Abstract
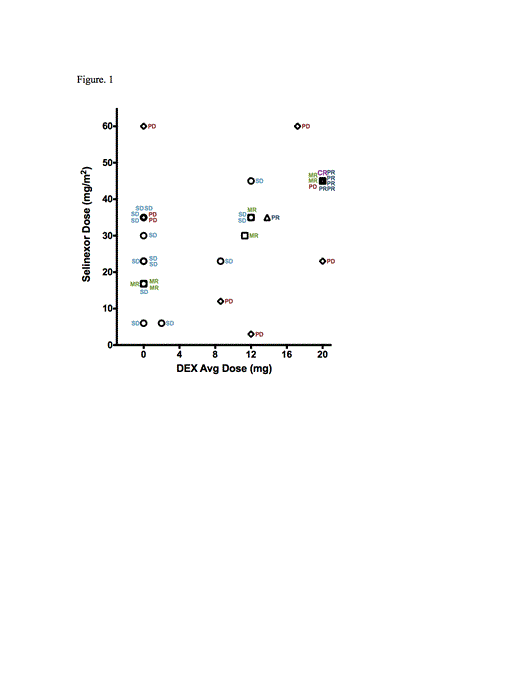
Introduction: The nuclear export protein exportin 1, (XPO1) is overexpressed in a wide variety of cancers including MM and often correlate with poor prognosis. Selinexor (KPT-330) is an oral Selective Inhibitor of Nuclear Export (SINE) XPO1 antagonist in Phase 1 and 2 clinical studies. Selinexor forces nuclear retention and reactivation of tumor suppressor proteins (TSPs) and reduction of many proto-oncogenes, including MDM2, MYC and Cyclin D. In addition, selinexor potently deactivates NF-κB, through forced nuclear retention of IκBα. Together these effects induce selective apoptosis in MM cells and inhibition of NF-κB dependent osteoclast activation. XPO1 is also responsible for nuclear export of the glucocorticoid receptor (GR). We hypothesized that selinexor will enhance the activity of dexamethasone (DEX)-bound GR, resulting in synergistic tumor cell killing. Methods: In vitro tumor cell viability measurements were based on MTT (CellTiter 96¨/Promega) and combination indices were calculated using CalcuSyn software. For xenograft studies, utilized NOD-SCID mice with subcutaneous inoculation of MM.1s cells. GR nuclear localization was measured with immunofluorescent anti-GR (phosphor-S211) antibody and quantitative imaging. To assess GR transcriptional activation, GR binding to a GCR consensus sequence was measured in nuclear extracts using an ELISA method (GR ELISA kit/Affymetrix). Patients (pts) with heavily pretreated refractory MM were dosed with oral selinexor at doses of up to 60 mg/m2 (8-10 doses/4 wk cycle) as part of a Phase 1 program in advanced hematological malignancies. Response we defined based on the IMWG criteria. The effect of combining DEX with selinexor was analyzed in all pts who received selinexor at moderate to high doses (30-60 mg/m2). Safety and efficacy were analyzed separately in three groups: no DEX, <20 mg DEX and 20 mgs DEX. Results: In MM.1s cells Sel-Dex showed synergy for nuclear retention of the DEX activated GR (Ser211-phosphorylated) and concomitant GR transcriptional activation. Sel-Dex showed highly synergistic cytotoxicity in MM.1s cells in vitro and in vivo, with a corresponding increase in apoptosis. Selinexor alone was potently cytotoxic in the DEX resistant MM cell lines MM.1R and ANBL6, but addition of DEX provided no additional effect. Twenty-eight pts with heavily pretreated refractory MM (16 M, 12 F; median age 62; ECOG PS 0/1: 7/21; median prior regimens: 6) received selinexor at 30 – 60 mg/m2 with either 0, <20, or 20 mgs DEX. All pts have received a proteasome inhibitor and an Imid and the majority of the pts have received pomalidomide (68%) and/or carfilzomib (36%). The most common Grade 1/2 AEs for these three groups were: nausea (82%/86%/70%), fatigue (55%/86%/40%), anorexia (36%/71%/60%), and vomiting (36%/57%/10%). Of the 28 pts treated; 10 heavily pretreated refractory MM pts treated with a combination of selinexor (45 mg/m2 twice weekly) and DEX (20 mg with each selinexor dose) were found to have dramatically improved disease response (n=10, ORR 60%), with one stringent complete response (sCR, 10%), 5 partial responses (PR, 50%) and clinical benefit rate (CBR) rate of 80% (Figure 1). Treatment with ³30mg/m2 selinexor and <20 mg DEX (n=7), resulted in ORR of 14% and CBR of 86%, while treatment with selinexor (30-60 mg/m2) without DEX (n=12) showed best response of stable disease (50%). Sel-Dex was also associated with an increase in time on study relative to selinexor alone, with 7 of out 10 pts in the 20 mg DEX combo group still on study (11-25 weeks). Five additional pts were treated with selinexor at a dose of 60 mg/m2 in combination with 20 mg DEX. Response evaluation is pending. Conclusions: Sel-Dex combination is markedly synergistic in preclinical models, which is supported by the preliminary clinical data presented. One potential mechanism underlying this synergy is the amplification of GR activity due the combined effects of selinexor-induced nuclear retention of activated GR coupled with DEX-mediated GR agonism. These results provide a promising basis for the continuing study of Sel-Dex for treatment of pts with refractory MM. Phase 2 studies of Sel-Dex in pts with MM refractory to both pomalidomide and carfilzomib are planned for early 2015.
Chen:Celgene: Honoraria; Janssen: Honoraria. Off Label Use: Lenalidomide maintenance therapy after ASCT. Gutierrez:Senesco: PI Other. Siegel:Celgene, Millennium, Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Baz:Celgene: Research Funding; Millennium: Research Funding; Bristol Myers Squibb: Research Funding; Karyopharm: Research Funding; Sanofi: Research Funding. Kukreti:Celgene: Honoraria. Azmi:Karyopharm Therpeutics: Research Funding. Kashyap:Karyopharm Therapeutics: Employment. Landesman:Karyopharm Therapeutics: Employment. Marshall:Karyopharm Therpeutics: Employment. McCartney:Karyopharm Therpeutics: Employment. Saint-Martin:Karyopharm Therpeutics: Employment. Norori:Karyopharm Therpeutics: Consultancy. Savona:Karyopharm Therpeutics: Membership on an entity's Board of Directors or advisory committees. Rashal:Karyopharm Therapeutics: Employment. Carlson:Karyopharm Therapeutics: Employment. Mirza:Karyopharm Therpeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shacham:Karyopharm Therapeutics Inc.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Kauffman:Karyopharm Therapeutics: Employment, Equity Ownership. Reece:Millennium: Honoraria, Research Funding; Millennium: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Merck: Research Funding; Merck: Research Funding; BMS: Research Funding; BMS: Research Funding; Novartis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen : Honoraria; Amgen : Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal