Key Points
Integrin α3β1 (VLA-3; CD49c/CD29) is significantly upregulated on neutrophils from human septic patients and mouse models of sepsis.
α3β1high granulocytes displayed hyperinflammatory phenotypes and inhibition of α3β1 improved survival in septic mice.
Abstract
Integrin-mediated migration of neutrophils to infected tissue sites is vital for pathogen clearance and therefore host survival. Although β2 integrins have been shown to mediate neutrophil transendothelial migration during systemic and local inflammation, relatively little information is available regarding neutrophil migration in sepsis beyond the endothelial cell layer. In this study, we report that integrin α3β1 (VLA-3; CD49c/CD29) is dramatically upregulated on neutrophils isolated from both human septic patients and in mouse models of sepsis. Compared with the α3β1low granulocytes, α3β1high cells from septic animals displayed hyperinflammatory phenotypes. Administration of a α3β1 blocking peptide and conditional deletion of α3 in granulocytes significantly reduced the number of extravasating neutrophils and improved survival in septic mice. In addition, expression of α3β1 on neutrophils was associated with Toll-like receptor–induced inflammatory responses and cytokine productions. Thus, our results show that α3β1 is a novel marker of tissue homing and hyperresponsive neutrophil subtypes in sepsis, and blocking of α3β1 may represent a new therapeutic approach in sepsis treatment.
Introduction
Severe sepsis, a systemic inflammatory response to infections associated with acute organ dysfunction, is an increasing cause of morbidity and mortality among children and adults and has been one of the most significant challenges in critical care.1,2 Pro-inflammatory signals arise at the early stage of sepsis and allow circulating neutrophils to access sites of inflammation and to phagocytose foreign pathogens and necrotic/apoptotic cells. Although these inflammatory mediators are important for the host defense, they also participate in endothelial and extravascular host tissue damage.3 Thus, in uncontrolled inflammatory conditions, such as sepsis, during which many neutrophils become activated at the endothelial interface and in the underlying tissue, excessive inflammatory activities lead to further microvascular dysfunction and tissue damage.4
Integrins are heterodimeric transmembrane receptors that play a critical role in cell migration.5,6 Although β2/CD18 integrins play an important role in intravascular leukocyte migration processes such as initial endothelial adhesion and vascular transmigration of leukocytes during inflammation, the β1/CD29 and β3/CD51 integrins mediate cell–matrix adhesion and promote leukocyte motility in the perivascular space and extracellular matrix (ECM) area.7-9 Integrins not only mediate cell–cell and cell–matrix interaction, but also influence differentiation, proliferation, and functioning of cells in the interstitial spaces.10 During inflammation, integrins are upregulated on leukocytes in response to various cytokines and chemokines and influence progression and prognosis of numerous inflammatory and autoimmune diseases as well as cancers.8,11-13
In this study, we report that, unlike other ECM binding integrins, only α3β1 (VLA-3; CD49c/CD29) expression on neutrophils becomes dramatically increased in both human and mouse sepsis. Blocking or genetic ablation of α3β1 results in improvement to the host’s innate immune response, such as reduced neutrophil tissue infiltration and Toll-like receptor 2 (TLR2)-induced cytokine response, and increases survival in septic animals. Therefore, we conclude that selective targeting of α3β1 on neutrophils could represent a new therapeutic approach in sepsis treatment.
Materials and methods
Sepsis mouse models
Endotoxemia and cecal ligation and puncture (CLP) were performed according to Animal Resource Protocol approved by the Committee at University of Rochester. For endotoxemia assay, 8- to 12-week-old C57BL/6 (Harlan) male mice were weighed and lipopolysaccharide (LPS) (Escherichia coli O55:B, Sigma-Aldrich) was administered by intraperitoneal injection to achieve LD80 mortality. For CLP, the cecum was ligated and punctured through and through with a 21-gauge needle. Mice were resuscitated with 1 mL Ringers lactate injected subcutaneously.
Generation of conditional knockout animals
For generation of granulocyte-specific integrin knockout mice, granulocyte elastase 2 (Ela)-Cre knock-in mice were purchased from The European Mouse Mutant Archive. In these mice, the Cre-recombinase is inserted after the first exon of the Ela gene within the myeloid cells, thereby deleting the functional expression of Ela.14 Ela-Cre mice were crossed with Itga3 floxed mice for 5 to 6 generations to achieve deletion of integrin α3 in the transgenic mice. Mice were genotyped by polymerase chain reaction from DNA isolated from tail tissues.
Data analysis
Values are expressed as means ± standard error of the mean (SEM) or means ± standard deviation (SD), as indicated. Data from human samples were analyzed by nonparametric Wilcoxon rank-sum test. Data from murine experiments were analyzed by Student t test or nonparametric Mann-Whitney, where appropriate. CD11b expression, phospho-Syk, phospho-focal adhesion kinase (FAK), and MyD88 time course experiments were analyzed by 2-way repeated measures analysis of variance (ANOVA). Survival curves were analyzed by Kaplan-Meier log-rank test. All statistics were performed using the GraphPad Prism 5.0 software. P values <.05 were considered significant.
Detailed information can be found in the supplemental Methods on the Blood Web site.
Results
Integrin α3β1 expression is upregulated on human and mouse neutrophils during sepsis
Neutrophils express several cell-surface integrins that can bind to ECM proteins.15 To determine which ECM-binding integrin is important for neutrophil trafficking during sepsis, circulating neutrophils were harvested from patients with severe sepsis, patients with noninfectious severe systemic inflammatory response syndrome (SIRS), and healthy volunteers (donor information summarized in supplemental Table 1). Unlike other ECM-binding integrins, the surface expression levels of α3β1 (VLA-3; CD49c/CD29) on neutrophils from patients with severe sepsis were significantly elevated compared with the healthy control subjects. Interestingly, upregulation of α3β1 was not observed in subjects with severe noninfectious SIRS (Figure 1A), suggesting that α3β1 is a novel cell surface marker that can discriminate sepsis from SIRS. The increases in messenger RNA (mRNA) and cell-surface levels of α3β1 were further investigated in neutrophils isolated from the peripheral blood of healthy subjects and incubated with various stimuli. Neutrophil activation by phorbol 12-myristate 13-acetate (PMA) and LPS induced significant increases in both the mRNA and protein levels of α3β1 within 1 and 3 hours of stimulation (Figure 1B). Tumor necrosis factor-α (TNF-α) stimulation significantly increased cell surface level of α3β1 within 1 hour (Figure 1B).
Integrin α3β1 surface expression increases on human neutrophils during sepsis. (A) Integrin surface expression on neutrophils from SIRS patients (n = 9), sepsis patients (n = 15), and healthy donors (n = 7). Flow cytometry results expressed as a ratio of the integrin mean fluorescence intensity (MFI) to isotype control MFI of the same donor. *P < .05 (Wilcoxon rank-sum test). (B) Neutrophils isolated from healthy donors were stimulated with PMA (20 ng/mL), TNF-α (20 ng/mL), LPS (100 μg/mL), or fMLP (1 μM) for 1 or 3 hours. Fold changes in Itga3 gene expression, compared with unstimulated cells, were determined by reverse transcription polymerase chain reaction (upper panel), and surface expression of α3β1 was measured by flow cytometry (bottom panel). Data are expressed as mean ± SEM of 3 separate donors. *P < .05 (Mann-Whitney test). Integrin α3β1 is upregulated on mouse neutrophils in (C) CLP surgery and (D) endotoxemia models of sepsis. Cells isolated from bone marrow, peritoneal lavage, and peripheral blood of naive and septic mice at the indicated time points were gated for neutrophils using forward scatter/side scatter and Gr1high expression. The results are expressed as % increases in MFI compared with naive controls (naive blood MFI was used to calculate the % change in peritoneal lavage [PL] expression). Data are expressed as mean ± SEM of 4 animals/time point.
Integrin α3β1 surface expression increases on human neutrophils during sepsis. (A) Integrin surface expression on neutrophils from SIRS patients (n = 9), sepsis patients (n = 15), and healthy donors (n = 7). Flow cytometry results expressed as a ratio of the integrin mean fluorescence intensity (MFI) to isotype control MFI of the same donor. *P < .05 (Wilcoxon rank-sum test). (B) Neutrophils isolated from healthy donors were stimulated with PMA (20 ng/mL), TNF-α (20 ng/mL), LPS (100 μg/mL), or fMLP (1 μM) for 1 or 3 hours. Fold changes in Itga3 gene expression, compared with unstimulated cells, were determined by reverse transcription polymerase chain reaction (upper panel), and surface expression of α3β1 was measured by flow cytometry (bottom panel). Data are expressed as mean ± SEM of 3 separate donors. *P < .05 (Mann-Whitney test). Integrin α3β1 is upregulated on mouse neutrophils in (C) CLP surgery and (D) endotoxemia models of sepsis. Cells isolated from bone marrow, peritoneal lavage, and peripheral blood of naive and septic mice at the indicated time points were gated for neutrophils using forward scatter/side scatter and Gr1high expression. The results are expressed as % increases in MFI compared with naive controls (naive blood MFI was used to calculate the % change in peritoneal lavage [PL] expression). Data are expressed as mean ± SEM of 4 animals/time point.
To perform detailed measurement of the expression kinetics of α3β1 and other ECM protein-binding integrins during sepsis, we used the following 2 mouse models: LPS-induced endotoxemia and CLP surgery. In both models, neutrophil stimulation was confirmed by the elevated surface expression of CD18, a well-known marker of neutrophil activation (Figure 1C-D). Flow cytometry analysis of neutrophils (Gr-1highCD11bhigh cells) isolated from the bone marrow, peripheral blood, and peritoneal lavage of LPS- and CLP-treated mice revealed that, of the ECM protein-binding integrins analyzed, only α3β1 expression was dramatically enhanced (Figure 1C-D). Recently published data from our laboratory implicated α3β1 in transmigration toward CXCL2 (MIP-2) in vivo,16 whereas other studies have described involvement of other integrins such as α6β1 (VLA-6; CD49f/CD29)17-19 and αvβ3 (CD51/CD61)20,21 in neutrophil and monocyte transendothelial migration during local inflammation. Interestingly, neither α6β1 nor αvβ3 has undergone such drastic upregulation over time as α3β1 did during systemic inflammation induced in our study. Our results show that the expression of α3β1 on neutrophils is enhanced during systemic inflammation; this finding may be critical for the recruitment of neutrophils into peripheral tissues during sepsis.
Increased α3β1 expression on neutrophils correlates with a highly pro-inflammatory phenotype
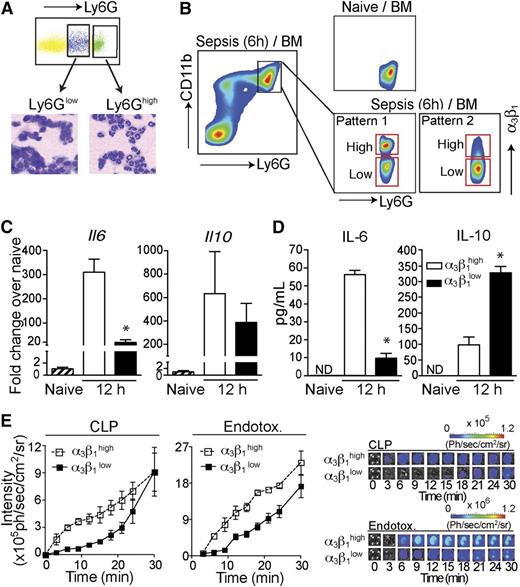
Though upregulated compared with healthy and SIRS groups, α3β1 expression on neutrophils from sepsis patients exhibited a large spread and became separated into 2 distinct populations (α3β1high and α3β1low; supplemental Figure 1) in some patients. The α3β1high neutrophil population was not observed in SIRS patients or healthy subjects. Similar to human patients, neutrophils from septic mice also exhibited a spread in α3β1 expression, as shown in Figure 2B. This was confirmed using both the Gr-1 and Ly6G antibodies to gate on neutrophils.
Gr1highCD11bhighα3β1high neutrophils have pro-inflammatory phenotypes. (A) Total bone marrow (BM) cells from C57BL/6 mice (6 hours after CLP) were stained with Ly6G and CD11b antibodies. Cells were sorted into Ly6Ghigh and Ly6Glow populations, as indicated, and stained with hematoxylin and eosin after cytospin. Ly6Ghigh cells demonstrate neutrophil morphology with a multilobed nucleus, and Ly6Glow cells have a monocyte-like appearance. (B) The gating strategy for α3β1high and α3β1low neutrophil populations in mice is shown. The pseudo color plots demonstrate α3β1 upregulation on mouse BM neutrophils (gated as Ly6GhighCD11bhigh single cells) 6 hours after CLP compared with neutrophils from naive mice. (C) Neutrophils from total BM of endotoxemia-treated mice were gated as Ly6GhighCD11bhigh cells and fluorescence-activated cell sorted based on their α3β1 expression levels into α3β1high and α3β1low populations, as shown in panel B. Fold changes in Il6 and Il10 gene expression 12 hours after sepsis induction, compared with neutrophils from naive mice, were quantified by reverse transcription polymerase chain reaction. Data are expressed as mean ± SD of 3 separate experiments. *P < .05 (Mann-Whitney test). (D) Fluorescence-activated cell -sorted α3β1high and α3β1low neutrophils isolated from endotoxemia mice 12 hours after sepsis induction were in vitro stimulated with LPS (100 ng/mL) to induce cytokine production. Culture supernatants were collected after 24 hours of stimulation and secreted IL-6 and IL-10 levels were measured by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD of 3 separate experiments. *P < .05 (Mann-Whitney test). (E) MPO activity of α3β1high and α3β1low BM neutrophils from CLP and endotoxemia mice was measured using bioluminescence. Sorted cells (5 × 104 cells/well) were stimulated with PMA (1 μM) in the presence of luminol (1 mg/well), and the luminescence intensity was imaged. The graphs of MPO activity kinetics (left) in α3β1high and α3β1low cells and the representative luminescence images (right) are shown. Endotox., endotoxin.
Gr1highCD11bhighα3β1high neutrophils have pro-inflammatory phenotypes. (A) Total bone marrow (BM) cells from C57BL/6 mice (6 hours after CLP) were stained with Ly6G and CD11b antibodies. Cells were sorted into Ly6Ghigh and Ly6Glow populations, as indicated, and stained with hematoxylin and eosin after cytospin. Ly6Ghigh cells demonstrate neutrophil morphology with a multilobed nucleus, and Ly6Glow cells have a monocyte-like appearance. (B) The gating strategy for α3β1high and α3β1low neutrophil populations in mice is shown. The pseudo color plots demonstrate α3β1 upregulation on mouse BM neutrophils (gated as Ly6GhighCD11bhigh single cells) 6 hours after CLP compared with neutrophils from naive mice. (C) Neutrophils from total BM of endotoxemia-treated mice were gated as Ly6GhighCD11bhigh cells and fluorescence-activated cell sorted based on their α3β1 expression levels into α3β1high and α3β1low populations, as shown in panel B. Fold changes in Il6 and Il10 gene expression 12 hours after sepsis induction, compared with neutrophils from naive mice, were quantified by reverse transcription polymerase chain reaction. Data are expressed as mean ± SD of 3 separate experiments. *P < .05 (Mann-Whitney test). (D) Fluorescence-activated cell -sorted α3β1high and α3β1low neutrophils isolated from endotoxemia mice 12 hours after sepsis induction were in vitro stimulated with LPS (100 ng/mL) to induce cytokine production. Culture supernatants were collected after 24 hours of stimulation and secreted IL-6 and IL-10 levels were measured by enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD of 3 separate experiments. *P < .05 (Mann-Whitney test). (E) MPO activity of α3β1high and α3β1low BM neutrophils from CLP and endotoxemia mice was measured using bioluminescence. Sorted cells (5 × 104 cells/well) were stimulated with PMA (1 μM) in the presence of luminol (1 mg/well), and the luminescence intensity was imaged. The graphs of MPO activity kinetics (left) in α3β1high and α3β1low cells and the representative luminescence images (right) are shown. Endotox., endotoxin.
It has previously been proposed that a hyperinflammatory neutrophil subtype arises during sepsis, which may cause tissue damage and lead to multiple organ failure in sepsis.4 Increased systemic release of pro-inflammatory cytokines and elevated myeloperoxidase (MPO) activity are biomarkers of hyperresponsive neutrophils.3 To determine whether the Gr1highCD11bhighα3β1high cells are associated with a hyperinflammatory phenotype, we measured the mRNA levels of pro-inflammatory cytokines. At 12 hours after sepsis induction, the expression level of interleukin-6 (IL-6) mRNA in the α3β1high neutrophils was significantly increased compared with its levels in α3β1low neutrophils from the same mice or naive neutrophils from untreated controls (Figure 2C). IL-10 mRNA was also significantly but similarly upregulated between α3β1high and α3β1low populations (Figure 2C), whereas TNF-α mRNA was only weakly induced (∼4-fold) compared with naive controls and was also similarly expressed by α3β1high and α3β1low neutrophils (data not shown). Consistent with mRNA data, α3β1high neutrophils release a significantly higher amount of IL-6 compared with IL-6 secretion from α3β1low cells in the presence of LPS (Figure 2D). Interestingly, unlike the IL-10 mRNA data, α3β1low neutrophils secreted significantly more IL-10 compared with α3β1high cells (Figure 2D).
Next, we measured the enzymatic activity of MPO in the 2 neutrophil subgroups (α3β1high vs α3β1low). As shown in Figure 2E, the MPO activity of the α3β1high neutrophils from both the CLP- and LPS-treated mice exhibited a higher sensitivity to PMA stimulation than the α3β1low cells. Taken together, our data suggest that integrin α3β1 upregulation is a novel marker for hyperinflammatory neutrophils.
Blocking or conditional deletion of integrin α3β1 improves survival during sepsis by interfering with neutrophil extravasation
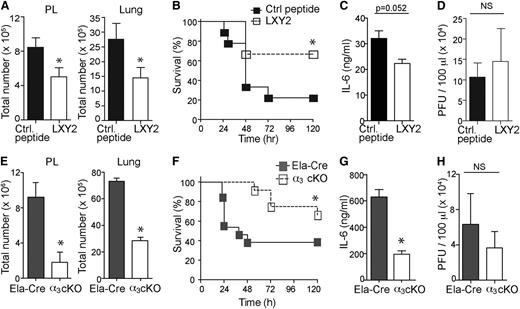
General inhibition of neutrophil extravasation or transendothelial migration could prove fatal by leaving all activated neutrophils within the circulation. Interestingly, partial depletion of CD18 was shown to improve inflammation.22 Integrin α3β1 expression on mouse neutrophils during sepsis showed a wide spread, with some cells being relatively high expressors of α3β1 and some being low expressors. These results led us to hypothesize that blocking integrin α3β1 may partially inhibit neutrophil infiltration during sepsis. Such targeting could be beneficial in reducing the severity of multiorgan dysfunction and sepsis mortality because the higher α3β1 expressors also demonstrate a more pro-inflammatory phenotype. Although there are no blocking antibodies for integrin α3β1 in mice, a combinatorial screen by Yao et al identified a cyclic peptide LXY2 as a high-affinity binding ligand (50% inhibition/inhibitory concentration = 57 nM) for α3β1.23 They have mapped the peptide–α3β1 interaction site and determined it to be overlapping, though not completely identical with the laminin-binding site on the α3 integrin subunit.23 We used LXY2 in our study to block mouse neutrophil migration in vivo. LXY2 administration (100 nmole, IV) significantly reduced the number of tissue-infiltrating neutrophils in mice 8 hours after CLP-induced sepsis (Figure 3A) without altering local bacterial clearance (Figure 3D). Moreover, periodic LXY2 injection (as shown in supplemental Figure 2A) protected mice from sepsis lethality (Figure 3B) and slightly reduced the levels of IL-6 in serum 8 hours after CLP (Figure 3C). Similar results were obtained using the endotoxemia model of sepsis (supplemental Figure 2B-C). Even at low doses (10 nmole), LXY2 injection reduced neutrophil transmigration of α3β1high and α3β1low neutrophils to the infectious site of the peritoneal cavity (supplemental Figure 2D). On the other hand, only α3β1high neutrophil numbers were reduced in whole lungs of LXY2-treated mice, with no significant change in α3β1low neutrophil numbers (supplemental Figure 2E). These findings parallel the in vitro adhesion assays with α3β1high and α3β1low neutrophils on laminin-coated slides (supplemental Figure 2F), where fMLP induced robust adhesion of α3β1high neutrophils, which was completely reversed by preincubation of cells with 10 μM LXY2. α3β1low cells exhibited a wide spread in N-formyl-methionine-leucine-phenylalanine (fMLP)-induced adhesion to laminin from experiment to experiment and, although on average their adhesion was also reduced by LXY2, statistically significant differences could not be obtained among any of the groups for the α3β1low neutrophils.
Blocking and conditional deletion of α3β1 inhibits neutrophil migration and improves survival. (A) Total cells were isolated from PL and lungs of septic mice (8 hours after CLP) injected either with LXY2 or control peptide (88 μg or 100 nmole/dose, IV) 2 hours after CLP. Bar graphs show total number of neutrophils (Gr1/Ly6GhighCD11bhigh cells), 5 mice/group. (B) CLP surgery was performed and either LXY2 or control peptide (100 nmole/dose) was injected post-CLP, as shown in supplemental Figure 3C. Mouse survival was analyzed using Kaplan-Meier log-rank test (9/group). (C) Serum concentrations of IL-6 (8 hours after CLP) from LXY2- and control peptide–treated mice were measured by sandwich ELISA. Values are expressed in ng/mL (5 mice/group). (D) Bacterial loads from PL of LXY2- and control peptide–treated mice (5/group). Diluted samples were streaked on tryptone soya agar blood agar and colonies counted after 24 hours’ incubation at 37°C. (E) Total cells were isolated from PL and lungs of α3 cKO mice (Itga3flox/flox; Ela-Cre) and littermate Ela-Cre controls (Itga3wt/wt; Ela-Cre) 8 hours after CLP. Neutrophils were gated as described earlier (4 mice/group). (F) CLP surgery was performed and survival analyzed using Kaplan-Meier log-rank test to compare α3 cKO and Ela-Cre control mice (12/group). (G) Serum levels of IL-6 (8 hours after CLP, 4 mice/group). (H) Bacterial loads in PL of α3 cKO and Ela-Cre mice 8 hours after CLP (4 mice/group). (A,C,D,E,G,H) Data are expressed as mean ± SEM. *P < .05 (Mann-Whitney test).
Blocking and conditional deletion of α3β1 inhibits neutrophil migration and improves survival. (A) Total cells were isolated from PL and lungs of septic mice (8 hours after CLP) injected either with LXY2 or control peptide (88 μg or 100 nmole/dose, IV) 2 hours after CLP. Bar graphs show total number of neutrophils (Gr1/Ly6GhighCD11bhigh cells), 5 mice/group. (B) CLP surgery was performed and either LXY2 or control peptide (100 nmole/dose) was injected post-CLP, as shown in supplemental Figure 3C. Mouse survival was analyzed using Kaplan-Meier log-rank test (9/group). (C) Serum concentrations of IL-6 (8 hours after CLP) from LXY2- and control peptide–treated mice were measured by sandwich ELISA. Values are expressed in ng/mL (5 mice/group). (D) Bacterial loads from PL of LXY2- and control peptide–treated mice (5/group). Diluted samples were streaked on tryptone soya agar blood agar and colonies counted after 24 hours’ incubation at 37°C. (E) Total cells were isolated from PL and lungs of α3 cKO mice (Itga3flox/flox; Ela-Cre) and littermate Ela-Cre controls (Itga3wt/wt; Ela-Cre) 8 hours after CLP. Neutrophils were gated as described earlier (4 mice/group). (F) CLP surgery was performed and survival analyzed using Kaplan-Meier log-rank test to compare α3 cKO and Ela-Cre control mice (12/group). (G) Serum levels of IL-6 (8 hours after CLP, 4 mice/group). (H) Bacterial loads in PL of α3 cKO and Ela-Cre mice 8 hours after CLP (4 mice/group). (A,C,D,E,G,H) Data are expressed as mean ± SEM. *P < .05 (Mann-Whitney test).
Determining the contributions of integrin α3β1 to immune functions in vivo has been challenging because of the lethality of a full α3 integrin subunit knockout in mice, which die of developmental defects of the kidney and lungs.24 To circumvent this problem and to further assess the function of integrin α3β1 on neutrophils in sepsis, we generated a conditional knockout Itga3flox/flox; Ela-Cre (α3 cKO) by crossing Itga3 floxed mice with mice expressing Cre recombinase inserted after the first exon of the granulocyte elastase (Ela) gene. Deletion of the floxed α subunit alleles and the absence of protein expression were confirmed, as shown in supplemental Figure 3A-B. Itga3wt/wt; Ela-Cre (Ela-Cre) mice were used as littermate controls for all experiments, rather than Itga3flox/flox mice, to control for the effects of truncated elastase within our α3 cKO mice. As supplemental Figure 4A shows, the Ela-Cre mice deficient in elastase demonstrate a strikingly improved sepsis survival over Itga3 floxed mice. This is consistent with the previously reported findings,14 which described improved survival of elastase deficient mice over wild-type controls in a model of low-dose endotoxic shock in combination with the sensitizer d-galactosamine. Deletion of α3β1 on neutrophils resulted in improved survival compared with the control Ela-Cre and Itga3 floxed mice after LPS (supplemental Figure 4A) and CLP treatments (Figure 3F). This improved survival was likely due to the reduced number of neutrophils infiltrating into the lungs and the peritoneal space, as measured within 8 hours of CLP (Figure 3E). Serum levels of IL-6 were reduced in the α3 cKO mice compared with the controls, indicating the reduced severity of sepsis (Figure 3G), while peritoneal bacterial clearance, on the other hand, between these groups was unaffected (Figure 3H). Similar results were obtained upon endotoxemia treatment of α3 cKO and Ela-Cre mice (supplemental Figure 4B-C), demonstrating reduced neutrophils infiltration into lung tissues and reduced serum IL-6 levels in α3 cKO mice.
It is interesting to note that, through a compensatory mechanism, naive α3 cKO mice upregulated their integrin α6β1 surface expression by approximately 5-fold (supplemental Figure 3C). This α6β1 upregulation, however, could not functionally compensate for the deficiency in α3β1-mediated extravasation of neutrophils from α3 cKO mice during CLP-induced sepsis. Interestingly, integrin CD11b was also upregulated (1.8-fold) on naive α3 cKO mice (supplemental Figure 3C). Because neutrophils in the perivascular space adhere and migrate on endothelial and pericyte monolayers in a Mac-1–dependent manner,25 CD11b upregulation on α3 cKOs may be an attempt to overcome the blockage in neutrophil basement membrane transmigration resulting from α3β1 deletion. However, none of the compensatory effects in the α3 cKO could sufficiently make up for the neutrophil migration defect resulting from α3β1 deletion.
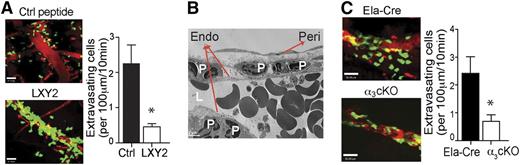
To access the inflamed tissues, neutrophils must cross the basement membrane underlying the vascular endothelial cells. Integrin α3β1 has been implicated in neutrophil and cancer cell adhesion and in vitro chemotaxis along ECM proteins, including laminin, fibronectin, and collagen IV,26-29 which are the predominant components of all basement membranes.10 Previous studies by Dangerfield et al17,19 showed that blocking antibodies against integrin α6β1, another laminin-binding integrin, could inhibit neutrophil subendothelial crawling in vivo. Interestingly, α6β1-mediated migration was only elicited by IL-1β stimulation, but not in response to TNF-α or fMLP. Thus, we proceeded to test whether integrin α3β1 is involved in neutrophil vascular extravasation in response to fMLP. Cremaster blood vessel imaging by intravital multiphoton microscopy revealed that mice injected with LXY2 (100 nmole, IV) demonstrated dramatically reduced neutrophil extravasation to fMLP (Figure 4A; supplemental Movies 1 and 2). Furthermore, ultrastructural analyses by electron microscopy showed that the majority of neutrophils accumulated within the space between the endothelium and the pericyte layer (Figure 4B). The number of extravasating neutrophils upon fMLP stimulation was also significantly reduced in the α3 cKO mice compared with control Ela-Cre animals (Figure 4C; supplemental Movies 3 and 4). Taken together, our data suggest that the α3β1-mediated migration through the vascular wall laminin is a critical step in neutrophil extravasation during bacterial infection–initiated inflammation and sepsis, in contrast to the α6β1-mediated transmigration previously reported to occur in IL-1β–dependent sterile inflammation. Moreover, selective deletion of the integrin α3β1 from granulocytes successfully decreases the massive tissue infiltration of neutrophils and improves survival during sepsis.
Integrin α3β1 blockade or targeted genetic depletion impedes neutrophil extravasation at the basement membrane. (A) The effect of α3β1 blockade on neutrophil migration in vivo was assessed by intravital multiphoton microscopy in the 1 μM fMLP-superfused cremaster vessels of LysM-GFP mice after IV injections of either control (Ctrl) peptide or LXY2 (88 μg or 100 nmole). (B) Transmission electron microscopy imaging of fMLP-superfused cremaster microvasculature of mice administered LXY2. Endo, endothelium; L, lumen; peri, pericyte; P, polymorphonuclear leukocytes. (C) The effect of α3β1 conditional deletion in α3 cKO (Itga3flox/flox; Ela-Cre) mice compared with control Ela-Cre (Itga3wt/wt; Ela-Cre) mice on neutrophil transmigration in response to fMLP (1 μM), assessed by intravital multiphoton microscopy. (A-C) Results are representative of 3 independent experiments. (A,C) Results were quantified and expressed as mean ± SEM. *P < .05 (Mann-Whitney test).
Integrin α3β1 blockade or targeted genetic depletion impedes neutrophil extravasation at the basement membrane. (A) The effect of α3β1 blockade on neutrophil migration in vivo was assessed by intravital multiphoton microscopy in the 1 μM fMLP-superfused cremaster vessels of LysM-GFP mice after IV injections of either control (Ctrl) peptide or LXY2 (88 μg or 100 nmole). (B) Transmission electron microscopy imaging of fMLP-superfused cremaster microvasculature of mice administered LXY2. Endo, endothelium; L, lumen; peri, pericyte; P, polymorphonuclear leukocytes. (C) The effect of α3β1 conditional deletion in α3 cKO (Itga3flox/flox; Ela-Cre) mice compared with control Ela-Cre (Itga3wt/wt; Ela-Cre) mice on neutrophil transmigration in response to fMLP (1 μM), assessed by intravital multiphoton microscopy. (A-C) Results are representative of 3 independent experiments. (A,C) Results were quantified and expressed as mean ± SEM. *P < .05 (Mann-Whitney test).
TLR2- but not TLR4-induced inflammatory responses are attenuated in α3β1-deficient neutrophils
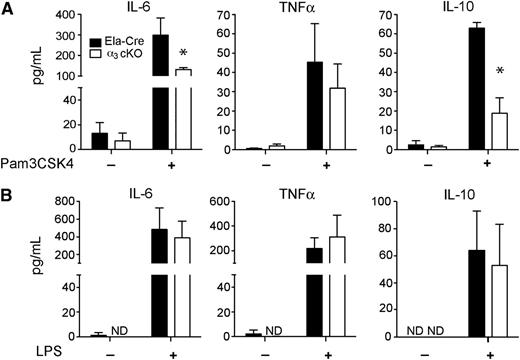
In addition to mediating cell migration, integrins can promote a host defense through cooperation with other innate immune receptors, such as TLRs. To determine whether α3β1 deficiency has an impact on TLR responses, bone marrow neutrophils isolated by negative selection from α3 cKO and control (Ela-Cre) mice were stimulated with TLR4- and TLR2/1-specific stimuli, LPS, and Pam3CSK4, respectively. As shown in Figure 5A, integrin α3β1-deficient neutrophils released less IL-6 and IL-10 after 24 hours of stimulation with Pam3CSK4 than did neutrophils from control mice. LPS stimulation, on the other hand, induced no significant changes in IL-6 or IL-10 secretion by neutrophils from either group (Figure 5B). Neither stimulus (administered at 100 ng/mL) produced any differences in TNF-α secretion from α3β1-deficient neutrophils compared with control neutrophils (Figure 5A-B). There were no differences in TLR2 and TLR4 surface expression levels before stimulation between α3β1-deficient neutrophils and neutrophils from control mice (supplemental Figure 5A-B). Cell survival, based on % Annexin V+ cells between the 2 groups at 24 hours after initial incubation, was also similar; the averages of 3 experiments are summarized in supplemental Figure 5C.
Integrin α3β1 regulates TLR2-, but not TLR4-induced cytokine responses. (A) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of α3 cKO (Itga3flox/flox; Ela-Cre) mice (white bars) and control Ela-Cre (Itga3wt/wt; Ela-Cre) mice (black bars). Cells (106) were stimulated for 24 hours with Pam3CSK4 (100 ng/mL) and supernatants tested in duplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test). (B) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of α3 cKO (white bars) and control (black bars) mice. Cells (106 cells/300 μL) were stimulated for 24 hours with ultrapure LPS (100 ng/mL) and supernatants tested in duplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test).
Integrin α3β1 regulates TLR2-, but not TLR4-induced cytokine responses. (A) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of α3 cKO (Itga3flox/flox; Ela-Cre) mice (white bars) and control Ela-Cre (Itga3wt/wt; Ela-Cre) mice (black bars). Cells (106) were stimulated for 24 hours with Pam3CSK4 (100 ng/mL) and supernatants tested in duplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test). (B) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of α3 cKO (white bars) and control (black bars) mice. Cells (106 cells/300 μL) were stimulated for 24 hours with ultrapure LPS (100 ng/mL) and supernatants tested in duplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test).
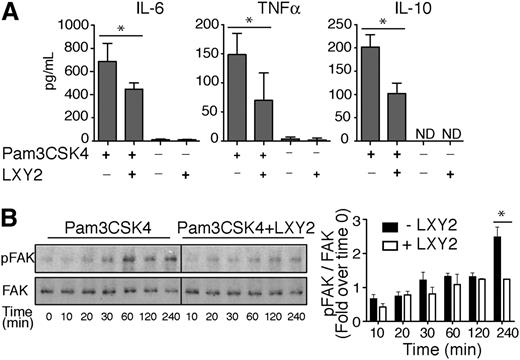
To further discern between direct effects of α3β1 on TLR2-dependent cytokine secretion vs compensatory effects from other integrins that are upregulated in α3 cKO mice, bone marrow neutrophils from wild-type C57BL/6 mice were stimulated for 24 hours with Pam3CSK4 alone or in combination with 1 μM LXY2. LXY2 binding to α3β1 resulted in mild attenuation of TLR2-induced responses, as evidenced by reduced secretion of IL-10, IL-6, and TNF-α (Figure 6A). Pam3CSK4 has been previously shown to induce FAK phosphorylation on Tyr397, and IL-6 release in primary mouse fibroblasts occurs thru a FAK-dependent pathway.30 To test whether α3β1 regulates Pam3CSK4-mediated cytokine release from neutrophil through FAK, we compared FAK phosphorylation levels in wild-type neutrophils upon Pam3CSK4 stimulation ± LXY2. Our data showed that a significant 2-fold decrease in FAK Tyr397 phosphorylation occurs 4 hours after coincubation with LXY2 and Pam3CSK4 vs Pam3CSK4 alone (Figure 6B). This result implies that disruption of a potential cooperative interaction between Pam3CSK4 and α3β1 attenuates cytokine secretion in mouse neutrophils in a FAK-dependent manner.
Integrin α3β1-binding peptide LXY2 differentially regulates cytokine secretion due to TLR2 and TLR4 stimulation. (A) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of wild-type C57Bl/6 mice. Cells (106 cells/300 μL) were incubated for 24 hours with Pam3CSK4 (100 ng/mL) with and without α3β1-binding peptide LXY2 (1 μM). Supernatants were tested in duplicate or triplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test). (B) Left: Representative western blot images of FAK phosphorylation kinetics in neutrophils (600 000 cells/100 μL) from C57BL/6 mice after Pam3CSK4 (10 μg/mL) stimulation ± LXY2 (100 μM) over indicated periods. Total cell lysates were analyzed by western blotting to determine the extent of FAK phosphorylation at Tyr397. Right: Densitometric analysis of FAK phosphorylation in WT neutrophils from C57BL/6 mice. Levels of phospho-FAK were normalized to total FAK at each time point and expressed as mean fold increase compared with control (time = 0 minutes). Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest).
Integrin α3β1-binding peptide LXY2 differentially regulates cytokine secretion due to TLR2 and TLR4 stimulation. (A) ELISA of IL-6, TNF-α, and IL-10 in supernatants of neutrophils, isolated by negative selection, from BM of wild-type C57Bl/6 mice. Cells (106 cells/300 μL) were incubated for 24 hours with Pam3CSK4 (100 ng/mL) with and without α3β1-binding peptide LXY2 (1 μM). Supernatants were tested in duplicate or triplicate. The results from 3 independent experiments are expressed as mean ± SD. *P < .05 (Mann-Whitney test). (B) Left: Representative western blot images of FAK phosphorylation kinetics in neutrophils (600 000 cells/100 μL) from C57BL/6 mice after Pam3CSK4 (10 μg/mL) stimulation ± LXY2 (100 μM) over indicated periods. Total cell lysates were analyzed by western blotting to determine the extent of FAK phosphorylation at Tyr397. Right: Densitometric analysis of FAK phosphorylation in WT neutrophils from C57BL/6 mice. Levels of phospho-FAK were normalized to total FAK at each time point and expressed as mean fold increase compared with control (time = 0 minutes). Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest).
α3β1 deficiency promotes compensatory CD11b upregulation and impacts TLR signal transduction
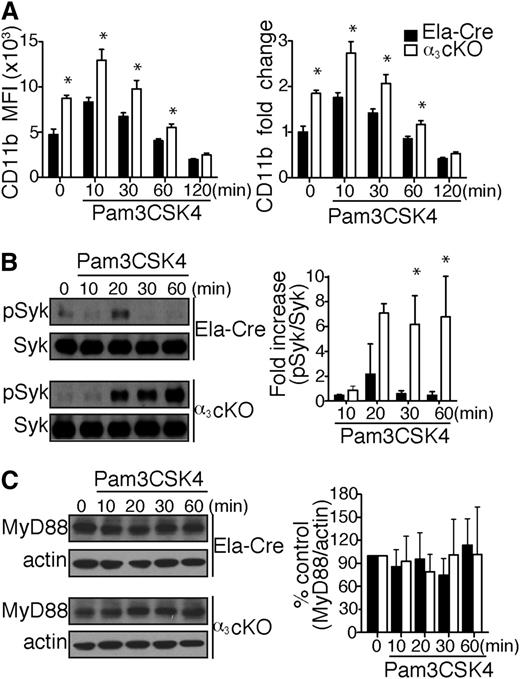
Interestingly, naive α3β1-deficient neutrophils demonstrate a 1.8-fold increase in CD11b expression compared with control mice (Figure 7A). Upon in vitro stimulation with Pam3CSK4, the difference in expression was maintained, though integrin recycling progressively diminished the fold change between groups until it became negligible 2 hours after initial stimulation. Han et al reported that CD11b negatively regulates TLR4 pro-inflammatory responses in macrophages by activating Syk and promoting MyD88 and TRIF degradation via Cbl-b.31 Although the role of CD11b in TLR regulation is not yet known in neutrophils, we hypothesized that CD11b compensatory upregulation on α3β1-deficient neutrophils contributes to the regulation of TLR2 inflammatory responses. Thus, we proceeded to test neutrophil whole cell lysates from α3 cKO and control (Ela-Cre) mice for Syk phosphorylation at different time points after Pam3CSK4 stimulation in vitro. Syk phosphorylation occurred transiently in the control mice 20 minutes after stimulation, whereas in α3 cKO mice, Syk remained stably phosphorylated over the 20- to 60-minute period (Figure 7B). We did not find any correlation between CD11b-induced Syk activation and MyD88 degradation in α3 cKO and Ela-Cre control neutrophils (Figure 7C). Longer sampling at 2 and 4 hours did not reveal significant differences in normalized MyD88 levels between our experimental groups (data not shown). Thus, CD11b-induced Cbl-b–dependent MyD88 degradation is not required for dampening TLR cytokine responses in mouse neutrophils.
Compensatory CD11b upregulation in α3β1-depleted mice negatively regulates TLR2 responses. (A) Surface expression of CD11b levels on BM neutrophils (gated as Ly6GhighCD115low cells) from α3 cKO and control Ela-Cre mice. Total BM cells (106) were stimulated with Pam3CSK4 (1 μg/mL) over time, as indicated (n = 4/time point). Data are expressed as mean MFI ± SD (top) or mean fold change compared with unstimulated neutrophils ± SD (bottom). *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest). (B) Left: Representative western blot images of Syk phosphorylation kinetics in neutrophils from α3 cKO and Ela-Cre mice after Pam3CSK4 (1 μg/mL) stimulation over indicated periods. Total cell lysates were analyzed by western blotting to determine the extent of Syk phosphorylation at Tyr346 (equivalent to Tyr352 for humans). Right: Densitometric analysis of Syk phosphorylation. phospho-Syk was normalized to total Syk at each time point and expressed as mean fold increase compared with time = 0 minutes. Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest). (C) Left: Representative western blot images of total MyD88 in neutrophils from α3 cKO and Ela-Cre mice after Pam3CSK4 (1 μg/mL) stimulation over time. Right: Densitometric analysis of bands expressed as % of control group (time = 0 minutes). MyD88 levels were normalized to actin at each time point. Data are expressed as mean % change ± SEM from 4 independent experiments.
Compensatory CD11b upregulation in α3β1-depleted mice negatively regulates TLR2 responses. (A) Surface expression of CD11b levels on BM neutrophils (gated as Ly6GhighCD115low cells) from α3 cKO and control Ela-Cre mice. Total BM cells (106) were stimulated with Pam3CSK4 (1 μg/mL) over time, as indicated (n = 4/time point). Data are expressed as mean MFI ± SD (top) or mean fold change compared with unstimulated neutrophils ± SD (bottom). *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest). (B) Left: Representative western blot images of Syk phosphorylation kinetics in neutrophils from α3 cKO and Ela-Cre mice after Pam3CSK4 (1 μg/mL) stimulation over indicated periods. Total cell lysates were analyzed by western blotting to determine the extent of Syk phosphorylation at Tyr346 (equivalent to Tyr352 for humans). Right: Densitometric analysis of Syk phosphorylation. phospho-Syk was normalized to total Syk at each time point and expressed as mean fold increase compared with time = 0 minutes. Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 (2-way repeated measures ANOVA with Bonferroni posttest). (C) Left: Representative western blot images of total MyD88 in neutrophils from α3 cKO and Ela-Cre mice after Pam3CSK4 (1 μg/mL) stimulation over time. Right: Densitometric analysis of bands expressed as % of control group (time = 0 minutes). MyD88 levels were normalized to actin at each time point. Data are expressed as mean % change ± SEM from 4 independent experiments.
Discussion
The dynamic migration of neutrophils first through the endothelium and subsequently through the ECM is a key step in the pathophysiology of sepsis-associated tissue injury and organ dysfunction.4,32 After diapedesis across the capillary endothelium, neutrophils must cross the subendothelial basement membrane to migrate through the ECM and gain access to the tissue space.33 α3β1 is a key integrin that mediates neutrophil chemotaxis through the basement membrane.16 In this study, we show that integrin α3β1 deletion in neutrophils attenuates their tissue infiltration and improves survivals during sepsis.
As the first line of defense, neutrophils are often considered to be a short-lived and homogeneous cell population. During inflammation, however, neutrophils actively migrate into infected or inflamed tissues and present themselves to local inflammatory mediators that prolong their survival and retention during disease progression.34 In this report, several lines of evidence suggest that α3β1high expression serves as a marker for hyperinflammatory neutrophils during sepsis. First, flow cytometry analysis of circulating and tissue-infiltrating neutrophils from septic human patients and murine sepsis models revealed that a subset of activated neutrophils expresses high levels of cell-surface α3β1. Furthermore, the dramatic upregulation of α3β1 during sepsis correlated with increased IL-6 production and secretion, as well as elevated MPO activity in the α3β1high neutrophil population. Although treatment with LXY2 blocking peptide resulted in global reduction of neutrophil tissue infiltration in vivo and laminin adhesion in vitro, α3β1high neutrophils were more robustly affected compared with lower α3β1 expressors. Consistent with our findings on neutrophil heterogeneity during sepsis, emerging evidence has suggested the existence of neutrophil subsets, including those that exhibit differential expression of fMLP35 and T-cell receptors.36 Moreover, subsets of neutrophils with distinct cytokine and chemokine production profiles, different effects on macrophage activation, and unique surface antigen expression have been identified in mice37 and humans.38 Our findings therefore further extend the current understanding of neutrophil characteristics in the mammalian immune system.
Whereas activated neutrophils can damage tissue, they can also kill bacteria. Antibodies directed against highly expressed adhesion molecules important for leukocyte extravasation, such as β2 (CD18) integrins and ICAM-1, have shown benefit in certain settings, but have been associated with devastating infections. Because life-threatening infections generally appear much sooner in neutropenic conditions than in lymphopenic patients, the administration of agents that block the recruitment of neutrophils into infected tissue sites has a greater risk of infection and immune response impairment compared with that seen upon the use of agents that interfere with lymphocyte recruitment. A spread in α3β1 expression levels on neutrophils in the bone marrow, circulation, and tissues during sepsis allows partial therapeutic blockade of neutrophil infiltration in sepsis, as we have demonstrated here. Although the effectiveness of this approach remains to be tested with live bacterial infections, it aims to slow down the influx of pro-inflammatory neutrophils into tissue spaces, while still allowing some to infiltrate and carry out local bacterial clearance. In the mouse model of CLP described here, such therapeutic blocking has significantly reduced sepsis lethality in mice. This suggests that α3β1 may be a suitable target for anti-inflammatory therapy during sepsis.
Short hairpin RNA knockdown of integrin α3β1 in U937 macrophages reduced their endocytosis of Borrelia burgdorferi spirochetes, indicating that α3β1 may be important for phagocytosis in other cells.36 Our findings using CLP did not indicate a difference in peritoneal cavity bacterial clearance between neutrophil-specific α3 cKO mice and corresponding Ela-Cre controls. However, these results are likely due to the inherent inability of neutrophils to phagocytose bacteria in blood- or fluid-filled body cavities, where macrophages are primarily responsible for bacterial clearance. Recently, increasing evidence is coming to light with regard to the role of TLR239 and lipoproteins,40 which are widely expressed in both gram-negative and gram-positive bacteria,41 in mediating sepsis mortality and better mimicking latter stage cell dysfunction observed in CLP animals as opposed to LPS. Our in vitro work showed another aspect to integrin α3β1, in addition to migration, in mediating neutrophil inflammatory responses in sepsis by cooperating with TLR2/1 and enhancing downstream cytokine secretion from neutrophils. Treatment with LXY2, a high-affinity peptide that blocks α3β1 both in vitro and in vivo, attenuated both FAK phosphorylation and cytokine release. Integrin α3β1 has already been implicated in IL-6 production from endolysosomes in macrophages,42 and CD14-integrin interactions were shown to enhance bacterial phagocytosis.43,44 TLR2/1 has also been shown to form a transient complex with integrin αvβ3, which promoted recognition of bacterial lipopeptides bound to vitronectin and subsequent TNF-α release.45 It is tempting to speculate that mouse neutrophils in our study, incubated in vitro in protein-free media for 24 hours for cytokine collection, may produce and secrete laminin, which in turn promotes complex formation between TLR2/1 and integrin α3β1, aiding in recognition and signal transduction by Pam3CSK4 lipopeptide.46 LXY2 binding to α3β1 on wild-type neutrophils may disrupt the multireceptor complex by inhibiting its interaction with laminin-associated Pam3CSK4 and thus reduce its internalization and/or signaling for cytokine production and release.
Previously, a study by Werr et al7 showed that cell surface expression of β1 integrins is highly upregulated on extravasated polymorphonuclear leukocytes during inflammation and that members of the β1 integrin subfamily other than α4β1 and α5β1 are important for extravascular polymorphonuclear leukocyte motility. As demonstrated in the current report, there was a significant difference in α3β1 expression levels in SIRS and severe sepsis patients. SIRS is commonly seen after major surgery or trauma and involves severe inflammation. If SIRS is accompanied by a confirmed infection, it is diagnosed as sepsis. Thus, our results indicate that strong TLR ligand-mediated effects of microbial components in addition to the pro-inflammatory milieu could be important for α3β1 upregulation on neutrophils during sepsis. Because the prompt diagnosis of sepsis warrants rapid therapy, including the early administration of antibiotics and control of the source of sepsis, the accurate differentiation between sepsis and SIRS is critical. Thus, many biomarkers have been proposed in clinical settings. Our data suggest that α3β1 is of value for differentiating between sepsis and SIRS in critically ill patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jennifer Wong and Sumir Shah for their technical assistance with mouse genotyping, Nathan Laniewski for cell sorting, and Susan Mroczek, David Oldfield, and Rebecca Schultz for assistance in compiling patient diagnostic data.
This work was supported by the National Institutes of Health (grants HL087088, HL018208, and HL094797) (M.K.) and (grant T32 DA07232) (Y.V.L.).
Authorship
Contribution: Y.V.L. and P.P.S. performed experiments and analyzed data; M.K. and P.P.S. conceived the study; Y.-M.H. performed intravital multiphoton microscopy and prepared samples for electron microscopy; K.L. performed the western blotting procedure; K.L.F. collected samples and data from intensive care unit patients; H.Y. analyzed human septic patient data; A.P.P. designed and conducted the clinical research; A.S. provided the Itga3 flox mice; and Y.V.L., M.K., and P.P.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.P.S. is Department of Biotechnology, Indian Institute of Technology-Roorkee, Roorkee, India.
Correspondence: Minsoo Kim, Department of Microbiology & Immunology, David H. Smith Center for Vaccine Biology and Immunology, University of Rochester Medical Center, 601 Elmwood Ave, Box 609, Rochester, NY 14642; e-mail: minsoo_kim@urmc.rochester.edu; and Pranita P. Sarangi, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand 247667, India; e-mail: psarafbt@iitr.ac.in.

![Figure 1. Integrin α3β1 surface expression increases on human neutrophils during sepsis. (A) Integrin surface expression on neutrophils from SIRS patients (n = 9), sepsis patients (n = 15), and healthy donors (n = 7). Flow cytometry results expressed as a ratio of the integrin mean fluorescence intensity (MFI) to isotype control MFI of the same donor. *P < .05 (Wilcoxon rank-sum test). (B) Neutrophils isolated from healthy donors were stimulated with PMA (20 ng/mL), TNF-α (20 ng/mL), LPS (100 μg/mL), or fMLP (1 μM) for 1 or 3 hours. Fold changes in Itga3 gene expression, compared with unstimulated cells, were determined by reverse transcription polymerase chain reaction (upper panel), and surface expression of α3β1 was measured by flow cytometry (bottom panel). Data are expressed as mean ± SEM of 3 separate donors. *P < .05 (Mann-Whitney test). Integrin α3β1 is upregulated on mouse neutrophils in (C) CLP surgery and (D) endotoxemia models of sepsis. Cells isolated from bone marrow, peritoneal lavage, and peripheral blood of naive and septic mice at the indicated time points were gated for neutrophils using forward scatter/side scatter and Gr1high expression. The results are expressed as % increases in MFI compared with naive controls (naive blood MFI was used to calculate the % change in peritoneal lavage [PL] expression). Data are expressed as mean ± SEM of 4 animals/time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/24/10.1182_blood-2014-01-552943/4/m_3515f1.jpeg?Expires=1763544195&Signature=k80MWFNtk2wOMVeJmrL5kmKy99v5njHwjijJ0onw6nR~UtxWXg0YPUf05SqbZaWmTS4F~aQtVQILK0CSX91rrMul0jf5RK3uefGEQgvAQXTcpvZrPzVpHtiA04fU2YXSBvC2F5-uHXDrgJCfzHpR6XvnBDyim1rQ1HneGAg12r1-rk1ZnC5ScVHXcMaJaiXLEJTwZSQYlG0HJvzFDapbeYe96aWOEeisgYxSesHWJbczlyHQbI5-WJ4K72VlRSNRjYOIFy5AO7iXXDaTF5--xV1lh~bpTArqx4xCv-g3qEK22a14JABH8nLSMigHBPriAoEeWQd1I3FQ-bqCFrMakA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal