Abstract
Background: Higher-risk MDS and CMML comprise a spectrum of disorders associated with cytopenias, high risk of transformation to acute myeloid leukemia (AML), and truncated survival. Initial treatment with a hypomethylating agent such as azacitidine (AZA) is considered standard of care. Whether addition of the histone deacetylase inhibitor vorinostat (VOR), which acts synergistically with AZA to reactivate epigenetically silenced genes, or addition of lenalidomide (LEN), which impacts the bone marrow microenvironment, improves response rates compared to AZA monotherapy is unknown.
Methods: This Phase II study (ClinTrials.gov # NCT01522976) randomized higher-risk MDS (International Prognostic Scoring System (IPSS) Int-2 or High and/or bone marrow blasts >5%) and CMML patients (pts) with <20% blasts to receive AZA (75 mg/m2/d on d1-7 of a 28d cycle), AZA + LEN (10 mg/d on d1-21), or AZA + VOR (300 mg BID on d3-9). Eligibility criteria included: >18 years (yrs), no previous allogeneic transplant, no prior treatment with any of the study drugs, and adequate organ function; therapy-related (t)MDS was allowed. Pts continued treatment until disease progression, relapse, unacceptable toxicity, or lack of response. Dose reductions occurred for unresolved grade >3 adverse events (per NCI CTCAE) or delayed count recovery. The primary endpoint was improvement in overall response rate (ORR), by intention to treat and reviewed centrally, of one of the combination arms vs. AZA monotherapy per 2006 International Working Group MDS response criteria (complete response (CR) + partial response (PR) + hematologic improvement (HI)). Relapse-free survival (RFS) was from time of response. The study had 81% power to detect a 20% improvement in ORR from 35% to 55%.
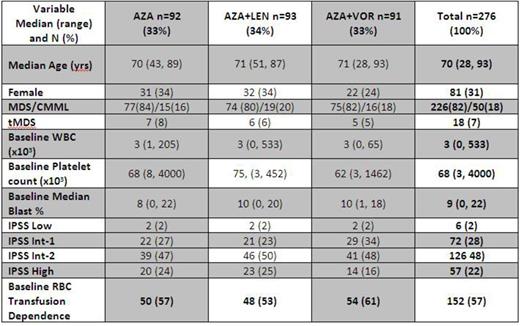
Results: Of 282 pts enrolled from 3/12–6/14, 276 are included in analyses (6 ineligible pts excluded): 92 on the AZA arm, 93 on AZA+LEN, and 91 on AZA+VOR. Baseline characteristics were well-balanced across arms (Table). Pts received a median of 23 weeks of therapy: 25 of AZA; 24 of AZA+LEN; and 20 of AZA+VOR and were followed for a median of 9 months (range: 0-26). Numbers of pts with notable adverse events >grade 3 for AZA:AZA+LEN:AZA+VOR included febrile neutropenia (10:13:13); gastrointestinal disorders (4:11:23); infections (2:3:3); and rash (2:12:1). Responses were assessable in 260 pts (94%). ORR for the entire cohort was 33%: 19% CR, 1% PR, and 13% HI, with a median RFS of 7 months. ORR was similar across study arms: 36% for AZA, 37% for AZA+LEN (p=1.0 vs. AZA), and 22% for AZA+VOR (p=.07 vs. AZA). CR/PR/HI rates across arms were also similar: 23%/0%/13% for AZA; 18%/1%/17% for AZA+LEN (CR p=.47 vs. AZA); and 14%/1%/7% for AZA+VOR (CR p=.18 vs. AZA); rates of bone marrow exams to assess response were 76%, 67%, and 73%, respectively. HI-P/HI-E/HI-N rates were 21%/15%/5% for AZA, 26%/14%/15% for AZA+LEN, and 12%/8%/4% for AZA+VOR. HI-N rates were higher in AZA+LEN vs. AZA (p=.05) but otherwise were similar across arms. Median time to best response across arms was 15 weeks in AZA, 16 weeks in AZA+LEN, and 16 weeks in AZA+VOR. ORR did not vary significantly across arms in subgroup analyses for tMDS, baseline red blood cell (RBC) transfusion dependence, and by IPSS risk group. ORR for CMML pts for AZA:AZA+LEN:AZA+VOR was 33%:53%(p=.15 vs. AZA):12%(p=.41 vs. AZA). Allogeneic transplantation rates were: 7 pts on AZA, 6 on AZA+LEN, and 9 on AZA+VOR. For AZA:AZA+LEN:AZA+VOR, median RFS was: 6:8:11 months (log-rank p=.3 for combination arms vs. AZA, Figure); and for pts on therapy >6 months, it was 7:7.5:13 months (log-rank p=.11 for AZA+VOR, .74 for AZA+LEN vs. AZA).
Conclusions: In higher-risk MDS pts, ORR was similar for AZA monotherapy compared to AZA-containing combination arms, though some subgroups may have benefitted from combination therapy. Differences in types of response may have resulted from differential rates of follow-up bone marrow assessments. While a non-significant signal of a DFS advantage for combination therapy was observed, longer-term outcome data are being assessed.
Sekeres:Boehringer-Ingelheim: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Off Label Use: lenalidomide, vorinostat for higher-risk MDS. List:Celgene Corporation: Consultancy. Gore:Celgene: Consultancy, Research Funding. Attar:Celgene: Consultancy. Erba:Seattle Genetics: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda Pharmaceuticals International Co.: Research Funding; Astellas Pharma: Research Funding; Celgene: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal