 although this is outweighed by improved outcomes.3,9,30 However, this rate may be increased when dexamethasone is given in combination with anthracylines,31 which may, in part, account for the increased risk in those receiving the 4-drug induction. Again, enhanced supportive care measures, such as antibiotic and antifungal prophylaxis or the use of intravenous immunoglobulin replacement, should be considered in the intensive phases of these regimens.
Bacterial infections were the leading cause of IRM. However, in contrast to research that has highlighted the prevalence of Gram-positive infections in pediatric ALL,13 we found that Gram-negative bacteria were most frequently implicated in IRM. In particular, Pseudomonas was the single most common cause of TRM, as found in the Nordic study (Nordic Society for Paediatric Haematology and Oncology ALL 92).6 It is therefore imperative that antibiotics used in the treatment and prophylaxis of infections in pediatric ALL have adequate efficacy against pseudomonal species and other Gram-negative bacteria. However, the use of antibiotic prophylaxis to reduce IRM needs to be weighed up against the potential to increase antibiotic resistance. Furthermore, fungal infections accounted for 20% of cases of IRM. This is a significant proportion of cases and highlights the need for consideration of antifungal prophylaxis and awareness of the potential risk for invasive fungal infections, as early recognition and treatment can reduce mortality. Notably, no deaths occurred because of P jiroveci or influenza. This may reflect the use of routine P jiroveci prophylaxis and annual influenza vaccination in children undergoing treatment for ALL.
Despite several limitations, such as the retrospective methodology, this study reports the largest cohort of IRM in pediatric ALL, thus providing a detailed picture of IRM in this patient group. Although a minority of the cases were classified as IRM without microbiological confirmation, each case had compelling clinical features implicating an infection etiology, warranting inclusion in the analysis. Although it is possible that some positive microbiological results were contaminants, all patients had clinical features of infection. The use of strict criteria for the presence of fungal infection, requiring microbiological proof, may have underestimated the true proportion of infectious deaths that occurred as a result of fungal infection, but we feel that this is the most accurate means of classifying fungal infection. In addition, distinguishing definite CVC infection from alternative sources of sepsis remains difficult. We opted to use the CDC definition in which a CABSI is defined as signs of sepsis with at least a single positive blood culture in the presence of a CVC, with no other focus. This definition does not confirm causation but gives an indication as to the potential burden of CVC infection. In addition, further data are required to explore other potential factors such as the effect of time between presentation and first antibiotics and to determine whether there is a center effect (tertiary specialized center vs local treatment center) with regard to survival outcomes in febrile neutropenia.
In summary, our analysis has demonstrated that IRM is a major factor in the outcome of children and young people treated for ALL. Importantly, in the low-risk group, infectious death is now almost as common as death from ALL. Therefore, further improvements in overall survival will require a reduction in TRM, which represents a significant scientific and logistic challenge and confirms the pressing need to harmonize TRM definitions across clinical trials. Furthermore, DS is identified as a major risk factor for sepsis death on UKALL 2003, accounting for the majority of deaths in this group. For such a high-risk cohort, consideration should be given to the use of enhanced supportive care and increased antibiotic prophylaxis in an attempt to reduce IRM.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients, families and clinical teams who participated in the trial.
The trial was supported by grants from Leukaemia and Lymphoma Research and the Medical Research Council.
Authorship
Contribution: A.V. was the trial’s chief investigator; D.O. and S.S. wrote the first draft of the manuscript; D.O., J.B., R.W., R.C., and S.D. collected and analyzed data; and N.G. and R.H. reviewed and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David O’Connor, Centre for Haematology, Commonwealth Building, Du Cane Road, Imperial College London, London W12 0NN, United Kingdom; e-mail d.oconnor@imperial.ac.uk.