Abstract
Chronic graft-versus-host disease (cGVHD) after allogeneic hematopoietic cell transplantation (HCT) results from incomplete reconstitution of immune tolerance. CD4+CD25+FOXP3+ regulatory T cells (Treg) are required for tolerance and function as dominant suppressors of innate and adaptive immune effector cells. In our prior phase 1 cGVHD study daily subcutaneous (SC) low-dose interleukin-2 (IL-2) for 8 weeks induced Treg expansion in vivo and objective clinical responses in 12 of 23 evaluable participants (NEJM 2011). We now report on a phase 2 trial of daily low-dose SC IL-2 at 1x106 IU/m2/d for 12 weeks in steroid-refractory cGVHD.
The study comprised 35 HCT recipients (51% male, 91% HLA-matched PBSC grafts). Median participant age was 51 years (range, 22-72). Median time from HCT and from cGVHD onset to start of IL-2 treatment was 616 days (range, 270-2145) and 252 days (range, 28-1880) respectively. Participants had a median of 4 cGVHD organ sites (range, 1-7), and 2 concurrent cGVHD therapies (range, 1-3) at enrollment. The median baseline prednisone dose was 20 mg (range, 2.5-50). The median follow-up in survivors was 21 months (range, 4-35).
12 week low dose IL-2 was well tolerated: 2 participants withdrew and 5 required IL-2 dose reduction for constitutional AE (n=6) and thrombocytopenia (n=1); 1 had Gr 3 infection (bacteremia); and none experienced relapse. At week 12, objective cGVHD responses (PR) were documented in 21 of 33 evaluable participants (64%). Two (6%) had cGVHD progression. cGVHD response sites included skin (n=9), joint/fascia/muscle (n=4), liver (n=7), lung (n=3), and GI tract (n=4). Overall 2-year OS/PFS was 91% (responders 94%; non-responders 83%). 23 participants with clinical benefit (PR or SD with minor response) proceeded on extended IL-2 therapy.
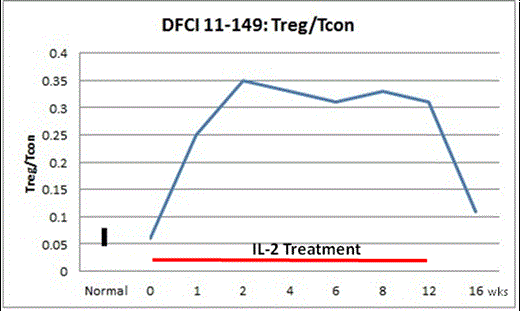
Immunologically, low dose IL-2 induced a >4-fold increase in median Treg count/µL (p<0.001): a rapid rise from a baseline of 17.1 (Q1-Q3, 8.6-40.6) to a week 4 peak of 137.9 (Q1-Q3, 51.8-188) and subsequent stabilization with a week 12 count of 104.1 (Q1-Q3, 53.9-167.1). No significant change in CD4 conventional T cell (Tcon), CD8 T cell, or CD20 B cell count was noted. NK cell count increased >3-fold (p<0.001). The median Treg:Tcon ratio increased >4-fold (p<0.001): a rapid rise from baseline of 0.06 (Q1-Q3, 0.05-0.13) to a week 2 peak of 0.35 (Q1-Q3, 0.26-0.48) that remained elevated through a week 12 ratio of 0.31 (Q1-Q3, 0.27-0.39) (Figure). Treg count and Treg:Tcon ratio declined during 4 weeks off IL-2 and rose thereafter on restarting IL-2.
Clinical responders were younger (50 vs. 61.5 years, p=0.01) and initiated IL-2 earlier (499 vs. 903 days post HCT, p=0.015). Responders had a higher median Treg:Tcon ratio at study baseline (0.09 vs. 0.06, p=0.052) and at week 1 of IL-2 (0.3 vs. 0.14, p=0.01). Combining phase 1 and 2 data, Treg:Tcon ratios of ≥0.07 at baseline and ≥0.2 at week 1 of IL-2 were highly predictive of clinical response (p=0.007; p=0.0013 respectively).
The combined phase 1 and 2 extended IL-2 cohort comprised 35 participants with a median follow up of 16.2 months (range, 4.1-66.8), with 20 and 12 participants receiving over 1 and 2 years of IL-2 respectively. Extended daily low dose IL-2 was well tolerated, and only 4 participants had Gr 3 AEs deemed IL-2 related: lung infection (n=1), arthralgia (n=1), and injection site induration (n=2). 5 participants required IL-2 dose reduction and 1 had hematologic malignancy relapse. Clinical responses were typically sustained during taper of concomitant immunosuppression. Treg augmentation persisted for the duration of IL-2 therapy. Tcon count slowly recovered to normal levels and Treg:Tcon ratio gradually normalized over a 2 year period. There was no change in CD8 count. The median steroid taper was 50% (range, -20-100).
In summary, daily low dose IL-2 therapy induced profound Treg enhancement, and clinical responses in over half of refractory cGVHD patients. Early clinical response predictors suggest IL-2 is more effective earlier in the cGVHD course and when starting numbers of Treg are higher. Sustained clinical and immunologic response during extended IL-2 was documented. Long term tolerance induction with daily low dose IL-2 is a promising and feasible strategy. Optimizing IL-2 clinical response by further augmenting Treg and the Treg;Tcon ratio early in the course of cGVHD is worth exploring.
Koreth:Prometheus Laboratories Inc: Research Funding; Millennium Pharmaceuticals Inc: Research Funding; Takeda Pharmaceuticals Inc: Membership on an entity's Board of Directors or advisory committees. Off Label Use: Low-dose Interleukin-2 for immune tolerance. Chen:Bayer Pharmaceuticals, Inc.: Other, Research Funding. Avigan:Astex Pharmaceuticals : Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal