Abstract
LY2510924 is a novel selective peptidic CXCR4 antagonist that blocks SDF-1α from binding to its receptor. We have demonstrated that LY2510924 at nanomolar concentrations durably disrupts the SDF-1α/CXCR4 axis in acute myeloid leukemia (AML) cells and exerts anti-leukemia effects as a single agent (AACR 2014: #4768). We further investigated the pronounced anti-leukemia activity of LY2510924 and the mechanisms underlying the anti-leukemia effect.
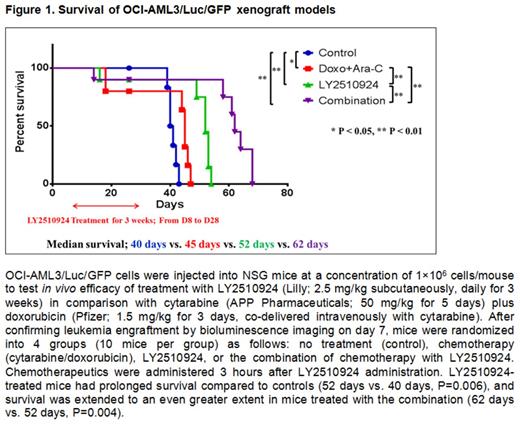
To test the efficacy of LY2510924 in combination with chemotherapy, we injected OCI-AML3/luc/GFP cells into NSG mice. Mice were randomized into 4 groups (10 mice per group) on day 8: control, chemotherapy (cytarabine [50 mg/kg, daily for 5 days, intravenous or intraperitoneal]/doxorubicin [1.5 mg/kg, daily for 3 days, co-delivered intravenously]), LY2510924 (2.5 mg/kg, daily for 3 weeks, subcutaneously), or chemotherapy and LY2510924. Bioluminescence imaging demonstrated that LY2510924 exerted an anti-leukemia effect equal to that achieved with chemotherapy (P=0.249), and the combination therapy group had the lowest luciferase activity. LY2510924-treated mice had prolonged survival (Figure 1) compared to controls (52 days vs. 40 days, p=0.006), and combination therapy extended survival even further (62 days vs. 52 days, p=0.004).
Next, we examined anti-leukemia efficacy of LY2510924 in primary human AML xenograft models. NSG mice were injected with primary AML cells and randomized into 2 groups on day 25, after engraftment was documented: control (n=13) and treatment with LY2510924 (n=15; 2.5 mg/kg subcutaneously, daily). First, we examined AML cell mobilization by measuring the proportion of circulating leukemic cells after daily LY2510924 administration. Mice treated with LY2519024 had a significant increase of circulating leukemic cells at 3 hours (2.1-fold, P=0.008), and further increases at 24 hours (2.7-fold, P=0.008) and 48 hours (3.0-fold, P=0.009) compared to controls. Flow cytometry showed a sustained inhibition of CXCR4 12G5 surface expression at 3 and 24 hours after the first LY2510924 injection. Thereafter, weekly examination of circulating leukemic cells in both groups revealed slower progression of leukemia in the LY2510924-treated group (54% vs. 86% circulating AML cells on day 45, P<0.001). Additionally, we sacrificed 3 mice per group on days 35 and 45 and demonstrated that LY2510924-treated mice had significantly lower leukemic cell burden in the spleen (22% vs. 51%, P=0.001) on day 35, and in both spleen (20% vs. 60%, P<0.001) and bone marrow (72% vs. 90%, P=0.012) on day 45 by flow cytometry. CXCR4 blockade with LY2510924 was associated with reduced AKT and/or ERK signaling in leukemic cells of spleen, bone marrow, and blood as measured by multi-parametric phospho-flow cytometry. This anti-leukemia effect translated into a significant prolongation of survival in LY2510924-treated mice (56 days vs. 44 days, p<0.001, Figure 2).
Our previous study (AACR 2014:#4768) demonstrated that LY2510924 did not induce AML cell death in vitro on its own but inhibited AML cell growth in co-cultures with human marrow stromal cells (hMSC). To explore how CXCR4-mediated signaling in AML cells elicits anti-leukemia effects, we performed whole gene expression profiling of FACS-sorted OCI-AML3 cells co-cultured with hMSC for 48 hours and co-treated with LY2510924, in duplicates. Among genes modified by CXCR4 antagonist, we found that CTNNB1 (human beta-catenin), JARID1C (lysine-specific demethylase 5C), RARA (retinoic acid receptor alpha), RARRES2 (chemerin), and COQ4 (coenzyme Q) were downregulated in co-cultured OCI-AML3 cells treated with LY2510925, when compared to either mono-cultured cells or co-cultured cells without LY2510924. These findings are currently being validated by using functional in vitro assays.
In conclusion, our findings demonstrate that CXCR4 antagonist LY2510924 inhibits AML progression in leukemia xenograft models in vivo and has a synergistic anti-leukemia effect in combination with chemotherapy. LY2510924 efficiently inhibits CXCR4 signaling in primary AML cells in vivo and induces mobilization of leukemic cells into circulation. This results in pronounced anti-leukemia activity as a single agent. LY2510924's potency and durable occupancy of CXCR4 receptors will likely translate into greater anti-leukemia potency in future clinical applications.
Peng:Eli Lilly & Company: Employment. Thornton:Eli Lilly & Company: Employment, stocks Other.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal