Key Points
SCD increases release of HMGB1.
HMGB1 plays a major role in increasing TLR4 activity in SCD.
Abstract
High mobility group box 1 (HMGB1) is a chromatin-binding protein that maintains DNA structure. On cellular activation or injury, HMGB1 is released from activated immune cells or necrotic tissues and acts as a damage-associated molecular pattern to activate Toll-like receptor 4 (TLR4). Little is known concerning HMGB1 release and TLR4 activity and their role in the pathology of inflammation of sickle cell disease (SCD). Circulating HMGB1 levels were increased in both humans and mice with SCD compared with controls. Furthermore, sickle plasma increased HMGB1-dependent TLR4 activity compared with control plasma. HMGB1 levels were further increased during acute sickling events (vasoocclusive crises in humans or hypoxia/reoxygenation injury in mice). Anti-HMGB1 neutralizing antibodies reduced the majority of sickle plasma-induced TLR4 activity both in vitro and in vivo. These findings show that HMGB1 is the major TLR4 ligand in SCD and likely plays a critical role in SCD-mediated inflammation.
Introduction
Sickle cell disease (SCD) induces chronic states of oxidative stress and inflammation. Myeloperoxidase,1,2 xanthine oxidase,3,4 uncoupled eNOS activity,5 NADPH oxidase,6 Fenton reactions of membrane iron,7,8 ischemia-reperfusion physiology,9 as well as cell-free hemoglobin/heme released via hemolysis have all been shown to contribute to oxidative stress in SCD. Individuals with SCD suffer from repeated bouts of sickling and reversible vasoocclusion, which results in ischemia/reperfusion injury, leukocytosis, immune cell activation, and endothelial cell injury.10,11 Interestingly many of these sequelae also induce the release of high mobility group box 1 (HMGB1). HMGB1 is a nuclear chromatin-binding protein that aids in regulating gene expression and maintaining nuclear DNA structure. It is secreted from activated immune cells12-14 and is passively released from injured or necrotic cells.14,15 Subsequent to release, HMGB1 acts as a damage-associated molecular pattern (DAMP) molecule that increases inflammation by binding to and activating Toll-like receptor 4 (TLR4) and/or advanced glycation end-product receptor.14,16
When released from activated/damaged cells, HMGB1 becomes a potent agonist of TLR4 or advanced glycation end-product receptor that can propagate sterile inflammatory responses.15,17,18 Furthermore, studies suggest that hemin may also amplify TLR4 signaling in SCD.19,20 Given that SCD is characterized by repeated bouts of ischemia/reperfusion injury and oxidative stress, it is likely that SCD increases HMGB1 release, with subsequent activation of TLR4, vascular inflammation, and tissue injury. Therefore, in this study we investigate the effects of chronic SCD and acute sickling on the release of HMGB1 and HMGB1-dependent TLR4 receptor activity.
Study design
Human subjects
Children’s Hospital of Wisconsin Institutional Review Board approved the studies involving plasma samples from human subjects. Subjects and/or guardians of children (with assent as appropriate) provided written informed consent in accordance with the Declaration of Helsinki. SCD subjects were diagnosed with either Hb SS or Hb S-βo thalassemia and not were transfused within the previous 2 months.
Mice
C57BL/6J mice (control mice) were purchased from The Jackson Laboratory (#000664, Bar Harbor, ME). Berkeley SCD mice (SS mice; Tag [Hu-miniLCRα1GγAγδβS] Hba0//Hba0Hbb0//Hbb0) have been previously described21,22 and were backcrossed to >60% C57BL/6J. Mice were cared for according to Association for Assessment and Accreditation of Laboratory Animal Care specifications. The Institutional Animal Care and Use Committee of the Medical College of Wisconsin approved animal experiments. Experimental groups contained comparable numbers of male and female mice.
Methods
TLR4 receptor reporter activity assay was performed using TLR4/NF-kB/SEAP Stable Reporter Cells (IML-104) from Imgenex (San Diego, CA) as described.23 (See also supplemental Methods, available at the Blood Web site.)
Hypoxia/reoxygenation (H/R) injury (acute sickling) was induced by placing mice into a Plexiglas chamber where nitrogen inflow was increased to reduce FIO2 to 10% for 3 hours as previously described.24 The animals were returned to room air (FIO2 = 21%) for 2 hours before phlebotomy via cardiac puncture under deep anesthesia, perfusion of all tissues with saline, and harvest of lungs and liver (see supplemental Methods).
Statistics
Data are presented as mean ± standard deviation. Analysis was by Student t test (2-sample or paired) or analysis of variance for normal data and Mann-Whitney U test or Fisher’s exact test for nonparametric data using Prism Graph Pad (v5.0).
Results and discussion
We found that SCD increased plasma HMGB1 in humans at baseline (Figure 1A). In a subset of the SCD patients with paired baseline and acute painful episodes (“crisis”) samples, plasma HMGB1 was even further increased during crisis (Figure 1B). Using a “TLR4 reporter cell line” that specifically recognizes ligands that bind and activate TLR4, plasma from healthy control subjects induced low levels of TLR4 receptor activity (Figure 1C). In contrast, plasma from SCD subjects at baseline induced higher levels of TLR4 receptor activity compared with controls. Plasma from SCD subjects in crisis induced even further increases in plasma TLR4 receptor activity.
SCD increases plasma HMGB1 and TLR4 receptor activity in humans. (A) Plasma concentrations of HMGB1 in control and SCD individuals (n = 19, 26, respectively, **P = .047); (B) HMGB1 concentrations in plasma from SCD individuals at baseline and crisis (n = 19, *P = .0362); (C) Total TLR4 receptor activity induced by control and SCD plasma (n = 12, *P < .05, ***P < .01); (D) HMGB1-dependent TLR4 receptor activity in plasma from SCD individuals at baseline and crisis. Data represent TLR4 reporter cell activity inhibited by pretreating plasma with anti-HMGB1 antibodies (n = 12, *P < .05); and (E) HMGB1-dependent TLR4 receptor activity represented as a percentage of total TLR4 receptor activity (n = 11 to 12, ***P < .01).
SCD increases plasma HMGB1 and TLR4 receptor activity in humans. (A) Plasma concentrations of HMGB1 in control and SCD individuals (n = 19, 26, respectively, **P = .047); (B) HMGB1 concentrations in plasma from SCD individuals at baseline and crisis (n = 19, *P = .0362); (C) Total TLR4 receptor activity induced by control and SCD plasma (n = 12, *P < .05, ***P < .01); (D) HMGB1-dependent TLR4 receptor activity in plasma from SCD individuals at baseline and crisis. Data represent TLR4 reporter cell activity inhibited by pretreating plasma with anti-HMGB1 antibodies (n = 12, *P < .05); and (E) HMGB1-dependent TLR4 receptor activity represented as a percentage of total TLR4 receptor activity (n = 11 to 12, ***P < .01).
HMGB1-dependent TLR4 receptor activity is estimated by subtracting TLR4 receptor activity in samples treated with anti-HMGB1 neutralizing antibodies from the total TLR4 receptor activity.25 HMGB1-dependent TLR4 receptor activity was increased in the plasma of SCD subjects in crisis compared with paired samples at baseline (Figure 1D). We also found that plasma HMGB1 contributed to only ∼10% of total TLR4 receptor activity in control subjects (Figure 1E). In contrast, HMGB1 in SCD subjects, whether at baseline or in crisis, specifically contributed to nearly 60% to 70% of total TLR4 receptor activity.
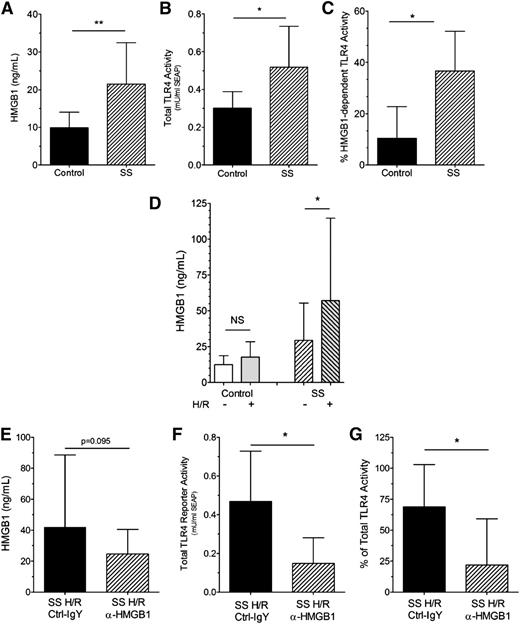
Plasma HMGB1 (Figure 2A) and total TLR4 receptor reporter activity (Figure 2B) were also increased in SS mice compared with control mice. Similar to humans, HMGB1-dependent TLR4 receptor activity in control mice accounts for only ∼10% of total TLR4 receptor activity compared with ∼37% that is HMGB1-dependent in SS mice (Figure 2C).
HMGB1 levels, TLR4 receptor activity, and HMGB1-dependent TLR4 receptor activity are increased in mice with SCD. (A) Plasma HMGB1 levels in SCD (SS) (hatched bar) compared with control (black bar, n = 9, 11, **P < .01) mice; (B) Plasma-induced total TLR4 receptor activity is increased in SS (hatched bar) compared with control (black bar, n = 6, *P < .05) mice; (C) % HMGB1-dependent TLR4 receptor activity in SS (hatched bar) compared with control (black bar, n = 6, *P < .05) mice; (D) Effect of H/R injury (see Study design) on HMGB1 levels. Left bars: control normoxia (open bar, n = 13) vs control H/R (gray open bar, n = 7) mice; NS, not significant. Right bars: SS normoxia (right-hatched open bar, n = 20) vs SS H/R (left-hatched gray bar, n = 17) mice (*P < .05); (E) Pretreatment of SS mice with isotype control (black bar) or anti-HMGB1 (hatched bar) antibodies did not significantly decrease HMGB1 levels (P = .095, n = 14 to 15); (F) Pretreatment of SS mice with anti-HMGB1 (hatched bar) but not isotype control (black bar) antibodies significantly decreases the ability of SS plasma to induce total TLR4 receptor activity in cultured TLR4 reporter cells (n = 6, *P < .05); and (G) Pretreatment of SS mice with anti-HMGB1 (hatched bar) but not isotype control (black bar) antibodies significantly decreased the % of HMGB1-dependent TLR4 receptor activity in SS plasma (n = 6, *P < .05).
HMGB1 levels, TLR4 receptor activity, and HMGB1-dependent TLR4 receptor activity are increased in mice with SCD. (A) Plasma HMGB1 levels in SCD (SS) (hatched bar) compared with control (black bar, n = 9, 11, **P < .01) mice; (B) Plasma-induced total TLR4 receptor activity is increased in SS (hatched bar) compared with control (black bar, n = 6, *P < .05) mice; (C) % HMGB1-dependent TLR4 receptor activity in SS (hatched bar) compared with control (black bar, n = 6, *P < .05) mice; (D) Effect of H/R injury (see Study design) on HMGB1 levels. Left bars: control normoxia (open bar, n = 13) vs control H/R (gray open bar, n = 7) mice; NS, not significant. Right bars: SS normoxia (right-hatched open bar, n = 20) vs SS H/R (left-hatched gray bar, n = 17) mice (*P < .05); (E) Pretreatment of SS mice with isotype control (black bar) or anti-HMGB1 (hatched bar) antibodies did not significantly decrease HMGB1 levels (P = .095, n = 14 to 15); (F) Pretreatment of SS mice with anti-HMGB1 (hatched bar) but not isotype control (black bar) antibodies significantly decreases the ability of SS plasma to induce total TLR4 receptor activity in cultured TLR4 reporter cells (n = 6, *P < .05); and (G) Pretreatment of SS mice with anti-HMGB1 (hatched bar) but not isotype control (black bar) antibodies significantly decreased the % of HMGB1-dependent TLR4 receptor activity in SS plasma (n = 6, *P < .05).
Hypoxia induces acute sickling in SCD mice, resulting in H/R injury that mimics many features of human acute vasoocclusion.10,24,26 As expected, H/R treatment had no effect on HMGB1 levels in control mice (Figure 2D). In contrast, H/R injury increased plasma HMGB1 concentrations in SS mice (H/R +) compared with HMGB1 levels in SS mice at baseline (H/R −).
Pretreatment of SS H/R mice with anti-HMGB1 antibodies did not significantly decrease plasma HMGB1 levels compared with isotype control antibody SS H/R mice (Figure 2E, P = .095). However, pretreatment of SS mice with anti-HMGB1 neutralizing antibodies reduced total TLR4 receptor activity in the SS H/R mice by >60% (Figure 2F). Furthermore, HMGB1-dependent TLR4 receptor activity in the plasma of SS H/R mice treated with isotype control antibody was ∼67% and was similar to human crisis samples. In contrast, HMGB1-dependent TLR4 receptor activity in SS H/R mice treated with anti-HMGB1 neutralizing antibodies dropped to only ∼12% (Figure 2G), which was similar to levels observed in healthy controls.
Although there was no significant improvement in histopathologic scores for vascular congestion of lungs or livers from anti-HMGB1–treated mice compared with control-IgY–treated mice, there was a trend for reduced liver ischemia/infarcts in anti-HMGB1–treated SS mice (P = .077, supplemental Figure). We also found no change in serum amyloid P or soluble vascular cell adhesion molecule-1 levels (see supplemental Results), suggesting that limiting HMGB1 and/or the associated TRL4 signaling may not rapidly improve these measures in the acute setting.
In conclusion, these data show for the first time that SCD increases the release of HMGB1 in humans and mice with concomitant increases in plasma TLR4 receptor activity. Using anti-HMGB1 antibodies, we have also shown that HMGB1 is responsible for most of the TLR4 receptor activity in human and mouse SCD plasma at both baseline and during acute sickling/crisis.
HMGB1 mediates inflammation and apoptosis via multiple mechanisms. Although oxidation of HMGB1 directs it to different receptors, this DAMP also binds other cytokines, modified proteins, and/or endotoxin to form unique proinflammatory complexes that can synergistically activate DAMP receptors to a greater level than either one of the agents can do alone.27 For example, HMGB1 binds cell-free hemoglobin to form a complex that increases inflammation by non-TLR–dependent mechanisms28 ; hemopexin blocks and/or disrupts this HMGB1-Hb-induced inflammation in nonsickle models.28 Cell-free heme, released in hemolysis, also synergizes with HMGB1 to increase inflammation.28 Our data suggest that HMGB1 could join hemin, which is released by hemolysis in the mechanisms by which SCD increases TLR4 signaling.19,20
Taken together, these reports and our data demonstrate that HMGB1 plays the predominant role in increasing TLR4 receptor activity in SCD and is likely a critical mechanism for SCD-induced tissue injury. Accordingly, therapies targeting HMGB1 directly and/or disrupting its binding to proinflammatory receptors or forming proinflammatory complexes may prove valuable in reducing inflammation and subsequent tissue injury in SCD.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Thomas D. Foster and Dawn Retherford for technical assistance and expertise in working with the murine model of sickle cell disease, and the authors thank Meghann Sytsma for assistance with manuscript preparation.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL-102996 (K.A.P.), HL-102836 (K.A.P., C.A.H.), U54 HL-090503 (C.A.H., N.H., N.J.W.), 5T32HL007209-33 (M.S.H.), Midwest Athletes Against Childhood Cancer Fund (C.A.H., N.J.W.), Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 5R01HD062347-01, Emergency Medical Services for Children program of the Maternal and Child Health Bureau grant U03MC22684 (D.C.B.), National Center for Advancing Translational Sciences, National Institutes of Health grant 8KL2TR000056 (J.C.D.), and CTSI Award 1-UL1-RR031973.
Authorship
Contribution: K.A.P. and C.A.H. conceived and designed experiments and wrote the manuscript; H.X. and N.J.W. contributed to experimental design, data analysis, and writing of the manuscript; H.X., Y.G., D.W.J., S.L.H., and M.S.H. performed experiments; E.M. and D.C.B. contributed clinical samples and edited the manuscript; and M.S.H., N.H., J.C.D., and S.K. contributed to experimental design and editing of the manuscript.
Conflict of interest disclosure: C.A.H. and N.J.W. are consultants for Bayer Pharmaceuticals. C.A.H. is a consultant for Biogen Idec. K.A.P. is the principal owner in K&E Diagnostics, LLC. D.C.B. is a consultant for Mast Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Kirkwood A. Pritchard Jr, Department of Surgery, Division of Pediatric Surgery, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: kpritch@mcw.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal