In this issue of Blood, Yee et al1 have demonstrated that expression or infusion of a truncated von Willebrand factor (VWF) fragment containing the factor VIII (FVIII)-binding D′D3 region of VWF is sufficient to stabilize endogenous FVIII levels in VWF-deficient mice. In the absence of the carrier function of VWF, FVIII is susceptible to rapid proteolysis and clearance resulting in markedly reduced plasma levels of FVIII that contribute to a bleeding diathesis.
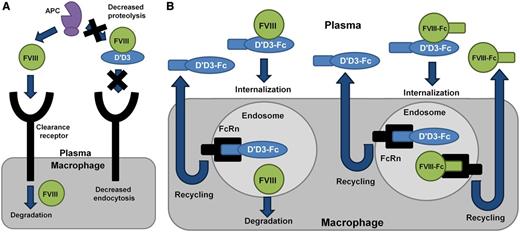
Mechanisms regulating plasma levels of FVIII. (A) In the absence of VWF, plasma levels of FVIII are reduced because of proteolysis by activated protein C (APC) and accelerated receptor-mediated endocytosis. VWF D′D3 is able to stabilize plasma levels of FVIII presumably by regulating these clearance mechanisms. (B) VWF D′D3-Fc has a prolonged half-life relative to VWF D′D3 because of interactions with the Fc receptor FcRn recycling pathway. VWF D′D3-Fc is able to stabilize endogenous FVIII levels in VWF-deficient mice because of continual secretion of FVIII into the plasma. However, in hemophilia A mice, exogenous FVIII is not adequately protected from clearance and rapid degradation. Coinfusion of VWF D′D3-Fc and FVIII-Fc might result in recycling of both molecules through the FcRn endosomal pathway, leading to an extended half-life beyond that observed for FVIII-Fc alone.
Mechanisms regulating plasma levels of FVIII. (A) In the absence of VWF, plasma levels of FVIII are reduced because of proteolysis by activated protein C (APC) and accelerated receptor-mediated endocytosis. VWF D′D3 is able to stabilize plasma levels of FVIII presumably by regulating these clearance mechanisms. (B) VWF D′D3-Fc has a prolonged half-life relative to VWF D′D3 because of interactions with the Fc receptor FcRn recycling pathway. VWF D′D3-Fc is able to stabilize endogenous FVIII levels in VWF-deficient mice because of continual secretion of FVIII into the plasma. However, in hemophilia A mice, exogenous FVIII is not adequately protected from clearance and rapid degradation. Coinfusion of VWF D′D3-Fc and FVIII-Fc might result in recycling of both molecules through the FcRn endosomal pathway, leading to an extended half-life beyond that observed for FVIII-Fc alone.
VWF and FVIII circulate in the plasma in a tight noncovalent complex. The FVIII binding site of VWF (residues 767-1031),2 contained within the D′D3 region, interacts with several sites in the light chain of FVIII (acidic a3, C1, and C2 domains3,4 ). Type 2N von Willebrand disease (VWD) involves mutations in the VWF D′D3 region that impair binding to FVIII, resulting in reduced plasma FVIII levels (5% to 30% of normal) but normal VWF.5 A recently solved solution structure for the VWF D′ region (residues 766-864), currently in press in Blood,6 describes D′ as being comprised of 2 independently folding domains termed TIL′ (trypsin-inhibitor-like, residues 766-827) and E′ (residues 829-864).7 It was observed that β-sheets in the TIL′ and E′ domains provide scaffold support to a flexible TIL′ loop. This conformation results in a dynamic TIL′ structure that contains a high positive charge density that may allow for binding to the negatively charged a3 domain of FVIII. The majority of type 2N VWD mutations are localized to the D′ region, with a preponderance of severe type 2N mutations residing in the TIL′ domain and involving either a loss of cysteine residues or positive charge.
Treatment of quantitative FVIII deficiency is dependent on the biological mechanism by which plasma FVIII levels are reduced. For type 2N VWD, administration of exogenous VWF is required to stabilize levels of endogenous FVIII as a substitute for the dysfunctional mutant VWF. In contrast, in hemophilia A, the normal endogenous VWF is able to act as a carrier for intravenously administered FVIII replacement products. The mean circulating half-life of recombinant or plasma-derived FVIII is between 8 and 12 hours, necessitating prophylactic treatment regimens for hemophilia A patients that are both frequent and expensive. Recent strategies designed to prolong FVIII half-life have focused on chemical (PEGylation)8 or genetic modification (Fc-fusion)9 of the FVIII molecule. Although these strategies have resulted in 1.5- to 1.8-fold increases in FVIII half-life relative to the native molecule, it is likely that this observed ceiling on FVIII half-life extension is related to the dominant carrier influence of VWF.
In the studies described by Yee et al, the authors generated a series of truncated VWF variants containing the FVIII-binding region of VWF. These fragments were either expressed or infused into VWF deficient mice, and their influence on stabilization of FVIII was observed. Interestingly, despite the fact that the minimal FVIII-binding VWF fragment (residues 764-1035) bound FVIII with high affinity, plasma FVIII was only stabilized by fragments containing the full VWF D′D3 region (residues 764-1247). The mechanism by which the VWF D′D3 fragment was able to stabilize FVIII in plasma, whereas the truncated VWF D′D3 molecule did not, is unknown, but may involve regulation of protein conformation or posttranslational modifications. It may also be related to the inhibition of interactions between FVIII and plasma proteases such as activated protein C, or clearance receptors such as LRP-1 by the extended VWF D′D3 molecule (see figure).
One limitation to the potential use of these truncated variants in clinical practice is the significant reduction in half-life of truncated VWF relative to the full-length molecule. To resolve this issue, Yee and colleagues generated a VWF D′D3 fragment fused to the Fc portion of immunoglobulin G1 at its C terminus (VWF D′D3-Fc). This molecule demonstrated a prolonged half-life relative to the VWF D′D3 fragment alone, likely by recycling of the VWF D′D3-Fc molecule through the FcRn endosomal pathway. Interestingly, expression of the VWF D′D3-Fc chimera resulted in durable stabilization of endogenous FVIII in VWF-deficient mice, but coinfusion of VWF D′D3-Fc with FVIII resulted in the rapid clearance of the exogenously administered FVIII in FVIII-deficient mice. The failure of VWF D′D3-Fc to stabilize coinfused FVIII in FVIII-deficient mice may be related to competition of VWF D′D3-Fc for FVIII binding with endogenous VWF, or endocytosis of the VWF D′D3-Fc/FVIII complex with dissociation of VWF D′D3-Fc and FVIII in the acidic endosomal compartment, leading to recycling of VWF D′D3-Fc and degradation of the FVIII molecule (see figure).
The stabilization of endogenous FVIII by the VWF D′D3-Fc fragment represents up to a 10-fold increase in plasma FVIII levels that can be sustained for at least 7 days postinfusion. This may be a viable strategy for management of type 2N VWD in which the VWF carrier function is compromised but endogenous FVIII biosynthesis is normal. Although this strategy does not prolong the half-life of exogenous FVIII, it is an intriguing prospect that coinfusion of D′D3-Fc with an extended FVIII half-life product such as FVIII-Fc might overcome the existing barrier in terms of FVIII half-life extension. Combinations of these half-life extension strategies could maintain plasma levels of FVIII above the 1% threshold required to prevent spontaneous bleeding in hemophilia A patients for a prolonged period, reducing both the frequency and cost of prophylactic FVIII replacement.
The functional data involving VWF D′D3 provided in this study by Yee et al, combined with the information imparted by the recently described solution structure of the D′ region of VWF, will likely serve as the basis for future studies aimed at optimizing FVIII stabilization with VWF fragments containing the D′D3 region. These studies may also lead the way toward improving the carrier function of VWF by manipulating the binding affinity between truncated VWF fragments and FVIII, optimizing the length of the VWF fragment necessary for FVIII stabilization, or increasing the plasma half-life of the VWF fragment. The studies presented in this issue by Yee and colleagues provide proof of principle that truncated VWF fragments can stabilize plasma FVIII levels, and suggest strategies for the ultimate translation of these findings to the clinic.
Conflict-of-interest disclosure: The authors declare no competing financial interests.