Key Points
Factor XIII-A is exposed in protruding caps on the activated platelet surface.
Platelet FXIII-A exerts antifibrinolytic function by cross-linking α2AP to fibrin.
Abstract
Factor XIII (FXIII) stabilizes thrombi against fibrinolysis by cross-linking α2-antiplasmin (α2AP) to fibrin. Cellular FXIII (FXIII-A) is abundant in platelets, but the extracellular functions of this pool are unclear because it is not released by classical secretion mechanisms. We examined the function of platelet FXIII-A using Chandler model thrombi formed from FXIII-depleted plasma. Platelets stabilized FXIII-depleted thrombi in a transglutaminase-dependent manner. FXIII-A activity on activated platelets was unstable and was rapidly lost over 1 hour. Inhibiting platelet activation abrogated the ability of platelets to stabilize thrombi. Incorporating a neutralizing antibody to α2AP into FXIII-depleted thrombi revealed that the stabilizing effect of platelet FXIII-A on lysis was α2AP dependent. Platelet FXIII-A activity and antigen were associated with the cytoplasm and membrane fraction of unstimulated platelets, and these fractions were functional in stabilizing FXIII-depleted thrombi against lysis. Fluorescence confocal microscopy and flow cytometry revealed exposure of FXIII-A on activated membranes, with maximal signal detected with thrombin and collagen stimulation. FXIII-A was evident in protruding caps on the surface of phosphatidylserine-positive platelets. Our data show a functional role for platelet FXIII-A through exposure on the activated platelet membrane where it exerts antifibrinolytic function by cross-linking α2AP to fibrin.
Introduction
Factor XIII (FXIII) plays an essential role in normal hemostasis where it contributes to the regulation of fibrinolysis,1-3 the maintenance of pregnancy,4 wound healing, and angiogenesis.5 The function of FXIII in hemostasis is further emphasized in its deficiency, which results in hemorrhage6,7 and slow wound healing.4,7 FXIII circulates in plasma as a heterotetramer (FXIII-A2B2) where the catalytic A (FXIII-A2) subunits are almost exclusively in complex with the inhibitory carrier B (FXIII-B2) subunits.8
FXIII is activated by the combined action of thrombin and Ca2+ to form the active transglutaminase (TG) FXIIIa.9,10 FXIIIa confers stability to the fibrin matrix by cross-linking fibrin, substantially altering its rheologic properties.11,12 TG catalyzes formation of covalent ε-(γ-glutamyl) lysyl bonds13 in which the lysine ε-amino group is cross-linked to the glutamine γ-carboxymide group.14 The cross-linking of γ-chains confers a degree of rigidity to the fibrin network, which is further stabilized by the cross-linking of α-chains15 to generate high-molecular-weight polymers.10,16
FXIIIa also cross-links inhibitors of fibrinolysis to fibrin, further increasing its insolubility and resistance to plasmin. These inhibitors include α2-antiplasmin (α2AP),17 thrombin activatable fibrinolysis inhibitor,18 and plasminogen activator inhibitor-2.19 α2AP is a serpin inhibitor that forms an irreversible complex with plasmin20 and interferes with binding of plasminogen to fibrin.21 FXIIIa cross-links Gln-2 of α2AP to Lys-303 of the Aα chain of fibrin(ogen).22 The crucial role of cross-linked α2AP in stabilizing fibrin is demonstrated by the rapid lysis of clots formed in the absence of α2AP,1,3,17 or when α2AP activity is neutralized.1
FXIII-A is present in platelets22-27 in large quantities; a single platelet contains 60 ± 10 fg,28 making local platelet FXIII-A concentration around 150-fold greater than that in plasma.29 Platelet FXIII-A, also known as cellular FXIII,30 is largely stored in the cytoplasm23,24 and, in contrast to FXIII-A2B2, can be nonproteolytically activated by Ca2+ alone.31 Platelet-rich clots in vivo are much more resistant to lysis than whole blood clots.32 Platelets are known to stabilize static clots,33,34 increase high-molecular-weight α-polymers and γ-γ dimer formation,33-36 and augment cross-linking of α2AP to fibrin.33-35 However, it is unclear how platelet FXIII-A elicits these functions because it is not released during activation37 and does not follow classical routes of secretion in platelets or other cells.38
We have previously shown, using thrombi formed under flow, that FXIII increases resistance to fibrinolysis,39 specifically via cross-linking of α2AP to fibrin.1 Here we show that activated platelets externalize their cytoplasmic pool of FXIII-A onto their membranes. FXIII-A exposed on the platelet surface is functional in cross-linking α2AP and confers fibrinolytic resistance to model thrombi formed under flow.
Methods
Collection of blood and preparation of plasma
Peripheral blood was collected into vacutainers containing acid-citrate-dextrose solution A (Greiner Bio-One LTD, Stonehouse, UK); the first 3 mL was discarded. Pooled normal plasma (PNP) that was essentially free of platelets was prepared from 20 normal donors by centrifugation at 1850g for 30 minutes at 4°C. Platelet-rich plasma (PRP) was prepared by centrifugation at 170g for 10 minutes at 22°C, and platelet-poor plasma (PPP) was prepared by centrifugation at 1850g for 30 minutes at 4°C.40
Preparation of washed platelets
Purified platelets were prepared from outdated human apheresis platelets (donated by Scottish National Blood Transfusion Service, Aberdeen, UK) as previously described.41 Alternatively, platelets were isolated from freshly drawn blood by centrifugation at 260g for 15 minutes at 22°C to collect PRP. PRP was added to 80 mM trisodium citrate, 52 mM citric acid, 183 mM glucose, and 0.1 U/mL apyrase (Sigma-Aldrich, Poole, UK), and centrifuged at 870g for 15 minutes at 22°C. The platelet pellet was washed in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) wash buffer (10 mM HEPES [pH 6.6], 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, 0.1% bovine serum albumin [BSA]) in the presence of 0.1 U/mL apyrase and centrifuged at 870g for an additional 15 minutes at 22°C. The platelet pellet was resuspended in HEPES resuspension buffer (10 mM HEPES [pH 7.45], 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, 0.1% BSA) in the presence of 0.1 U/mL apyrase. Platelet counts were performed on a Siemens ADVIA 2120i Hematology System by the Haematology Department, Aberdeen Royal Infirmary.
Preparation of platelet fractions
Washed apheresis platelets (10 × 109/mL) were activated with 200 μg/mL equine type 1 collagen (American Biochemical and Pharmaceuticals, Surrey, UK) and 200 µM thrombin receptor activator peptide 6 (TRAP-6) (Sigma-Aldrich) for 5 minutes at 37°C. Activated platelets were centrifuged at 13 000g, and the supernatant termed platelet releasate was collected. Platelet lysate was prepared by sonicating platelets for 30 seconds in a sonic dismembrator (ARTEK Systems Corporation, Farmingdale, NY). Platelet membranes were prepared from platelet lysates by centrifuging at 12 000g to remove unlysed platelets. Supernatants were then ultracentrifuged at 100 000g for 1 hour in a Beckman Coulter Optima MAX Ultracentrifuge. The supernatant was collected as the platelet cytoplasmic fraction, and the residual pellet (or membrane fraction) was resuspended in the original volume of 20 mM tris(hydroxymethyl)aminomethane (Tris) –HCl [pH 7.6], 150 mM NaCl, 5 mM glucose, and 0.1 M CaCl2.
FXIII activity assay
FXIII activity was quantified using an in-house activity assay as previously described39 with the following modifications. The WHO First International Standard FXIII plasma (National Institute for Biological Standards and Control, Hertfordshire, UK) was used.42 The reaction was performed in 100 mM Tris-HCl [pH 7.4], 1 mM dithiothreitol, 1% BSA, 0.5 mM 5-(biotinamido) pentylamine (Pierce Thermo Fisher Scientific, Rockford, IL), and 5 mM CaCl2. Plates were washed with 0.01% Triton-X-100 in 100 mM Tris-HCl [pH 7.4] before adding para-nitrophenyl phosphate substrate (Sigma-Aldrich).
Chandler model thrombus lysis
Chandler model thrombus lysis was performed as previously described.39 PNP, FXIII-depleted plasma, or α2AP-depleted plasma (the latter two from Affinity Biologicals, Ancaster, ON, Canada) containing fluorescein isothiocyanate (FITC)-labeled fibrinogen (43.5 μg/mL) with an FITC:fibrinogen ratio of approximately 6:1 were recalcified with CaCl2 (10.9 mM). The loops were rotated for 90 minutes at 30 rpm. Apheresis platelets (5 × 108/mL) or platelet fractions at equivalent concentrations were added in some experiments. Initial experiments were performed with lysates prepared by freeze-thawing apheresis platelets. A nonreversible inhibitor of TG (1,3-dimethyl-2-[(2-oxopropyl) thio]imidazolium chloride) (1 mM)39,43 or a neutralizing antibody to α2AP (150 μg/mL; Technoclone)1 were incorporated prior to recalcification. Formed thrombi were washed in 0.9% (w/v) NaCl and bathed at 37°C in 10 mM Tris, 0.01% TWEEN 20 [pH 7.4] containing 1 μg/mL single-chain tissue-type plasminogen activator (Genentech, San Francisco, CA). Samples were taken at 30-minute intervals for 4 hours. Fluorescence was read (excitation 485 nm and emission 528 nm) on a Biotek Instruments Fluorometer. Alternatively, thrombi were dissolved in 8 M urea, 0.2 M Tris (pH 8), 40 mM dithiothreitol, and 4% sodium dodecyl sulfate at 72°C for 1 hour for western blotting. Model thrombi formed from FXIII-depleted plasma or α2AP-depleted plasma will be referred to as FXIII-depleted thrombi or α2AP-depleted thrombi, respectively.
Western blot
Isolated washed platelet fractions or dissolved thrombi were run on 4% to 12% polyacrylamide Bis-Tris NuPAGE gels with NuPAGE MOPS (morpholinepropanesulfonie) sodium dodecyl sulfate (SDS) running buffer (Life Sciences, Paisley, UK) under reducing conditions. Fibrogammin P (20 μg/mL; CSL Behring, Sussex, UK) was included as a positive control for FXIII-A2B2 and human umbilical vein endothelial cell lysates for TG2. Proteins were transferred to nitrocellulose and immunoblotted with a monoclonal antibody for FXIII-A (2 μg/mL; Ab1834, Abcam, Cambridge, UK), a polyclonal antibody to FXIII-B (200 ng/mL; sc-18015, Santa Cruz Biotechnology, Santa Cruz, CA), a polyclonal antibody to α2AP (2.5 μg/mL; SA2AP-IG, Affinity Biologicals), a polyclonal antibody to fibrinogen γ-chain (1 μg/mL; H-194, Santa Cruz Biotechnology), a polyclonal antibody to fibrinogen α-chain (1 μg/mL; H-300, Santa Cruz Biotechnology), or a monoclonal antibody to TG2 (100 ng/mL; CUB7402, Stratech Scientific Limited, Newmarket, UK). Appropriate horseradish peroxidase (HRP) –conjugated secondary antibodies were applied, and proteins were detected by using enhanced chemiluminescence (Thermo Fisher-Scientific, Leicestershire, UK) with a UVP Biospectrum 810 system and analyzed with UVP visionWorks LS Image Aquisition and Analysis software.
Flow cytometry
Freshly isolated platelets were incubated for 45 minutes with FITC-labeled polyclonal rabbit anti-human FXIII antibody (20 μg/mL; Zedira, Darmstadt, Germany) specific for FXIII-A subunit (supplemental Figure 1 available on the Blood Web site) or an isotype control (Stratech Scientific Limited). Platelets were stimulated in HEPES resuspension buffer (pH 7.45) containing CaCl2 (2 mM) for 45 minutes at room temperature with a range of single or dual agonists. Single agonists included 20 μM adenosine 5′-diphosphate (ADP; Sigma-Aldrich), 500 μg/mL arachidonic acid (Sigma-Aldrich), 20 μM TRAP-6, 100 ng/mL convulxin (CVX; Pentapharm, Basel, Switzerland), or 100 nM thrombin (Sigma-Aldrich). Dual agonists mixtures were CVX and ADP, CVX and arachidonic acid, CVX and TRAP-6, or CVX and thrombin at the described concentrations. In some samples, 5 mM Gly-Pro-Arg-Pro (GPRP; Sigma-Aldrich,) was included to inhibit fibrin polymerization. A Becton Dickinson LSR II flow cytometer with FACSDiva 6.1.3 software (Becton Dickinson) at excitation 488 nm was used for analysis of treated platelets with 10 000 events collected per sample. Platelets were gated forward scatter against side scatter to exclude aggregates and platelet fragments. Data were analyzed by using FlowJo V.X.0.6 software. Results are expressed as mean percent FXIII-A positive platelets ± standard error of the mean.
Confocal microscopy
Ibidi μ-slide VI0.4 uncoated chambers were washed with phosphate buffered saline [pH 7.4]. Washed platelets (5 × 107/mL) were activated with collagen (20 μg/mL) and TRAP-6 (20 μM) or collagen and thrombin (100 nM) in the presence of FITC-labeled polyclonal rabbit anti-human FXIII (20 μg/mL), Alexa-fluor 647 Annexin-V conjugate (1/20; Life Technologies, Paisley, UK), and CaCl2 (2 mM) and allowed to adhere to slides for 5 minutes before analysis. Images were taken by using brightfield and at excitation wavelengths of 488 nm and 633 nm on a Zeiss LSM710 confocal microscope with 63 × 1.40 oil immersion objective and analyzed by using Zen 2012 software.
Statistical analysis
Rates of lysis (FU/min−1) for Chandler model thrombi were determined by best fit of the slope to a centered second-order polynomial quadratic in GraphPad Prism 5.04 and used to calculate fold differences in lysis. Statistical analysis was performed on FXIII activity assay data and Chandler model thrombi lysis rates by using one-way analysis of variance with Dunnett’s multiple comparison post hoc test. Paired t tests were used to analyze flow cytometry results. P < .05 was considered to be significant.
Results
Platelet TG stabilizes FXIII-depleted thrombi
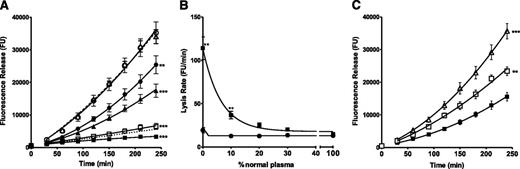
Total FXIII activity generated in plasma is 1.6-fold higher in PRP (1.5 ± 0.05 IU/mL) compared with PPP (0.9 ± 0.12; n = 4; P < .005), indicating that platelets represent an important pool of potential activity. No activity could be detected in FXIII-depleted plasma. We therefore analyzed whether platelet lysates could stabilize thrombi against the rapid lysis observed in the absence of FXIII-A2B2.39 Addition of platelet lysates stabilized FXIII-depleted thrombi in a dose-dependent manner (Figure 1A).
Platelets stabilize FXIII-depleted thrombi against fibrinolytic degradation in a concentration and transglutaminase dependent manner. (A) FXIII-depleted thrombi (△ dashed line) were prepared with the addition of platelet lysates over a range of platelet concentrations (all in platelets per milliliter (plts/mL)): ▪ 5 × 108, □ 2.5 × 108, ▲ 1 × 108, ● 0.5 × 108, and ○ 0.1 × 108. The dotted line represents PNP thrombus lysis. **P < .01; ***P < .001 compared with FXIII-depleted thrombi (n = 4). (B) Thrombi were prepared from PNP or mixtures of PNP with FXIII-depleted plasma in the (▪) absence and (●) presence of platelet lysates at 2.5 × 108 plts/mL. **P < .01 compared with thrombi + 2.5 × 108 plts/mL (n = 5). (C) (△) FXIII-depleted thrombi were prepared with the addition of 5 × 108 plts/mL in the (▪) absence and (□) presence of a TG inhibitor. **P < .01; ***P < .001 compared with FXIII-depleted thrombi + 5 × 108 plts/mL (n = 6).
Platelets stabilize FXIII-depleted thrombi against fibrinolytic degradation in a concentration and transglutaminase dependent manner. (A) FXIII-depleted thrombi (△ dashed line) were prepared with the addition of platelet lysates over a range of platelet concentrations (all in platelets per milliliter (plts/mL)): ▪ 5 × 108, □ 2.5 × 108, ▲ 1 × 108, ● 0.5 × 108, and ○ 0.1 × 108. The dotted line represents PNP thrombus lysis. **P < .01; ***P < .001 compared with FXIII-depleted thrombi (n = 4). (B) Thrombi were prepared from PNP or mixtures of PNP with FXIII-depleted plasma in the (▪) absence and (●) presence of platelet lysates at 2.5 × 108 plts/mL. **P < .01 compared with thrombi + 2.5 × 108 plts/mL (n = 5). (C) (△) FXIII-depleted thrombi were prepared with the addition of 5 × 108 plts/mL in the (▪) absence and (□) presence of a TG inhibitor. **P < .01; ***P < .001 compared with FXIII-depleted thrombi + 5 × 108 plts/mL (n = 6).
The experiments described in Figure 1A-B were performed on frozen platelet preparations, but fresh apheresis platelets (Figure 1C) still produced a 2.3-fold (P < .001) stabilizing effect on FXIII-depleted thrombi and were used throughout the rest of the study. Inclusion of a TG inhibitor39,43 abrogated the stabilizing effect of platelets in FXIII-depleted thrombi (P < .01; Figure 1C), revealing a TG-mediated effect. TG2 antigen was below the limit of detection in western blots of platelet lysates (1 × 109/mL) (data not shown). It is therefore assumed that the source of TG activity is platelet FXIII-A. Thrombi formed with mixtures of pooled normal and FXIII-depleted plasma lysed rapidly until FXIII-A2B2 concentrations reached approximately 20% of normal (Figure 1B). In contrast, no defect in lysis was observed in the presence of platelets (2.5 × 108/mL) (Figure 1B).
α2AP is required for platelet FXIII-A to stabilize thrombi
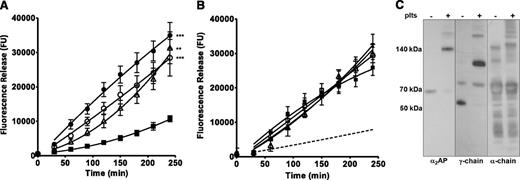
Cross-linking of α2AP is essential for FXIII-A2B2 to exhibit antifibrinolytic activity.1 Here we use α2AP as a probe to examine the functional activity of platelet FXIII-A. FXIII-depleted thrombi were formed with platelets in the absence or presence of an antibody to α2AP that inhibits activity but not its cross-linking to fibrin.1 Inclusion of the neutralizing antibody to α2AP reduced the lag time to lysis resulting in a more linear curve, but the overall change in lysis rate was not significantly different (Figure 2A). However, the antibody substantially reduced the stabilizing effect of platelets on FXIII-depleted thrombi (P < .001; Figure 2A). Similarly, thrombi formed from α2AP-depleted plasma lysed at a rapid rate compared with thrombi formed with PNP, but addition of platelets failed to stabilize against lysis (Figure 2B). Incorporation of a TG inhibitor also had no effect on the lysis of α2AP-depleted thrombi formed with or without platelets. Cross-linking of α2AP to fibrin and fibrin-fibrin crosslinking were also demonstrated by western blotting using extracts of FXIII-depleted thrombi formed with or without platelets (Figure 2C). These data indicate that platelet FXIII-A is functional in cross-linking α2AP to fibrin and stabilizing against lysis.
α2AP is required for platelet FXIII-A to stabilize thrombi. (A) (△) FXIII-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (○) 5 × 108 plts/mL with neutralizing antibody to α2AP, or (●) neutralizing antibody to α2AP with no added platelets. **P < .01; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL (n = 7). (B) (△) α2AP-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (□) with 5 × 108 plts/mL and TG inhibitor, and (▲) with TG inhibitor and no platelets. The dashed line represents PNP thrombus lysis (n = 3). (C) FXIII-depleted thrombi with and without the addition of 5 × 108 plts/mL were dissolved and separated under reducing conditions by sodium dodecyl sulfate- polyacrylamide gel electrophoresis; fibrinogen γ-chain (57 kDa), α-chain (60 kDa), or α2AP (70 kDa) antigens were detected by western blotting. Representative image of two separate experiments.
α2AP is required for platelet FXIII-A to stabilize thrombi. (A) (△) FXIII-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (○) 5 × 108 plts/mL with neutralizing antibody to α2AP, or (●) neutralizing antibody to α2AP with no added platelets. **P < .01; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL (n = 7). (B) (△) α2AP-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (□) with 5 × 108 plts/mL and TG inhibitor, and (▲) with TG inhibitor and no platelets. The dashed line represents PNP thrombus lysis (n = 3). (C) FXIII-depleted thrombi with and without the addition of 5 × 108 plts/mL were dissolved and separated under reducing conditions by sodium dodecyl sulfate- polyacrylamide gel electrophoresis; fibrinogen γ-chain (57 kDa), α-chain (60 kDa), or α2AP (70 kDa) antigens were detected by western blotting. Representative image of two separate experiments.
Platelet activation is essential for stabilization of thrombi
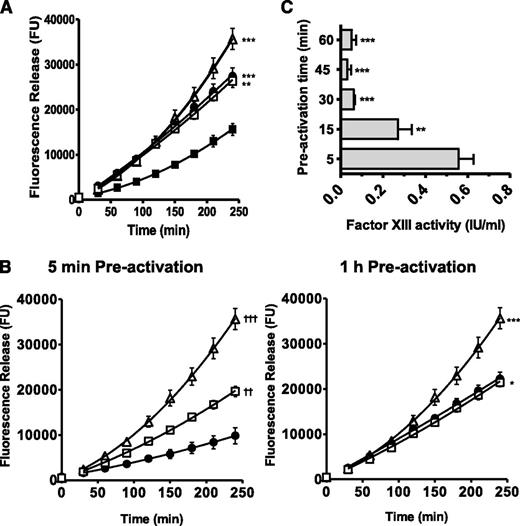
Inhibition of platelet activation with theophylline and prostaglandin E141 prevented platelets from stabilizing FXIII-depleted thrombi (P < .001). In line with this, inclusion of a TG inhibitor had no additional effect on lysis (Figure 3A). These results support our observation that platelets expose FXIII-A during activation.
Platelet FXIII-A function requires platelet activation. (A) (△) FXIII-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (●) 5 × 108 plts/mL preincubated with 100 nM prostaglandin E1 (PGE1) and 1 mM theophylline, or (□) 5 × 108 plts/mL preincubated with 100 nM PGE1 and 1 mM theophylline plus a TG inhibitor (1 mM). **P < .01; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL (n = 6). (B) (△) FXIII-depleted thrombi; (●) 5 × 108 plts/mL preactivated for 5 minutes or 1 hour at 37°C with 200 μg/mL collagen and 200 μM TRAP-6; (□) 5 × 108 plts/mL preactivated for 5 minutes or 1 hour at 37°C with 200 μg/mL collagen and 200 μM TRAP-6 including a TG inhibitor (1 mM). *P < .05; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL. ††P < .01; †††P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL preactivated for 5 minutes (n = 6). (C) Platelets (5 × 108 /mL) were preactivated with 200 μg/mL collagen and 200 μM TRAP-6 for up to 1 hour and FXIII activity (IU/mL) was measured. **P < .01; ***P < .001 vs 5 minutes preactivation. Data are expressed as mean with standard error of the mean n= 3.
Platelet FXIII-A function requires platelet activation. (A) (△) FXIII-depleted thrombi were prepared with (▪) 5 × 108 plts/mL, (●) 5 × 108 plts/mL preincubated with 100 nM prostaglandin E1 (PGE1) and 1 mM theophylline, or (□) 5 × 108 plts/mL preincubated with 100 nM PGE1 and 1 mM theophylline plus a TG inhibitor (1 mM). **P < .01; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL (n = 6). (B) (△) FXIII-depleted thrombi; (●) 5 × 108 plts/mL preactivated for 5 minutes or 1 hour at 37°C with 200 μg/mL collagen and 200 μM TRAP-6; (□) 5 × 108 plts/mL preactivated for 5 minutes or 1 hour at 37°C with 200 μg/mL collagen and 200 μM TRAP-6 including a TG inhibitor (1 mM). *P < .05; ***P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL. ††P < .01; †††P < .001 vs FXIII-depleted thrombi + 5 × 108 plts/mL preactivated for 5 minutes (n = 6). (C) Platelets (5 × 108 /mL) were preactivated with 200 μg/mL collagen and 200 μM TRAP-6 for up to 1 hour and FXIII activity (IU/mL) was measured. **P < .01; ***P < .001 vs 5 minutes preactivation. Data are expressed as mean with standard error of the mean n= 3.
Preactivating platelets for 5 minutes with TRAP-6 and collagen prior to thrombus formation stabilized FXIII-depleted thrombi against lysis (P < .001; Figure 3B). However, this effect was reduced following 1-hour preactivation (Figure 3B). Incorporation of TG inhibitor into thrombi formed with platelets preactivated for 1 hour did not increase lysis. Loss of FXIII-A activity following preactivation by TRAP-6/collagen was examined by activity assay in the absence of exogenous thrombin. Maximal FXIIIa activity was detected when platelets were preactivated for 5 minutes before incorporation into the cross-linking assay (0.56 ± 0.07 IU/mL) but decreased to 0.27 ± 0.07 IU/mL (P < .01) after 15 minutes of preactivation and was almost undetectable after 1 hour (0.05 ± 0.02; P < .001; Figure 3C). These data indicate that FXIII-A activity on the activated platelet membrane is unstable over time.
Platelet FXIII-A is stored in the cytoplasm and membrane fractions
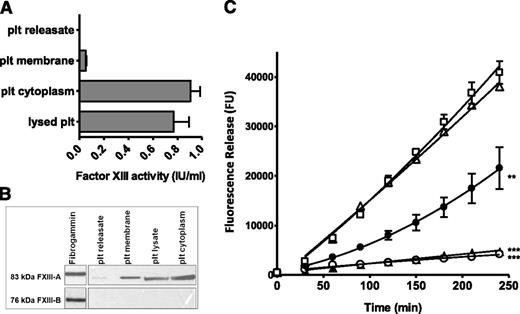
FXIIIa activity derived from unstimulated lysed platelets (0.77 IU/mL) was largely contained in the platelet cytoplasm (0.91 IU/mL), with only a small proportion found in the platelet membrane fraction (0.05 IU/mL). Minimal FXIIIa activity was detected in the platelet releasate (0.01 IU/mL; Figure 4A). FXIIIa activity derived from lysed platelet preparations closely correlates with that observed in platelets activated for 5 minutes (Figure 3C), suggesting that the majority of platelet FXIII-A is exposed upon activation. FXIII-A antigen was detected by western blotting in lysed platelet preparations and in membrane and cytoplasmic fractions, all at concentrations equivalent to 2 × 109 plts/mL. In contrast, FXIII-A was barely detectable in platelet releasates (Figure 4B). FXIII-B antigen was not detected in lysed platelets or isolated fractions, confirming that FXIII-A is platelet-derived and is not from contaminating FXIII-A2B2 on the surface of platelets or the result of uptake of the plasma pool into α-granules. Isolated platelet fractions were incorporated into FXIII-depleted thrombi in concentrations equivalent to 5 × 108 plts/mL (Figure 4C). Platelet lysate (P < .001) and platelet cytoplasm (P < .001) derived from unstimulated platelet preparations stabilized FXIII-depleted plasma thrombi 11.6-fold and 8.8-fold, respectively. The addition of unstimulated platelet membranes to FXIII-depleted thrombi partially stabilized thrombi against lysis (twofold; P < .01). Addition of releasates from stimulated platelets to FXIII-depleted thrombi were unable to stabilize against lysis. Due to the unstable nature of FXIIIa on the surface of activated platelets, we were unable to quantify activity in isolated membrane and cytoplasmic fractions following activation.
Platelet FXIII-A is associated with the membrane and cytoplasmic fractions. (A) Platelets (plt) were lysed or fractionated and FXIII-A activity (IU/mL) was measured in each fraction at the equivalent concentration (5 × 108 plts/mL). Data are shown as mean with standard error of the mean, n = 8. (B) Platelets were fractionated and FXIII-A and FXIII-B antigens were detected in fractions by western blotting. Image shown is representative of 3 experiments. (C) (△) FXIII-depleted thrombi were prepared with (○) unstimulated lysed platelets at 5 × 108 plts/mL, (▲) isolated cytoplasm from unstimulated platelets at 5 × 108 plts/mL, (●) isolated membranes from unstimulated platelets at 5 × 108 plts/mL, or (□) releasate collected from platelets (5 × 108/mL) stimulated with 200 μg/mL collagen and 200 μM TRAP-6. **P < .01; ***P < .001 vs FXIII-depleted thrombi (n = 3).
Platelet FXIII-A is associated with the membrane and cytoplasmic fractions. (A) Platelets (plt) were lysed or fractionated and FXIII-A activity (IU/mL) was measured in each fraction at the equivalent concentration (5 × 108 plts/mL). Data are shown as mean with standard error of the mean, n = 8. (B) Platelets were fractionated and FXIII-A and FXIII-B antigens were detected in fractions by western blotting. Image shown is representative of 3 experiments. (C) (△) FXIII-depleted thrombi were prepared with (○) unstimulated lysed platelets at 5 × 108 plts/mL, (▲) isolated cytoplasm from unstimulated platelets at 5 × 108 plts/mL, (●) isolated membranes from unstimulated platelets at 5 × 108 plts/mL, or (□) releasate collected from platelets (5 × 108/mL) stimulated with 200 μg/mL collagen and 200 μM TRAP-6. **P < .01; ***P < .001 vs FXIII-depleted thrombi (n = 3).
Platelet FXIII-A is exposed on the surface of activated platelets
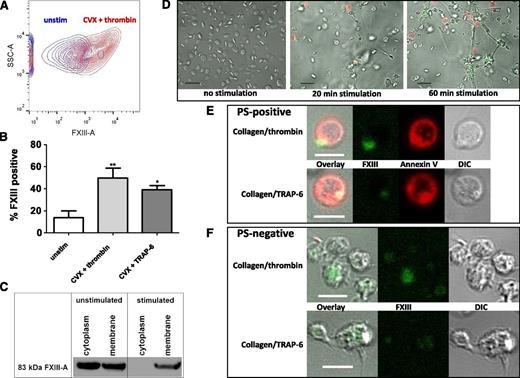
We next examined exposure of FXIII-A on activated platelets by flow cytometry. Stimulation of platelets with thrombin/CVX (49.8% ± 8.8%; P < .01) and to a lesser extent TRAP-6/CVX (39.2% ± 3.6%; P < .05) significantly increased exposure of FXIII-A compared with unstimulated platelets (13.7% ± 6.1%; Figure 5A-B). Single agonists, including ADP, arachidonic acid, CVX, TRAP-6, and thrombin increased platelet FXIII-A exposure compared with unstimulated platelets, but the only significant increase observed was with thrombin (data not shown). A small level of background staining, equivalent to 6% or less, was evident with the isotype control. Inclusion of GPRP to inhibit fibrin polymerization did not alter FXIII-A expression levels in TRAP-6/CVX stimulated platelets (41.4% ± 2.9%; not shown) but did result in a 6.4% reduction in total FXIII-A expression on thrombin/CVX-stimulated platelets (43.4% ± 9.5%; not shown). Western blot analysis revealed that FXIII-A was present in the platelet membrane pre- and poststimulation with TRAP-6/collagen; however, this approach does not differentiate between proteins on the inner and outer leaflets of the membrane (Figure 5C). In contrast, FXIII-A could not be detected in the cytoplasm poststimulation, suggesting that this pool translocates during platelet activation.
Activated platelets expose FXIII-A on their membrane surface. (A-B) Washed platelets were incubated with FITC-labeled anti-FXIII-A antibody and were left unstimulated (unstim) or stimulated with CVX (100 ng/mL) and TRAP-6 (20 μM) or thrombin (100 nM) for 45 minutes at room temperature before analyzing the number of positive cells by flow cytometry. (A) Representative contour plot showing side scatter (SSC-A) against FXIII-A-intensity or (B) mean data with standard error of the mean (SEM) from 4 experiments. *P < .05; **P < .01 vs unstimulated platelets. (C) Prior to fractionation, platelets were left unstimulated or were stimulated for 45 minutes at 37°C with 200 μM TRAP-6/200 μg/mL collagen. FXIII-A antigen was detected in the membrane and cytoplasm fractions by western blotting. Data are representative of 2 experiments. (D) Washed platelets (5 × 107/mL) were left unstimulated or were activated with 20 μg/mL collagen/20 μM TRAP-6 and stained using FITC-labeled anti-FXIII-A antibody (green) and Alexa-fluor647 Annexin-V to detect phosphatidylserine (red). Scale bar represents 10 μm. (E) PS and FXIII-A co-expressing platelets stimulated by 20 μg/mL collagen/20 μM TRAP-6 or 20 μg/mL collagen/100 nM thrombin. Differential interference contrast (DIC) image is shown and the scale bar represents 5 μm. Results are representative of 4 experiments. (F) PS-negative platelets stimulated with 20 μg/mL collagen/20 μM TRAP-6 or 20 μg/mL collagen/100 nM thrombin. Scale bar represents 5 μm. Results are representative of 4 experiments. (D-F) Images were obtained with a Zeiss LSM710 confocal microscope with a 63 × 1.40 oil immersion objective and were analyzed using Zen 2012 software.
Activated platelets expose FXIII-A on their membrane surface. (A-B) Washed platelets were incubated with FITC-labeled anti-FXIII-A antibody and were left unstimulated (unstim) or stimulated with CVX (100 ng/mL) and TRAP-6 (20 μM) or thrombin (100 nM) for 45 minutes at room temperature before analyzing the number of positive cells by flow cytometry. (A) Representative contour plot showing side scatter (SSC-A) against FXIII-A-intensity or (B) mean data with standard error of the mean (SEM) from 4 experiments. *P < .05; **P < .01 vs unstimulated platelets. (C) Prior to fractionation, platelets were left unstimulated or were stimulated for 45 minutes at 37°C with 200 μM TRAP-6/200 μg/mL collagen. FXIII-A antigen was detected in the membrane and cytoplasm fractions by western blotting. Data are representative of 2 experiments. (D) Washed platelets (5 × 107/mL) were left unstimulated or were activated with 20 μg/mL collagen/20 μM TRAP-6 and stained using FITC-labeled anti-FXIII-A antibody (green) and Alexa-fluor647 Annexin-V to detect phosphatidylserine (red). Scale bar represents 10 μm. (E) PS and FXIII-A co-expressing platelets stimulated by 20 μg/mL collagen/20 μM TRAP-6 or 20 μg/mL collagen/100 nM thrombin. Differential interference contrast (DIC) image is shown and the scale bar represents 5 μm. Results are representative of 4 experiments. (F) PS-negative platelets stimulated with 20 μg/mL collagen/20 μM TRAP-6 or 20 μg/mL collagen/100 nM thrombin. Scale bar represents 5 μm. Results are representative of 4 experiments. (D-F) Images were obtained with a Zeiss LSM710 confocal microscope with a 63 × 1.40 oil immersion objective and were analyzed using Zen 2012 software.
FXIII-A exposure on the surface of activated platelets was also examined by fluorescence confocal microscopy. Two subpopulations of activated platelets were identified; phosphatidylserine (PS) -positive platelets, which formed a balloon-like shape with a protruding PS-rich cap (Figure 5D-E and supplemental Video 1) and PS-negative platelets with extending filopodia, which aggregated and spread on collagen (Figure 5D,F). FXIII-A was visible on the surface of both PS-positive and PS-negative platelets activated with TRAP-6/collagen or thrombin/collagen (Figure 5, supplemental Videos 1, and 2). FXIII-A was localized exclusively in the PS-rich cap of PS-positive platelets (Figure 5D-E and supplemental Video 1). The intensity of FXIII-A staining in PS-rich caps was greater with thrombin/collagen compared with TRAP-6/collagen (Figure 5E), consistent with the flow cytometry results. The intensity of FXIII-A staining on the surface of activated PS-negative platelets increased over time, particularly in platelets associated with collagen fibers (supplemental Video 2).
Discussion
This study defines the contribution of platelet FXIII-A to the inhibition of fibrinolysis. We show that physiological platelet concentrations stabilize FXIII-depleted thrombi against fibrinolysis. The stabilizing effect was largely TG mediated, because it was abrogated by a specific TG inhibitor.39 Activity was attributed to FXIII-A, because TG2 antigen was undetectable in human platelets, consistent with the lack of TG2 messenger RNA.44 Our data support previous observations that platelets increase mechanical stability45 and the number of high-molecular-weight α-polymers and γ-γ dimers33-36 in FXIII-depleted clots while limiting fibrinolysis.33,34 We quantitatively assessed the subcellular localization of platelet FXIII-A and have shown for the first time that cytoplasmic FXIII-A is externalized onto the activated platelet membrane.
Expression of PS and platelet-derived FXIII-A on resting and activated platelets was visualized using confocal microscopy and flow cytometry. Approximately 50% of stimulated platelets express FXIII-A on their surface. FXIII-A was previously detected, but not quantified, on activated platelets by flow cytometry.46 Nagy et al47 reported that less than 5% of platelets expose FXIII-A after TRAP stimulation, values that differ markedly from this study. Differences in experimental set-up, including activation time and the sole use of TRAP-6, may account for these discrepancies. We observed a significant increase in FXIII-A exposure on platelets only when thrombin or dual agonists were used. Strong activation of platelets with dual agonists generates a large intracellular Ca2+ spike48 and a specific population termed “coated platelets”.49,50 Platelet TG activity is required for formation of coated platelets.51,52 However, they are detected in FXIII-deficient mice which, interestingly, exhibit higher total cellular TG activity compared with wild-type mice, suggesting a possible compensatory mechanism.52 Upon activation, we observed a subpopulation of PS-positive balloon-shaped procoagulant platelets characterized by a protruding cap, consistent with previous observations.48,53 We found that FXIII-A is expressed exclusively on caps of PS-positive balloon-shaped platelets in contrast to its wider distribution on PS-negative platelets. Secreted fibrinogen has also been detected in caps on the platelet surface,53 suggesting colocalization of FXIII-A and fibrin(ogen). Interestingly, GPRP only slightly reduced the number of thrombin/CVX-stimulated platelets exposing FXIII-A and had no effect on platelets stimulated with TRAP-6/CVX. This suggests that a small pool of FXIII-A associates with polymerized fibrin on the membrane but does not rule out an interaction with fibrinogen or monomeric fibrin. It is possible that fibrin(ogen) acts as an interface for FXIII-A to translocate from the platelet surface into the surrounding fibrin network extending from platelet aggregates.
FXIII-A is localized in the cytoplasmic fraction of resting platelets, with only a small contribution from the membrane, consistent with previous reports.23,24,26 FXIII-A in these subcellular fractions was functional, as evidenced in the activity assay and in their ability to stabilize FXIII-depleted thrombi against lysis. FXIII has previously been detected in platelet releasates,54,55 but these observations are not consistent with our data or other reports.23,24,37,46 FXIII-A levels are normal in platelets derived from patients with gray-platelet syndrome25 and plasma FXIII levels are unchanged in thrombocytopenic mice.38 Together these data indicate a very minor contribution of α-granule FXIII-A2B2 to the overall TG activity derived from platelet preparations.
Platelet activation is essential for FXIII-A–mediated stabilization of FXIII-depleted thrombi. FXIII-A activity assays on platelets preactivated with TRAP-6/collagen are carried out in the absence of exogenous thrombin, suggesting that FXIII-A is nonproteolytically activated by the high Ca2+ levels associated with platelet stimulation. Strong stimulation of platelets depletes the cytoplasm of FXIII-A and allows its accumulation on the outer membrane surface, as shown by flow cytometry and confocal microscopy. Inhibition of platelet activation and aggregation prior to thrombus formation prevented stabilization of FXIII-depleted thrombi. FXIII-A activity exposed on platelet membranes was unstable and was almost undetectable after a 1-hour preactivation, consistent with previous reports on plasma FXIII.10,56 The unstable nature of FXIII-A on the platelet membrane may have important physiological consequences, because it would prevent ongoing cross-linking, which is known to increase fibrinolytic resistance of thrombi.36,57
Thrombi formed under flow are a sensitive model in which to examine the effects of cross-linking39 and inhibitors58 on fibrinolysis. We have previously shown that α2AP-depleted thrombi, or normal thrombi formed with an antibody to α2AP, lyse rapidly irrespective of FXIII concentration, thereby illustrating the essential contribution of cross-linked α2AP to thrombus stability.1 Here we show that platelet FXIII-A also mediates cross-linking of α2AP to fibrin. Platelets have been implicated in cross-linking of α2AP in purified clots and plasma,34,35 but their contribution was considered minor. The antifibrinolytic effect of platelets was substantially reduced in the absence of functional α2AP. Platelets also contain a pool of plasminogen activator inhibitor-1,40 which may account for the small remaining effect on stabilization of FXIII-depleted thrombi. These data again underscore the principal role of cross-linked α2AP in regulating fibrinolysis.
Activated platelets do not externalize their cytoplasmic pool of FXIII-A by classical release mechanisms.37,38 One potential mechanism of FXIII-A transport to the membrane could be via sphingomyelin-rich lipid rafts. Sphingomyelin-rich rafts act as signaling platforms on the platelet membrane between intra- and extracellular clot retraction machinery. FXIII-A is highly expressed in the membrane raft fraction in stimulated but not resting platelets.59 Alternatively, secretion could be mediated by golgi matrix protein 130 (GP130), which colocalizes with FXIII-A in macrophages. GP130 functions in nonclassical protein secretion to the plasma membrane prior to excretion through membrane pores.38 Platelet-derived tissue factor pathway inhibitor is exposed on activated coated platelets by an unknown mechanism. Like FXIII-A, tissue factor pathway inhibitor is not localized in platelet granules and appears to be of cytoplasmic origin.60 Platelet microvesicles, released upon activation,61,62 contain cytoplasmic material, including FXIII-A, and could be implicated in its secretion.62 However, the latter release mechanism is not consistent with our observations that FXIII-A resides in caps on the membrane surface of activated platelets. Further studies are required to elucidate the precise signaling mechanisms involved in externalization of FXIII-A on activated platelets.
The fibrin network immediately adjacent to platelet aggregates is extremely resistant to lysis.63-66 This is attributed to the tightly contracted fibrin network; however, it may also result from cross-linking of additional α2AP to fibrin as a result of the high local concentration of FXIII-A. It has recently been shown that supplementing blood of thrombocytopenic patients with FXIII, thrombin activatable fibrinolysis inhibitor, or fibrinogen increases clot strength and decreases lysis.67 These proteins are all contained in platelets, and their presence indicates that local concentrations of these proteins, including surface-bound FXIII-A, mediate thrombus stabilization. Indeed, an increase in FXIII-A activity over time was observed on incorporation of intact platelets into plasma clots.37 We show that inhibiting platelet activation or FXIIIa activity dramatically reduces stabilization of thrombi by platelets. The impact of platelet FXIII-A on thrombus stabilization suggests it may translocate from the activated membrane into the adjacent fibrin network, but the extent of diffusion requires further clarification.
Under certain circumstances, the antifibrinolytic effect of platelet FXIII-A may be central in stabilizing thrombi, such as during surgery when plasma FXIII-A2B2 levels rapidly decline67 or following cardiopulmonary bypass with extracorporeal circuit. Acquired FXIII deficiency can also occur in the absence of surgery, as in the case of aortic dissection in which plasma FXIII-A2B2 levels deplete to 30% to 35% of normal.68 FXIII-B subunit deficiency results in loss of FXIII-A in plasma, presumed to be the result of instability,69-71 but levels of platelet FXIII-A are normal.70,72 FXIII-B deficiency is associated with milder symptoms than FXIII-A deficiency in humans69 and FXIII-B knockout mice.73 This is thought to be the result of the remaining levels of free FXIII-A in the plasma; however, our results suggest that platelet FXIII-A may also compensate under these circumstances. Our data demonstrate that when plasma FXIII-A2B2 is below 20% of normal, the antifibrinolytic effect of platelet FXIII-A becomes apparent, with levels of 10% of normal being statistically significant. High arterial shear rates result in increased platelet adhesion and deposition74 but reduced accumulation of fibrinogen in forming thrombi.74,75 In these platelet-rich thrombi, cellular sources of fibrin(ogen) and FXIII-A may contribute to stabilizing against fibrinolysis.
In conclusion, our study is the first to show that activated platelets externalize their pool of cytoplasmic FXIII-A in response to strong stimulation. FXIII-A exposed on the stimulated platelet membrane is active and is potentially transferred into the fibrin network adjacent to platelet aggregates. This mechanism may be important in the initial stabilization of the platelet-plug at the site of injury. The ability of FXIII-A to stabilize fibrin against lysis is entirely dependent on the presence of functional α2AP, consistent with our previous studies on FXIII-A2B2. Further work is necessary to define the exact mechanism involved in translocation of FXIII-A across the platelet membrane and the signaling events that elicit these responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Janne Koikkalainen for performing the western blot for TG2 in platelets, the Medical Statistics Team, the Microscopy and Histology Core Facility, the Iain Fraser Cytometry Centre at the University of Aberdeen for excellent advice and use of the facilities, and the Scottish National Blood Transfusion Service for outdated platelets.
The study was supported by grants FS/11/2/28579 (N.J.M., A.S.L.) and PG/11/1/28461 (N.J.M., C.S.W.) from the British Heart Foundation, and by the University of Aberdeen Development Trust (N.J.M., J.L.M.). N.A.B. held a Leverhulme Trust Emeritus Fellowship.
Authorship
Contribution: J.L.M. performed the research, analyzed data, and wrote the manuscript; A.S.L. and S.R.F. performed research and analyzed data; C.S.W. supervised the research and analyzed data; N.A.B. analyzed data and wrote the manuscript; and N.J.M. supervised the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola J. Mutch, School of Medicine and Dentistry, Institute of Medical Sciences, Foresterhill, University of Aberdeen, Aberdeen AB25 2ZD, United Kingdom; e-mail: n.j.mutch@abdn.ac.uk.